A Self-Healing Thermoset Epoxy Modulated by Dynamic Boronic Ester for Powder Coating
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
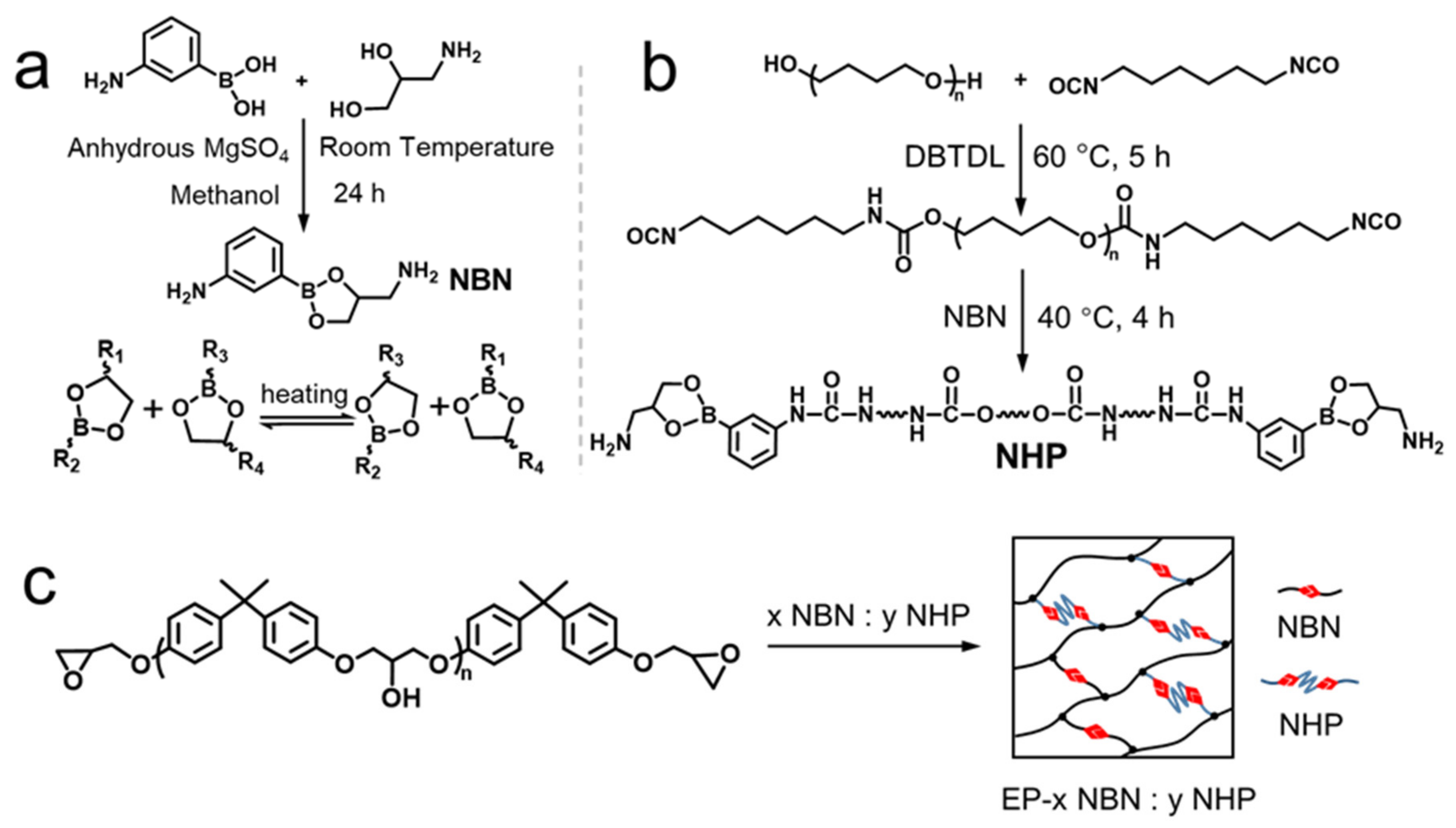
2.2. Synthesis of Small-Molecule Curing Agent (NBN) and Modified Curing Agent (NHP) Containing Boronic Ester Bonds
2.3. Synthesis of Dynamic Cross-Linking Networks of Epoxy Resin
2.4. Characterization of Synthesis
2.5. Characterization of Mechanical and Thermal Property
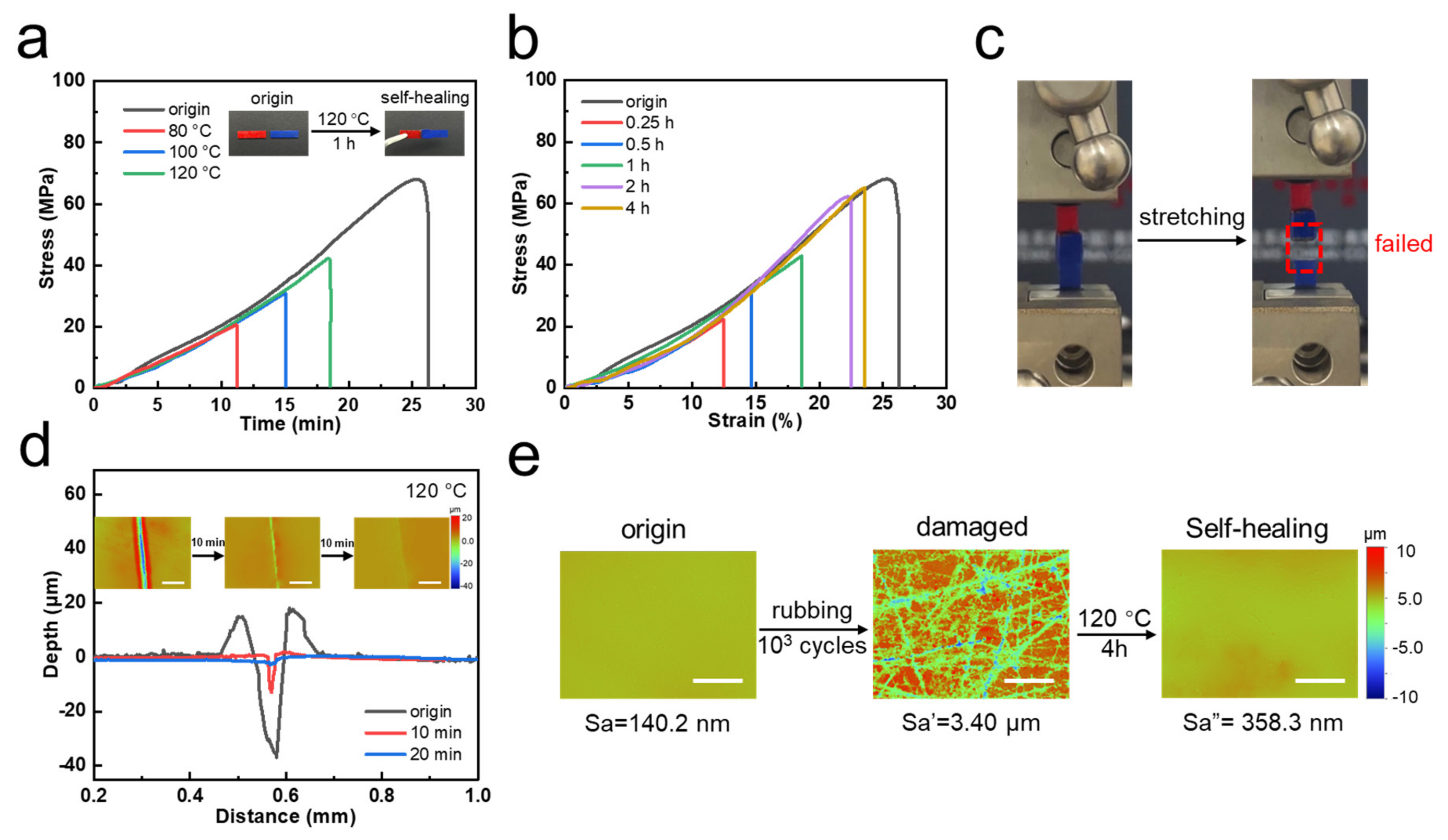
2.6. Self-Healing Test
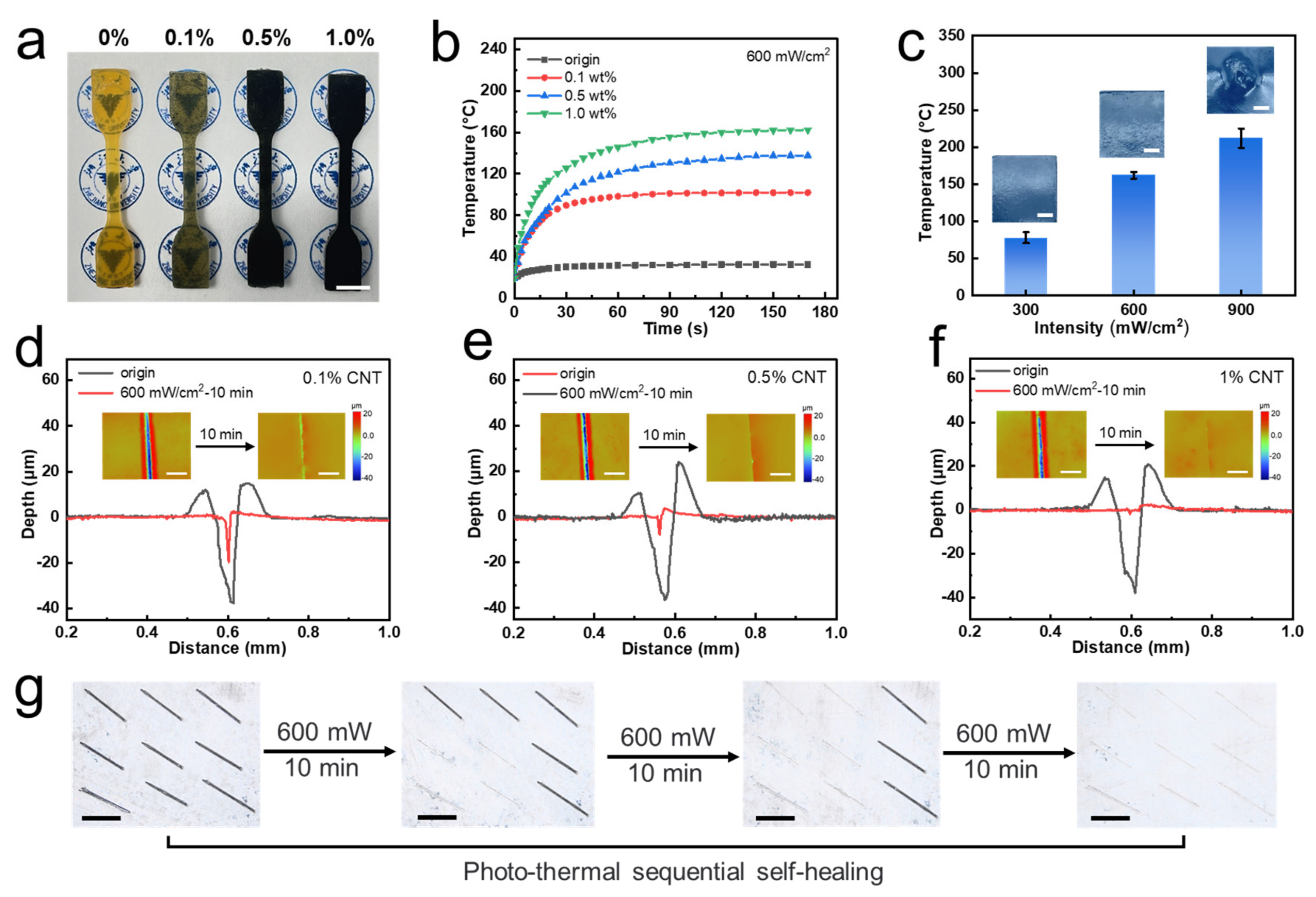
2.7. Photo-Thermal Test
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Medvedev, G.A.; Lee, E.W.; Kim, J.; Caruthers, J.M.; Strachan, A. Molecular dynamics simulations and experimental studies of the thermomechanical response of an epoxy thermoset polymer. Polymer 2012, 53, 4222–4230. [Google Scholar] [CrossRef]
- Kravchenko, O.G.; Li, C.; Strachan, A.; Kravchenko, S.G.; Pipes, R.B. Prediction of the chemical and thermal shrinkage in a thermoset polymer. Compos. Part A 2014, 66, 35–43. [Google Scholar] [CrossRef]
- Deka, M.; Saikia, C.N. Chemical modification of wood with thermosetting resin: Effect on dimensional stability and strength property. Bioresour. Technol. 2000, 73, 179–181. [Google Scholar] [CrossRef]
- Rusayyis, M.A.B.; Torkelson, J.M. Reprocessable covalent adaptable networks with excellent elevated-temperature creep resistance: Facilitation by dynamic, dissociative bis (hindered amino) disulfide bonds. Polym. Chem. 2021, 12, 2760–2771. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, F.S.; Lai, Y.C.; Shi, Z.E.; Yu, Y.H. Flexible epoxy graphene thermoset with excellent weather and corrosion resistance. Prog. Org. Coat. 2021, 151, 106052. [Google Scholar] [CrossRef]
- Linde, E.; Giron, N.H.; Celina, M.C. Water diffusion with temperature enabling predictions for sorption and transport behavior in thermoset materials. Polymer 2018, 153, 653–667. [Google Scholar] [CrossRef]
- Jin, Y.; Lei, Z.; Taynton, P.; Huang, S.; Zhang, W. Malleable and recyclable thermosets: The next generation of plastics. Matter 2019, 1, 1456–1493. [Google Scholar] [CrossRef]
