Increasing Functionality of Fish Leather by Chemical Surface Modifications
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Surface Modification of Leather with UC
2.3. Functionalization of Leather Surface Using PFDT
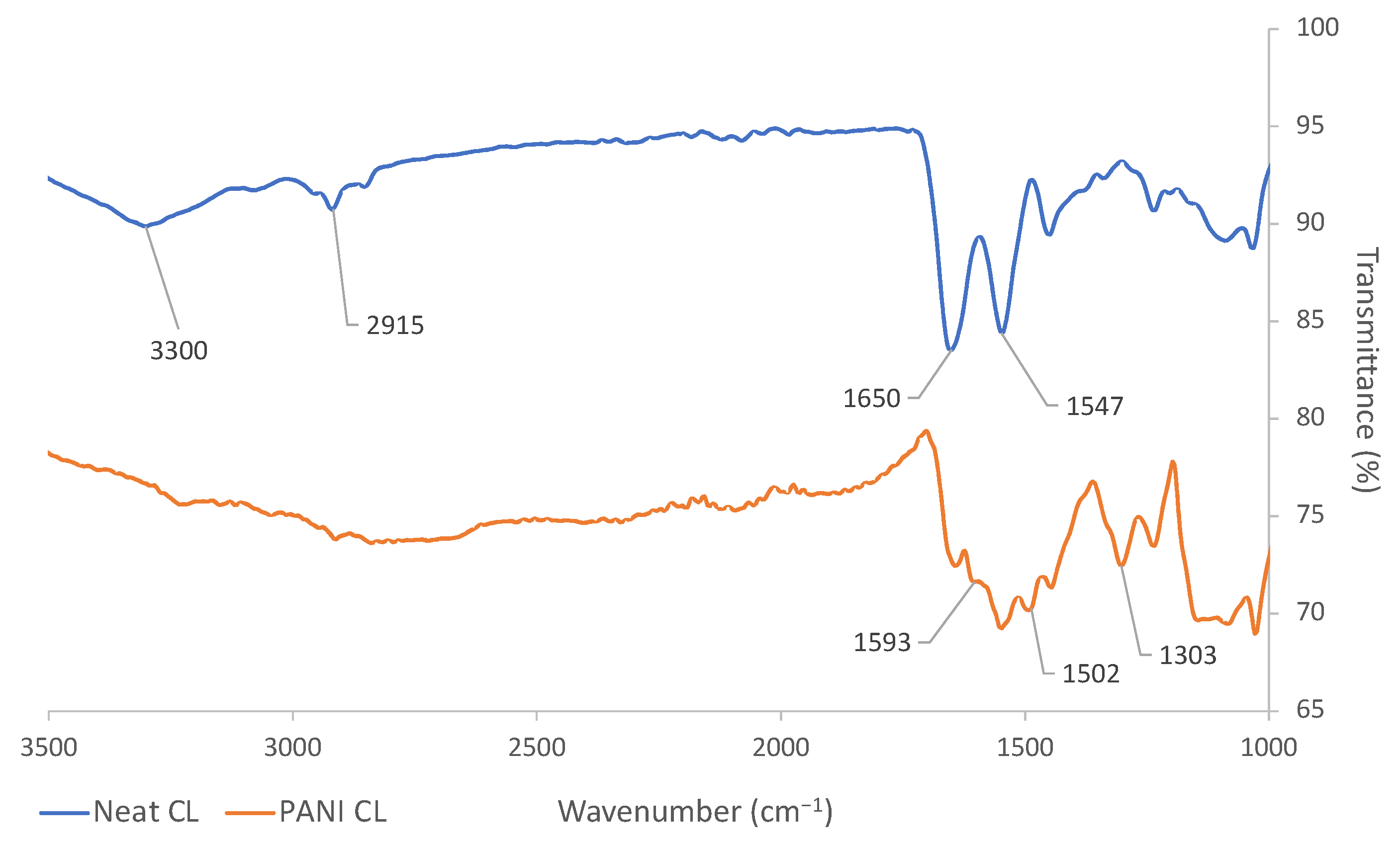
2.4. PANI Coating of Leather
2.5. PVDF-HFP/Nano-Silica Particle Coating Leather
2.6. Characterization Methods
3. Results and Discussion
3.1. Incorporation of Electro-Conductive Coating
3.2. Hydrophobic Surface Treatment
3.3. Amphiphobic Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Sheikh Abdullah, S.R.; Abu Hasan, H.; Othman, A.R.; Ismail, N.I. Aquaculture Industry: Supply and Demand, Best Practices, Effluent and Its Current Issues and Treatment Technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubcheno, J.; Shumway, S.E.; Troell, M. A 20-year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Maina, P.; Ollenga, M.A.; Nthiga, E.W. Trends in Leather Processing: A Review. Int. J. Sci. Res. Publ. 2019, 9, 9626. [Google Scholar] [CrossRef]
- Senthil, R.; Vedakumari, S.; Hemalatha, T.; Sumathi, V.; Gobi, N.; Sastry, T. New Approaches for the Effective Utilization of Fish Skin Wastes of Aluterus Monoceros. J. Earth Environ. Health Sci. 2016, 2, 50–55. [Google Scholar] [CrossRef]
- Muralidharan, V.; Palanivel, S.; Balaraman, M. Turning problem into possibility: A comprehensive review on leather solid waste intra-valorization attempts for leather processing. J. Clean. Prod. 2022, 367, 133021. [Google Scholar] [CrossRef]
- Sivakumar, V. Towards environmental protection and process safety in leather processing—A comprehensive analysis and review. Process. Saf. Environ. Prot. 2022, 163, 703–726. [Google Scholar] [CrossRef]
- Bhavya, K.S.; Selvarani, J.A.; Samrot, A.V.; Mohamed Javad, P.T.; Appalaraju, V.V.S.S. Leather Processing, Its Effect on Environment and Alternatives of Chrome Tanning. Int. J. Adv. Res. Eng. Technol. 2019, 10, 69–79. [Google Scholar] [CrossRef]
- Duraisamy, R.; Shamena, S.; Berekete, A.K. A Review of Bio-tanning Materials for Processing of Fish Skin into Leather. Int. J. Eng. Trends Technol. 2016, 39, 10–20. [Google Scholar]
- Kaygusuz, M.K.; Meyer, M.; Junghans, F.; Aslan, A. Modification of Leather Surface with Atmospheric Pressure Plasma and Nano-Finishing. Polym. Plast. Technol. Eng. 2017, 57, 260–268. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Bao, Y.; Zhu, Z. Synthesis and Application of Polyacrylate/Nano-SiO2 Composite Leather Finishing Agent with Polymerizable Surfactant. Polym. Plast. Tenchnol. Eng. 2012, 51, 1460–1467. [Google Scholar] [CrossRef]
