Processing of Grape Bagasse and Potato Wastes for the Co-Production of Bacterial Cellulose and Gluconic Acid in an Airlift Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Grape Bagasse and Discarded Potato Pre-Treatments
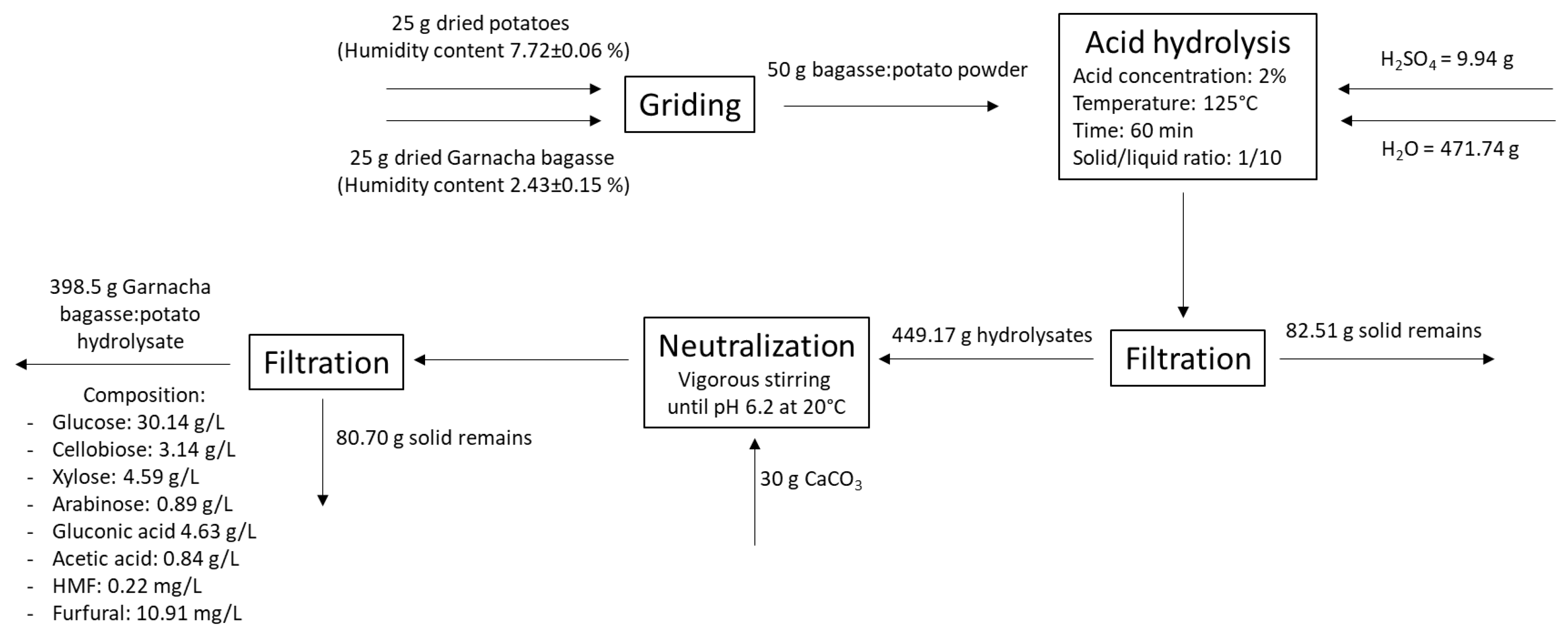
2.3. Acid Hydrolysis Treatment of Grape Bagasse
2.4. Analytical Methods
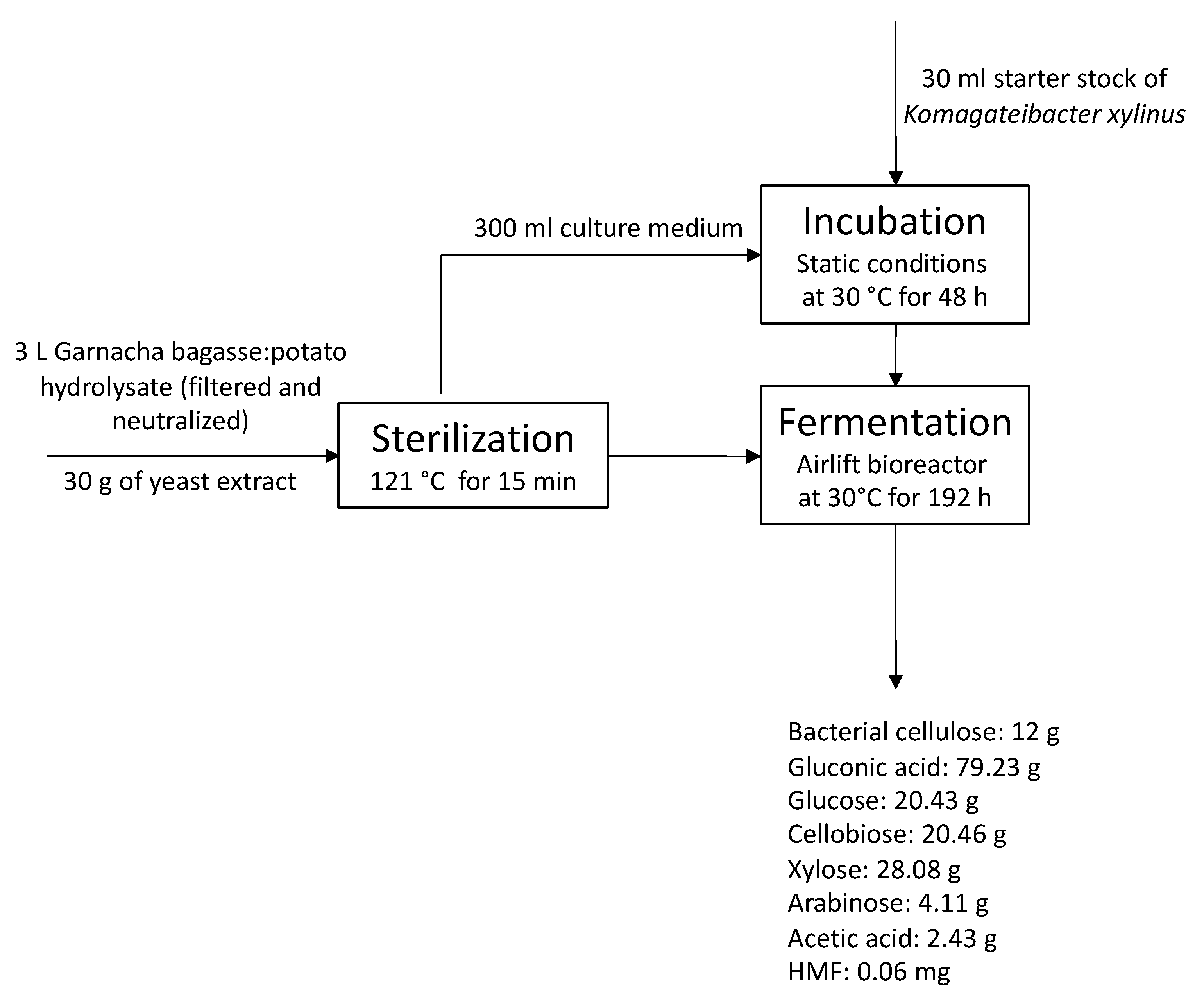
2.5. Culture Media and Bacterial Cellulose Production in Airlift Bioreactor
2.6. Statistical Analysis
3. Results
3.1. Modeling for the Acid Hydrolysis of Garnacha Bagasse
3.2. Optimization of the Acid Hydrolysis of Grape Bagasse
3.3. Sustainable Culture Media with Phenolic Compounds
3.4. Co-Production of Bacterial Cellulose and Gluconic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sufficiency, E.; Qamar, S.A.; Ferreira, L.F.R.; Franco, M.; Iqbal, H.M.N.; Bilal, M. Emerging Biotechnological Strategies for Food Waste Management: A Green Leap towards Achieving High-Value Products and Environmental Abatement. Energy Nexus 2022, 6, 100077. [Google Scholar] [CrossRef]
- Otoni, C.G.; Azeredo, H.M.C.; Mattos, B.D.; Beaumont, M.; Correa, D.S.; Rojas, O.J. The Food–Materials Nexus: Next Generation Bioplastics and Advanced Materials from Agri-Food Residues. Adv. Mater. 2021, 33, 2170342. [Google Scholar] [CrossRef]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape Marc as a Source of Carbohydrates for Bioethanol: Chemical Composition, Pre-Treatment and Saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ghalamara, S.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach. Processes 2023, 11, 495. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, E.; Portilla-Rivera, O.M.; Ramírez, J.A.; Vázquez, M. Modelling of the Acid Hydrolysis of Potato (Solanum tuberosum) for Fermentative Purposes. Biomass Bioenergy 2012, 42, 59–68. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial Cellulose as a Biodegradable Food Packaging Material: A Review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Fernandes, I.d.