- Du, Z.; Wen, S.; Wang, J.; Yin, C.; Yu, D.; Luo, J. The review of powder coatings. J. Mater. Sci. Chem. Eng. 2016, 4, 54–59. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Sonawane, N.; Siju, C.R. Epoxy powder coatings containing polyaniline for enhanced corrosion protection. Prog. Org. Coat. 2009, 64, 383–386. [Google Scholar] [CrossRef]
- Mafi, R.; Mirabedini, S.M.; Naderi, R.; Attar, M.M. Effect of curing characterization on the corrosion performance of polyester and polyester/epoxy powder coatings. Corros. Sci. 2008, 50, 3280–3286. [Google Scholar] [CrossRef]
- Americus. Recent progress in powder coatings. Pigm. Resin Technol. 1972, 1, 13–17. [Google Scholar] [CrossRef]
- Schmitt, F.; Wenning, A.; Weiss, J.V. Dimeric isocyanates in polyurethane powder coatings. Prog. Org. Coat. 1998, 34, 227–235. [Google Scholar] [CrossRef]
- Riese, W.A. Epoxy Powder Coatings—Current Status and Future Trends; SAE International: Warrendale, PA, USA, 1971. [Google Scholar]
- Abd El-Ghaffar, M.A.; Abdel-Wahab, N.A.; Sanad, M.A.; Sabaa, M.W. High performance anti-corrosive powder coatings based on phosphate pigments containing poly (o-aminophenol). Prog. Org. Coat. 2015, 78, 42–48. [Google Scholar] [CrossRef]
- Bello, S.A.; Agunsoye, J.O.; Adebisi, J.A.; Hassan, S.B. Effect of Aluminium Particles on Mechanical and Morphological Properties of Epoxy Nanocomposites; UNILAG Library: Lagos, Nigeria, 2017. [Google Scholar]
- Fu, J.; Krantz, M.; Zhang, H.; Zhu, J.; Kuo, H.; Wang, Y.M.; Lis, K. Investigation of the recyclability of powder coatings. Powder Technol. 2011, 211, 38–45. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.; Singh, V.; Xu, L.; Kabi, P.; Bele, E.; Tiwari, M.K. Digital light 3D printing of a polymer composite featuring robustness, self-healing, recyclability and tailorable mechanical properties. Addit. Manuf. 2023, 61, 103343. [Google Scholar] [CrossRef]