- Gargano, M.; Bacardit, A.; Sannia, G.; Lettera, V. From Leather Wastes back to Leather Manufacturing: The Development of New Bio-Based Finishing Systems. Coatings 2023, 13, 775. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.; Bao, Y.; Liu, J. A Facile Spraying Method for Fabricating Superhydrophobic Leather Coating. Colloids Surf. A Physicochem. Eng. Asp. 2015, 472, 21–25. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Mu, C.; Wang, C.; Lin, W. Advances in Antimicrobial Polymer Coatings in the Leather Industry: A Comprehensive Review. Ind. Eng. Chem. Res. 2021, 60, 15004–15018. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Yan, K. CQDs-MoS2 QDs loaded on Dendritic fibrous Nanosilica/Hydrophobic waterborne polyurethane acrylate for antibacterial coatings. J. Chem. Eng. 2022, 429, 132170. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, X.; Zheng, M.; Yue, O.; Xie, L.; Zha, S.; Dong, S.; Li, T.; Song, Y.; Huang, M.; et al. Leather for flexible multifunctional bio-based materials: A review. J. Leather Sci. Eng. 2022, 4, 1–16. [Google Scholar] [CrossRef]
- Türk, M.; Ehrmann, A.; Mahltig, B. Water-, Oil-, and Soil-Repellent Treatment of Textiles, Artificial Leather, and Leather. J. Text. Inst. 2015, 106, 611–620. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Liu, X.; Yue, O.; Wang, X.; Jiang, H. Advanced Collagen Nanofibers-based Functional Bio-composites for High-Value Utilization of Leather: A Review. J. Sci. Adv. Mater. Devices 2021, 6, 153–166. [Google Scholar] [CrossRef]
- Kayaoğlu, B.K.; Öztürk, E. Imparting Hydrophobicity to Natural Leather through Plasma Polymerization for Easy Care Effect. Fibers Polym. 2013, 14, 1706–1713. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Abu-Thabit, N.Y.; Uwaezuoke, O.J.; Azad, A.K. Superwetting cotton textiles for separation of oil/water mixtures. Cellulose 2023, 30, 7427–7462. [Google Scholar] [CrossRef]
- Kamely, N. “Fatliquors” for Leathers: An Application of Microemulsion-A Review. Polym. Bull. 2021, 79, 1977–2002. [Google Scholar] [CrossRef]
- Jankauskaitė, V.; Gulbinienė, A.; Jihembetova, I.; Širvaitytė, J.; Urbelis, V.; Mickus, K.V. Comparable Evalution of Leather Waterproofing Behavior upon Hide Quality. II. Influence of Finishing on Leather Properties. Mater. Sci. 2014, 20, 165–170. [Google Scholar]
- Jankauskaitė, V.; Jihembetova, I.; Gulbinienė, A.; Širvaitytė, J.; Beleška, K.; Urbelis, V. Comparable Evalution of Leather Waterproofing Behavior upon Hide Quality. II. Influence of Retanning and Fatliqouring Agents on Leather Structure and Properties. Mater. Sci. 2012, 20, 150–157. [Google Scholar]
- Wei, C.; Wang, X.; Wang, W.; Sun, S.; Liu, X. Bifunctional amphoteric polymer-based ecological integrated retanning/fatliquoring agents for leather manufacturing: Simplifying processes and reducing pollution. J. Clean. Prod. 2022, 369, 133229. [Google Scholar] [CrossRef]
- Samanta, D.; Murali, A.; Prakash, J.A.; Nagaraju, P.; Ramesh, R.; Mitra, T.; Gnanamani, A.; Jaisankar, S.N.; Mohan, R.; Md Alam, S.; et al. Chromium-Assisted Immobilization of N-Isopropylacrylamide-based Methacrylic Acid Copolymers on Collagen and Leather Surfaces: Thermo-Responsive Behaviour. RSC Adv. 2013, 3, 16626–16631. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, B.; Pang, H.; Lu, M.; Meng, Y. Preparation and Characterization of a Self-Matting Coating based on Waterborne Polyurethane-Polyacrylate Hybrid Dispersions. Prog. Org. Coat. 2020, 143, 105551. [Google Scholar] [CrossRef]