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial Cellulose: From Production Optimization to New Applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of Bacterial Cellulose from Industrial Wastes: A Review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of Bacterial Nanocellulose from Wine Industry Residues: Importance of Fermentation Time on Pellicle Characteristics. J. Appl. Polym. Sci. 2016, 133, 43109. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of Residues from Agro-Forest Industries in the Production of High Value Bacterial Cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Ogrizek, L.; Lamovšek, J.; Čuš, F.; Leskovšek, M.; Gorjanc, M. Properties of Bacterial Cellulose Produced Using White and Red Grape Bagasse as a Nutrient Source. Process 2021, 9, 1088. [Google Scholar] [CrossRef]
- Rani, M.U.; Udayasankar, K.; Appaiah, K.A.A. Properties of Bacterial Cellulose Produced in Grape Medium by Native Isolate Gluconacetobacter sp. J. Appl. Polym. Sci. 2011, 120, 2835–2841. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of Nano Bacterial Cellulose from Beverage Industrial Waste of Citrus Peel and Pomace Using Komagataeibacter Xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Production and Characterization of Bacterial Cellulose from Citrus Peels. Waste Biomass Valorization 2018, 10, 2165–2175. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose Using Gluconacetobacter Xylinus. Waste Biomass Valorization 2017, 10, 85–93. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of Nano Bacterial Cellulose from Waste Water of Candied Jujube-Processing Industry Using Acetobacter Xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of Various Fruit Juices as Carbon Source for Production of Bacterial Cellulose by Acetobacter Xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of Gluconic Acid: A Review towards Process Intensification for Green Production. Chem. Eng. Process. Process Intensif. 