- Garcia, S.J. Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Li, N.; Abell, C.; Scherman, O.A. Tough supramolecular polymer networks with extreme stretchability and fast room-temperature self-healing. Adv. Mater. 2017, 29, 1605325. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, Y.J.; Zhang, H.; Lu, X.; Sun, J. Room-temperature self-healing and recyclable tough polymer composites using nitrogen-coordinated boroxines. Adv. Funct. Mater. 2018, 28, 1800560. [Google Scholar] [CrossRef]
- Kim, C.; Ejima, H.; Yoshie, N. Polymers with autonomous self-healing ability and remarkable reprocessability under ambient humidity conditions. J. Mater. Chem. A 2018, 6, 19643–19652. [Google Scholar] [CrossRef]
- Yang, K.; He, J.; Zhou, Q.; Hao, X.; Yang, H.; You, Y. An anti-freezing/drying, adhesive and self-healing motion sensor with humidity-enhanced conductivity. Polymer 2021, 214, 123354. [Google Scholar] [CrossRef]
- Lee, J.H.; Hinchet, R.; Kim, S.K.; Kim, S.; Kim, S.W. Shape memory polymer-based self-healing triboelectric nanogenerator. Energy Environ. Sci. 2015, 8, 3605–3613. [Google Scholar] [CrossRef]
- Xu, W.; Li, G. Constitutive modeling of shape memory polymer based self-healing syntactic foam. Int. J. Solids Struct. 2010, 47, 1306–1316. [Google Scholar] [CrossRef]
- Sun, S.; Chen, C.; Zhang, J.; Hu, J. Biodegradable smart materials with self-healing and shape memory function for wound healing. RSC Adv. 2023, 13, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; White, S.R.; Sottos, N.R. Microcapsule induced toughening in a self-healing polymer composite. J. Mater. Sci. 2004, 39, 1703–1710. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, G.; Zhu, G.; Han, N.; Schlangen, E.; Xing, F. Synthesis and characterization of a new polymeric microcapsule and feasibility investigation in self-healing cementitious materials. Constr. Build. Mater. 2016, 105, 487–495. [Google Scholar] [CrossRef]
- Sato, K.; Nakajima, T.; Hisamatsu, T.; Nonoyama, T.; Kurokawa, T.; Gong, J.P. Phase-separation-induced anomalous stiffening, toughening, and self-healing of polyacrylamide gels. Adv. Mater. 2015, 27, 6990–6998. [Google Scholar] [CrossRef]