- Xu, W.; Hao, L. Synthesis of Novel Anionic Fluorinated Polyacrylate Emulsion and Its Application in Leather Waterproofing. Adv. Mater. Res. 2012, 496, 511–514. [Google Scholar]
- Ayyappan, V.G.; Prakash, D.; Jaisankar, S.N.; Sadhukhan, N.; Alam, M.S.; Samanta, D. Nanoconjugates of Methacrylic Polymers: Synthesis, Characterization, and Immobilization to Leather. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Fan, Q.; Ma, J.; Xu, Q. Insights into Functional Polymer-based Organic-Inorganic Nanocomposites as Leather Finishes. J. Leather Sci. Eng. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Ma, J.; Ma, L.; Zhang, L.; Zhang, W.; Fan, Q.; Han, B. Bio-based Waterborne Poly(Vanillin-Butyl Acrylate)/MXene Coatings for Leather with desired Warmth Retention and Antibacterial Properties. Engineering 2023. [Google Scholar] [CrossRef]
- Yilmaz, O.; Cheaburu, C.N.; Gülümser, G.; Vasile, C. Rheological Behaviour of Acrylate/Montmorillonite Nanocomposite Latexes and their Application in Leather Finishing as Binders. Prog. Org. Coat. 2011, 70, 52–57. [Google Scholar] [CrossRef]
- Yu, F.; Gao, J.; Liu, C.; Chen, Y.; Zhong, G.; Hodges, C.; Chen, M.; Zhang, H. Preparation and UV Aging of Nano-SiO2/Fluorinated Polyacrylate Polyurethane Hydrophobic Composite Coating. Prog. Org. Coat. 2020, 141, 105556. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Bao, Y.; Wang, J.; Tang, H.; Zhang, L. Polyacrylate/Surface-Modified ZnO Nanocomposite as Film-Forming Agent for Leather Finishing. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 809–814. [Google Scholar] [CrossRef]
- Ramkumar, S.C.; Murali, A.; Preethi, G.; Chandrasekaran, B.; Saravanan, P.; Jaisankar, S.N. Polycarbodiimide and Polyurethane Cross-Linkers for Leather Finishing. Leather Footwear J. 2017, 17, 181–192. [Google Scholar] [CrossRef]
- Su, S.; Wang, J.; Li, C.; Yuan, J.; Pan, Z.; Pan, M. Short-branched Fluorinated Polyurethane Coating Exhibiting Good Comprehensive Performance and Potential UV Degradation in Leather Waterproofing Modification. Coatings 2021, 11, 395. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Xiao, Y.; Mu, C.; Lin, W. Fabrication of Water-Resistance and Durable Antimicrobial Adhesion Polyurethane Coating Containing Weakly Amphiphilic Poly(isobornyl acrylate) Side Chains. Prog. Org. Coat. 2020, 147, 105812. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, J.; Chen, H.; Zhou, P.; Li, C.; Yang, K.; Hua, N.; Wang, J.; Weng, M. Multi-functional graphene/leather for versatile wearable electronics. J. Mater. Chem. A 2023, 11, 11773–11785. [Google Scholar] [CrossRef]
- Zong, Y.; Tan, S.; Ma, J. Flame-Retardant PEDOT:PSS/LDHs/Leather Flexible Strain Sensor for Human Motion Detection. Macromol. Rapid Commun. 2022, 43, 2100873. [Google Scholar] [CrossRef]
- Gao, D.; Guo, S.; Zhou, Y.; Lyu, B.; Ma, J.; Zhao, P.; Pan, D.; Chen, S. Hydrophobic, flexible electromagnetic interference shielding films derived from hydrolysate of waste leather scraps. J. Colloid Interface Sci. 2022, 613, 396–405. [Google Scholar] [CrossRef]