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Singh, O.V.; Kumar, R. Biotechnological Production of Gluconic Acid: Future Implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, K.; Zhang, H.; Wang, Y.; Yuan, Q.; Su, N.; Bao, J.; Fang, X. Gluconic Acid Production from Potato Waste by Gluconobacter Oxidans Using Sequential Hydrolysis and Fermentation. ACS Sustain. Chem. Eng. 2017, 5, 6116–6123. [Google Scholar] [CrossRef]
- Singh, O.V.; Singh, R.P. Bioconversion of Grape Must into Modulated Gluconic Acid Production by Aspergillus Niger ORS-4·410. J. Appl. Microbiol. 2006, 100, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.V.; Kapur, N.; Singh, R.P. Evaluation of Agro-Food Byproducts for Gluconic Acid Production by Aspergillus Niger ORS-4.410. World J. Microbiol. Biotechnol. 2005, 21, 519–524. [Google Scholar] [CrossRef]
- Wu, H.; Huang, T.; Cao, F.; Zou, Q.; Wei, P.; Ouyang, P. Co-Production of HMF and Gluconic Acid from Sucrose by Chemo-Enzymatic Method. Chem. Eng. J. 2017, 327, 228–234. [Google Scholar] [CrossRef]
- Qian, X.; Gorte, O.; Chen, L.; Zhang, W.; Dong, W.; Ma, J.; Jiang, M.; Xin, F.; Ochsenreither, K. Co-Production of Single Cell Oil and Gluconic Acid Using Oleaginous Cryptococcus Podzolicus DSM 27192. Biotechnol. Biofuels 2019, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yuan, H.; Wang, H.; Yu, H. Coproduction of Bacterial Cellulose and Pear Vinegar by Fermentation of Pear Peel and Pomace. Bioprocess Biosyst. Eng. 2021, 44, 2231–2244. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Niu, H.; Wang, D.; Xin, C.; Liang, C. Low-Energy Hemiacetal Dehydrogenation Pathway: Co-Production of Gluconic Acid and Green Hydrogen via Glucose Dehydrogenation. Chem. Asian J. 2022, 17, e202200138. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Huang, L.; Cao, R.; Xu, Y. Efficient Coproduction of Gluconic Acid and Xylonic Acid from Lignocellulosic Hydrolysate by Zn(II)-Selective Inhibition on Whole-Cell Catalysis by Gluconobacter Oxydans. Bioresour. Technol. 