- Sun, D.; Sun, G.; Zhu, X.; Pang, Q.; Yu, F.; Lin, T. Identification of wetting and molecular diffusion stages during self-healing process of asphalt binder via fluorescence microscope. Constr. Build. Mater. 2017, 132, 230–239. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, X.; Li, W.; Tang, Y.; Bai, Y. Quickly self-healing hydrogel at room temperature with high conductivity synthesized through simple free radical polymerization. J. Appl. Polym. Sci. 2019, 136, 47379. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Chang, Z.; Wu, W.; Wu, G.; Wu, R.; Li, J. Recent achievements in self-healing materials based on ionic liquids: A review. J. Mater. Sci. 2020, 55, 13543–13558. [Google Scholar] [CrossRef]
- Faghihnejad, A.; Feldman, K.E.; Yu, J.; Tirrell, M.V.; Israelachvili, J.N.; Hawker, C.J.; Zeng, H. Adhesion and surface interactions of a self-healing polymer with multiple hydrogen-bonding groups. Adv. Funct. Mater. 2014, 24, 2322–2333. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Harada, A. Highly flexible, tough, and self-healing supramolecular polymeric materials using host–guest interaction. Macromol. Rapid Commun. 2016, 37, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, G.; Turri, S.; Levi, M. Effect of the plasticizer on the self-healing properties of a polymer coating based on the thermoreversible Diels–Alder reaction. Prog. Org. Coat. 2015, 78, 526–531. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Hou, S.; Huang, Y. Rapid and efficient polymer/graphene based multichannel self-healing material via Diels-Alder reaction. Carbon 2019, 147, 398–407. [Google Scholar] [CrossRef]
- Banerjee, S.; Tawade, B.V.; Améduri, B. Functional fluorinated polymer materials and preliminary self-healing behavior. Polym. Chem. 2019, 10, 1993. [Google Scholar] [CrossRef]
- Azcune, I.; Odriozola, I. Aromatic disulfide crosslinks in polymer systems: Self-healing, reprocessability, recyclability and more. Eur. Polym. J. 2016, 84, 147–160. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Y.; Zhang, X.; Liu, J.; Xie, H. A Self-Healing and Nonflammable Cross-Linked Network Polymer Electrolyte with the Combination of Hydrogen Bonds and Dynamic Disulfide Bonds for Lithium Metal Batteries. Energy Environ. Mater. 2023, 6, e12568. [Google Scholar] [CrossRef]