- Stanca, M.; Gaidau, C.; Alexe, C.A.; Stanculescu, I.; Vasilca, S.; Matei, A.; Simion, D.; Constantinescu, R.R. Multifunctional Leather Surface Design by Using Carbon Nanotube-based Composites. Materials 2021, 14, 3003. [Google Scholar] [CrossRef]
- Hong, K.H. Preparation of Conductive Leather Gloves for Operating Capacitive Touch Screen Displays. Korea Sci. 2012, 14, 1018–1023. [Google Scholar]
- Ngwabebhoh, F.A.; Zandraa, O.; Sáha, T.; Stejskal, J.; Trchová, M.; Kopecký, D.; Pfleger, J.; Prokeš, J. In-situ coating of leather with conducting polyaniline in colloidal dispersion mode. Synth. Met. 2022, 291, 117191. [Google Scholar] [CrossRef]
- Wegene, J.D.; Thanikaivelan, P. Conducting Leathers for Smart Product Applications. Ind. Eng. Chem. Res. 2014, 53, 18209–18215. [Google Scholar] [CrossRef]
- Shabani, A.; Hylli, M.; Kazani, I.; Berberi, P. Resistivity Behavior of Leather After Electro-Conductive Treatment. Text. Leather Rev. 2019, 2, 15–22. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Zandraa, O.; Sáha, T.; Stejskal, J.; Kopecký, D.; Trchová, M.; Pfleger, J. Coating of Leather with Dye-Containing Antibacterial and Conducting Polypyrrole. Coatings 2023, 13, 608. [Google Scholar] [CrossRef]
- Vos, L.; Fah, A. Modified Silica Sol Coatings for Surface Enhancement of Leather. Acta Chim. Slov. 2012, 59, 331–337. [Google Scholar]
- Silvestre, C.R.; Blasco, M.P.C.; López, S.R.; Anguilar, H.P.; Limiñana, M.A.P.; Gil, E.B.; Calpena, E.O.; Ais, F.A. Hydrophobic Leather Coating for Footwear Applications by a Low-Pressure Plasma Polymerisation Process. Polymers 2021, 13, 3549. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, A.; Zohra, F.T.; Murad, A.B.M.W.; Ahmed, S. Enhancement of Waterproofing Properties of Finished Upper Leather Produced from Bangladeshi Cow Hides. Eur. J. Eng. Res. Sci. 2019, 4, 63–71. [Google Scholar] [CrossRef]
- Dan, Y.; Popowski, Y.; Buzhor, M.; Menashe, E.; Rachmani, O.; Amir, E. Covalent Surface Modification of Cellulose-Based Textiles for Oil-Water Separation Applications. Ind. Eng. Chem. Res. 2020, 59, 5456–5465. [Google Scholar] [CrossRef]
- Dan, Y.; Buzhor, M.; Raichman, D.; Amir, E. Covalent Surface Functionalization of Nonwoven Fabrics with Controlled Hydrophobicity, Water Absorption, and pH Regulation Properties. J. Appl. Polym. Sci. 2020, 138, 1–11. [Google Scholar] [CrossRef]
- Jarach, N.; Meridor, D.; Buzhor, M.; Raichman, D.; Dodiuk, H.; Kenig, S.; Amir, E. Hybrid Antibacterial and Electro-Conductive Coating for Textiles Based on Cationic Conjugated Polymer. Polymers 2020, 12, 1517. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, Self-healing Superamphiphobic Fabrics prepared by Two-Step Coating of Fluoro-Containing Polymer, Fluoroalkylsilane, and Modified Silica Nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- ISO 14268:2012; Leather–Physical and Mechanical Tests–Determination of Water Vapour Permeability. International Organization for Standardization: Geneva, Switzerland, 2012.