2017, 243, 855–859. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Teng, H.-Y.; Lee, C.-K. Knock-out of Glucose Dehydrogenase Gene in Gluconacetobacter Xylinus for Bacterial Cellulose Production Enhancement. Biotechnol. Bioprocess Eng. 2015, 20, 18–25. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, E.; Portilla-Rivera, O.M.; Jarquín-Enríquez, L.; Ramírez, J.A.; Vázquez, M. Acid Hydrolysis of Wheat Straw: A Kinetic Study. Biomass Bioenergy 2012, 36, 346–355. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Active Packaging Film of Chitosan and Santalum Album Essential Oil: Characterization and Application as Butter Sachet to Retard Lipid Oxidation. Food Packag. Shelf Life 2022, 34, 100938. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Antioxidant Extracts of Nettle (Urtica Dioica) Leaves: Evaluation of Extraction Techniques and Solvents. Molecules 2022, 27, 6015. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, O.M.; Jeong, J.H.; Jeon, Y.D.; Park, K.H.; Kim, H.S.; An, W.G.; Son, H.J. Production and Characterization of Cellulose by Acetobacter Sp. V6 Using a Cost-Effective Molasses-Corn Steep Liquor Medium. Appl. Biochem. Biotechnol. 2010, 162, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Puertas, G.; Vázquez, M. Production and Characterization of Active Bacterial Cellulose Films Obtained from the Fermentation of Wine Bagasse and Discarded Potatoes by Komagateibacter Xylinus. Polymers 2022, 14, 5194. [Google Scholar] [CrossRef]
- Cook, R.D. Detection of Influential Observations in Linear Regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
- Sakia, R.M. The Box-Cox Transformation Technique: A Review. J. R. Stat. Soc. Ser. D Stat. 1992, 41, 169. [Google Scholar] [CrossRef]