- Ahmed, S.; Bae, M.J.; Jeong, S.; Lee, J.H.; Kim, J.C.; Park, Y.I.; Cheong, I.W. Design of Eco-Friendly Self-Healing Polymers Containing Hindered Urea-Based Dynamic Reversible Bonds. ACS Appl. Polym. Mater. 2022, 4, 8136–8146. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Hu, J.; Wang, H.; Mei, H.; Zheng, S. Self-healable and reprocessable networks involving diblock copolymer and hindered urea bonds. Polymer 2022, 242, 124591. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Wang, D.P.; Zuo, J.L.; Li, C.H. A tough and self-healing polymer enabled by promoting bond exchange in boronic esters with neighboring hydroxyl groups. ACS Mater. Lett. 2021, 3, 1328–1338. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Zhang, X.; Liu, Y.; Wu, S.; Guo, B. Covalently cross-linked elastomers with self-healing and malleable abilities enabled by boronic ester bonds. ACS Appl. Mater. Interfaces 2018, 10, 24224–24231. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, X.; Sun, J.; Fang, Q. A facile conversion of a bio-based resveratrol to the high-performance polymer with high Tg and high char yield. Polymer 2020, 200, 122570. [Google Scholar] [CrossRef]
- Röttger, M.; Domenech, T.; van Der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, Z.; Lv, B.; Qiu, Z. Re-Assemblable, Recyclable, and Self-Healing Epoxy Resin Adhesive Based on Dynamic Boronic Esters. Polymers 2023, 15, 3488. [Google Scholar] [CrossRef] [PubMed]
- Groot, R.D.; Madden, T.J. Dynamic simulation of diblock copolymer microphase separation. J. Chem. Phys. 1998, 108, 8713–8724. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Influence of selected components on crosslink density in polymer structures. Eur. J. Oral Sci. 2001, 109, 282–285. [Google Scholar] [CrossRef]