- Abilevitch, L.; Mizrahi, L.; Cohen, G.; Kenig, S.; Amir, E. Polyaniline for Smart Textile Applications. In Trends and Developments in Modern Applications of Polyaniline, 1st ed.; Năstase, F., Ed.; IntechOpen: London, UK, 2023; in press. [Google Scholar]
- Moreno, H.M.; Montero, M.P.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen Characteristics of Farmed Atlantic Salmon with Firm and Soft Fillet Texture. Food Chem. 2012, 134, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Samsi, M.S.; Kamari, A.; Din, S.M.; Lazar, G. Synthesis, Characterization and Application of Gelatin–Carboxymethyl Cellulose Blend Films for Preservation of Cherry Tomatoes and Grapes. J. Food Sci. Technol. 2019, 56, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Ngwabebhoh, F.A.; Sáha, T.; Stejskal, J.; Trchová, M.; Kopecký, D.; Pfleger, J. Conducting polypyrrole-coated leathers. Pro. Org. Coatings. 2023, 179, 107495. [Google Scholar] [CrossRef]
- Hartman, C.; Popowski, Y.; Raichman, D.; Amir, E. Biodegradable Polymer Coating for Controlled Release of Hydrophobic Functional Molecules from Cotton Fabrics. J. Coat. Technol. Res. 2020, 17, 669–679. [Google Scholar] [CrossRef]
- Anavi, D.; Popowski, Y.; Slor, G.; Segal, M.; Frid, L.; Amir, R.J.; Amirav, A.; Amir, E. Covalent Functionalization of Solid Cellulose by Divergent Synthesis of Chemically Active Dendrons. J. Polym. Sci. Part. A Polym. Chem. 2018, 56, F2103–F2114. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-Step Coating of Fluoro-Containing Silicananoparticles for Univeral Generation of Surface Superhydrophobicity. Chem. Commun. 2008, 7, 877–879. [Google Scholar] [CrossRef]








| Leather Type | ) | ) |
|---|---|---|
| Neat CL | 11.4 ± 0.4 | 11.8 ± 0.3 |
| PANI-CL | 5.5 ± 0.4 | 5.2 ± 0.4 |
| Neat VL | 11.7 ± 0.2 | 11.7 ± 0.1 |
| PANI-VL | 6.8 ± 0.3 | 5.2 ± 0.2 |
| Leather | Neat | UC | PFDT |
|---|---|---|---|
| VL | 0 | 126° ± 6 | 134° ± 4 |
| CL | 0 | 126° ± 6 | 134° ± 4 |
| % Atomic Composition | ||||||
|---|---|---|---|---|---|---|
| C | O | N | F | Cr | S | |
| NEAT-CL | 62.26 | 26.12 | 6.2 | - | 2.09 | 1.54 |
| UC-CL | 68.77 | 20.87 | 6.17 | 0.36 | 1.47 | 1.36 |
| PFDT-CL | 42.89 | 13.23 | 2.12 | 36.46 | 0.56 | 2.25 |
| NEAT-VL | 78.45 | 16.46 | 0.93 | - | - | 1.35 |
| UC-VL | 68.58 | 22.17 | 5.43 | - | - | 1.23 |
| PFDT-VL | 51.42 | 18.72 | 4.29 | 19.58 | - | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zilberfarb, A.; Cohen, G.; Amir, E. Increasing Functionality of Fish Leather by Chemical Surface Modifications. Polymers 2023, 15, 3904. https://doi.org/10.3390/polym15193904
Zilberfarb A, Cohen G, Amir E. Increasing Functionality of Fish Leather by Chemical Surface Modifications. Polymers. 2023; 15(19):3904. https://doi.org/10.3390/polym15193904
Chicago/Turabian StyleZilberfarb, Achiad, Gali Cohen, and Elizabeth Amir. 2023. "Increasing Functionality of Fish Leather by Chemical Surface Modifications" Polymers 15, no. 19: 3904. https://doi.org/10.3390/polym15193904
APA StyleZilberfarb, A., Cohen, G., & Amir, E. (2023). Increasing Functionality of Fish Leather by Chemical Surface Modifications. Polymers, 15(19), 3904. https://doi.org/10.3390/polym15193904