- Allen, L.V. Quality Control: Water Activity Considerations for Beyond-Use Dates. Int. J. Pharm. Compd. 2018, 22, 288–293. [Google Scholar]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape Stalk Valorization for Fermentation Purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape Winery Waste as a Promising Feedstock for the Production of Polyhydroxyalkanoates and Other Value-Added Products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Brandelli, A.; Marczak, L.D.F.; Tessaro, I.C. Kinetic Modeling of Total Polyphenol Extraction from Grape Marc and Characterization of the Extracts. Sep. Purif. Technol. 2012, 100, 82–87. [Google Scholar] [CrossRef]
- Keshk, S. Vitamin C Enhances Bacterial Cellulose Production in Gluconacetobacter Xylinus. Carbohydr. Polym. 2014, 99, 98–100. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-Chemical Properties of Cell Wall Materials Obtained from Ten Grape Varieties and Their Byproducts: Grape Pomaces and Stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly Green Conversion of Potato Peel Wastes to High Productivity Bacterial Cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef]
- Lin, S.P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.R.; Demirci, A.; Cheng, K.C. Biosynthesis, Production and Applications of Bacterial Cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent Developments in the Production and Applications of Bacterial Cellulose Fibers and Nanocrystals. Crit. Rev. Biotechnol. 2017, 37, 510–524. [Google Scholar] [CrossRef]
- Kim, H.; Son, J.; Lee, J.; Yoo, H.Y.; Lee, T.; Jang, M.; Oh, J.M.; Park, C. Improved Production of Bacterial Cellulose through Investigation of Effects of Inhibitory Compounds from Lignocellulosic Hydrolysates. GCB Bioenergy 2021, 13, 436–444. [Google Scholar] [CrossRef]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial Cellulose Production by Acetobacter Xylinum in a 50-L Internal-Loop Airlift Reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
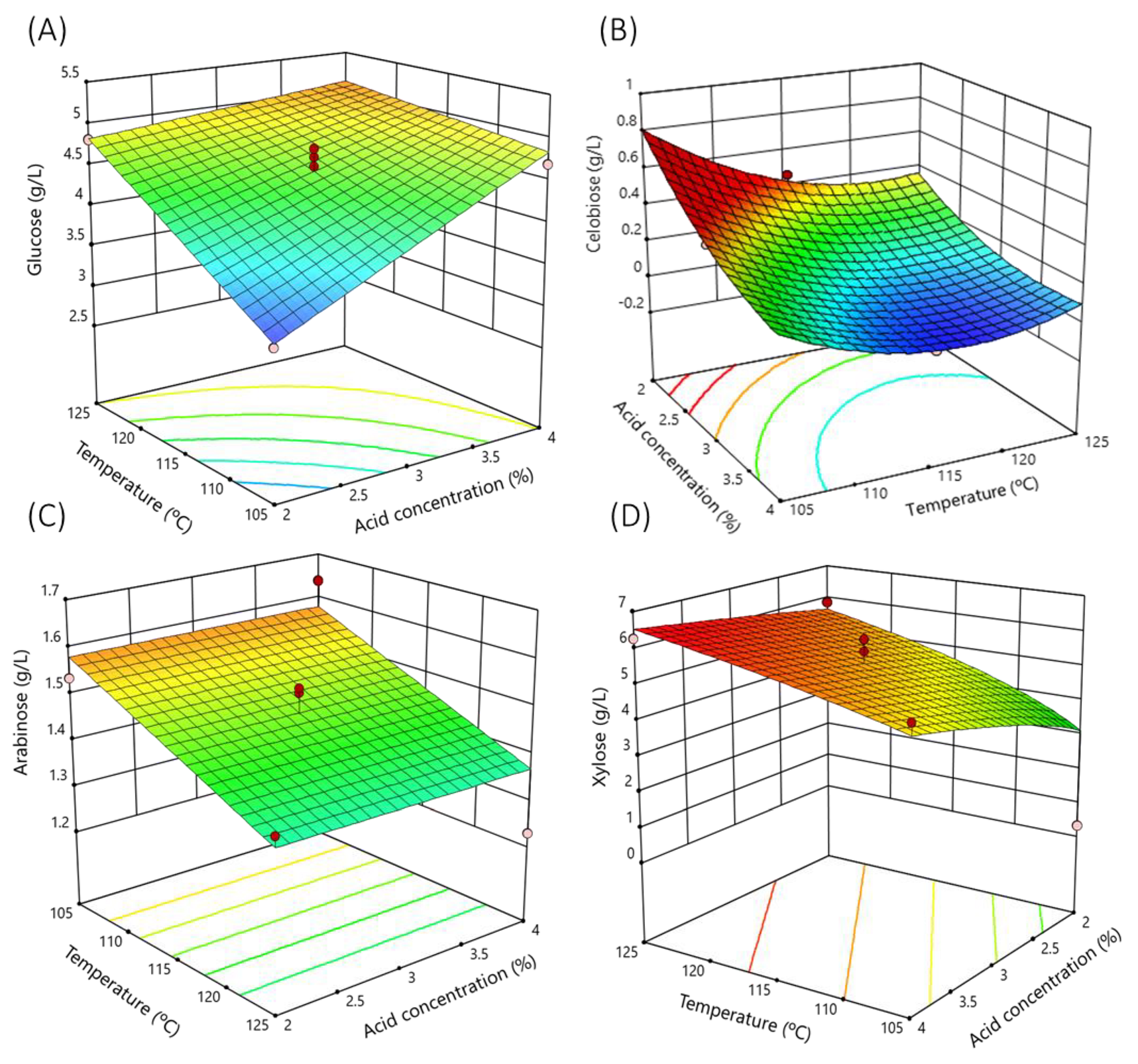



| Factors (1) | Nomenclature | Units | Variation Levels |
|---|---|---|---|
| Sulfuric acid | A | g/L | 2, 3, 4 |
| Time | B | min | 60, 120, 180 |