- Seaman, L.; Curran, D.R.; Shockey, D.A. Computational models for ductile and brittle fracture. J. Appl. Phys. 1976, 47, 4814–4826. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.L.; Li, R.W.; Zhang, Q. Hydrogen bonding in self-healing elastomers. ACS Omega 2021, 6, 9319. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Zhang, D.; Lou, Y.; Huang, L.; Ma, L.; Hao, X.; Dong, L.; Rosei, F.; Lau, W.M. Ultrafast and high-efficient self-healing epoxy coatings with active multiple hydrogen bonds for corrosion protection. Corros. Sci. 2021, 187, 109485. [Google Scholar] [CrossRef]
- Rault, J.; Ping, Z.H.; Nguyen, T. The Tg regulation effect in hydrophilic polymers. J. Non-Cryst. Solids 1994, 172, 733–736. [Google Scholar] [CrossRef]
- Song, W.L.; Guan, X.T.; Fan, L.Z.; Zhao, Y.B.; Cao, W.Q.; Wang, C.Y.; Cao, M.S. Strong and thermostable polymeric graphene/silica textile for lightweight practical microwave absorption composites. Carbon 2016, 100, 109–117. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, S.; Xu, X.; Chen, Y.; Zhang, F. Fabrication and curing properties of o-cresol formaldehyde epoxy resin with reversible cross-links by dynamic boronic ester bonds. Polymer 2020, 211, 123116. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, J.; Liu, S.; Yang, B. Rosin-based epoxy vitrimers with dynamic boronic ester bonds. Polymers 2021, 13, 3386. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.; Davey, T.; Subramanian, P.; Such, G. Self-healing organic coatings—Fundamental chemistry to commercial application. Prog. Org. Coat. 2023, 183, 107759. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Madhujith, T. Current trends in applications of enzymatic interesterification of fats and oils: A review. LWT 2020, 132, 109880. [Google Scholar] [CrossRef]
- Lai, Y.; Kuang, X.; Zhu, P.; Huang, M.; Dong, X.; Wang, D. Colorless, transparent, robust, and fast scratch-self-healing elastomers via a phase-locked dynamic bonds design. Adv. Mater. 2018, 30, 1802556. [Google Scholar] [CrossRef]
- Ning, S.; Wang, M.; Luo, S.; Yang, G.; Feng, Y.; Su, F.; Liu, C. Lightweight carbon fiber hybrid film for high-efficiency electromagnetic interference shielding and electro/photo-thermal conversion applications. J. Alloys Compd. 2023, 958, 170510. [Google Scholar] [CrossRef]
- Xing, J.; Dang, W.; Li, J.; Huang, J. Photo/thermal response of polypyrrole-modified calcium alginate/gelatin microspheres based on helix-coil structural transition and the controlled release of agrochemicals. Colloids Surf. B 2021, 204, 111776. [Google Scholar] [CrossRef]
- Holm, V.R.; Greve, M.M.; Holst, B. A theoretical investigation of the optical properties of metal nanoparticles in water for photo thermal conversion enhancement. Energy Convers. Manag. 2017, 149, 536–542. [Google Scholar] [CrossRef]
- Kwak, B.S.; Kim, B.S.; Song, S.H.; Kim, H.O.; Cho, H.H.; Jung, H.I. Direct measurement of the in vitro hemoglobin content of erythrocytes using the photo-thermal effect of the heme group. Analyst 2010, 135, 2365–2371. [Google Scholar] [CrossRef]
- Loomis, J.; Fan, X.; Khosravi, F.; Xu, P.; Fletcher, M.; Cohn, R.W.; Panchapakesan, B. Graphene/elastomer composite-based photo-thermal nanopositioners. Sci. Rep. 2013, 3, 1900. [Google Scholar] [CrossRef] [PubMed]
- Ayrilmis, N. A review on electrostatic powder coatings for the furniture industry. Int. J. Adhes. Adhes. 2022, 113, 103062. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Z.; Zhang, C.; Yang, B.; Ren, H. A Self-Healing Thermoset Epoxy Modulated by Dynamic Boronic Ester for Powder Coating. Polymers 2023, 15, 3894. https://doi.org/10.3390/polym15193894
Liu Y, Li Z, Zhang C, Yang B, Ren H. A Self-Healing Thermoset Epoxy Modulated by Dynamic Boronic Ester for Powder Coating. Polymers. 2023; 15(19):3894. https://doi.org/10.3390/polym15193894
Chicago/Turabian StyleLiu, Yongqi, Ziyuan Li, Caifu Zhang, Biru Yang, and Hua Ren. 2023. "A Self-Healing Thermoset Epoxy Modulated by Dynamic Boronic Ester for Powder Coating" Polymers 15, no. 19: 3894. https://doi.org/10.3390/polym15193894
APA StyleLiu, Y., Li, Z., Zhang, C., Yang, B., & Ren, H. (2023). A Self-Healing Thermoset Epoxy Modulated by Dynamic Boronic Ester for Powder Coating. Polymers, 15(19), 3894. https://doi.org/10.3390/polym15193894