| Temperature | C | °C | 105, 115, 125 |
| Dimensionless | |||
| Sulfuric acid | X1 | −1, 0, 1 | |
| Time | X2 | −1, 0, 1 | |
| Temperature | X3 | −1, 0, 1 | |
| Response (2) | |||
| Glucose | g/L | ||
| Celobiose | g/L | ||
| Arabinose | g/L | ||
| Acetic acid | g/L | ||
| Xylose | g/L | ||
| Furfural | mg/L | ||
| HMF (3) | mg/L | ||
| TPC (4) | mg GAE/g dried bagasse |
| Run | Sulfuric Acid | Time | Temperature |
|---|---|---|---|
| % | min | °C | |
| 1 | 3 | 180 | 125 |
| 2 | 4 | 60 | 115 |
| 3 | 2 | 120 | 125 |
| 4 | 3 | 120 | 115 |
| 5 | 3 | 60 | 105 |
| 6 | 4 | 120 | 125 |
| 7 | 3 | 120 | 115 |
| 8 | 2 | 180 | 115 |
| 9 | 2 | 120 | 105 |
| 10 | 3 | 120 | 115 |
| 11 | 3 | 60 | 125 |
| 12 | 4 | 120 | 105 |
| 13 | 2 | 60 | 115 |
| 14 | 4 | 180 | 115 |
| 15 | 3 | 180 | 105 |
| Run | Glucose | Cellobiose | Arabinose | Xylose | Acetic Acid | HMF | F | TPC |
|---|---|---|---|---|---|---|---|---|
| g/L | g/L | g/L | g/L | g/L | mg/L | mg/L | mg GAE * | |
| 1 | 4.97 | 0.39 | 0.66 | 5.87 | 0.88 | 70.39 | 1.26 | 12.35 |
| 2 | 4.72 | 0.00 | 1.45 | 5.17 | 0.75 | 24.22 | 2.14 | 9.65 |
| 3 | 4.80 | 0.32 | 1.39 | 5.86 | 0.85 | 124.79 | 0 | 11.68 |
| 4 | 4.58 | 0.00 | 1.43 | 5.66 | 0.84 | 31.53 | 8.68 | 9.68 |
| 5 | 3.39 | 0.42 | 1.48 | 3.44 | 0.56 | 0.22 | 0 | 12.72 |
| 6 | 5.02 | 0.26 | 1.23 | 6.26 | 0.94 | 67.85 | 146.35 | 11.31 |
| 7 | 4.79 | 0.00 | 1.53 | 6.01 | 0.86 | 23.23 | 6.85 | 10.59 |
| 8 | 4.57 | 0.27 | 1.52 | 5.48 | 0.76 | 15.24 | 3.48 | 9.64 |
| 9 | 3.24 | 0.48 | 1.53 | 0.31 | 0.48 | 0.31 | 0 | 10.30 |
| 10 | 4.69 | 0.07 | 1.52 | 6.01 | 0.85 | 30.52 | 10.08 | 8.74 |
| 11 | 4.81 | 0.11 | 1.40 | 6.12 | 0.88 | 64.19 | 32.03 | 12.32 |
| 12 | 4.69 | 0.10 | 1.64 | 5.29 | 0.72 | 13.99 | 1.39 | 11.00 |
| 13 | 3.50 | 0.46 | 1.49 | 3.54 | 0.48 | 0.57 | 0 | 9.45 |
| 14 | 5.40 | 0.12 | 1.52 | 6.11 | 0.77 | 58.77 | 43.68 | 12.51 |
| 15 | 4.63 | 0.18 | 1.61 | 4.77 | 0.64 | 70.87 | 2.53 | 7.23 |
| Glucose | Cellobiose | Arabinose | Xylose | |||||
| Source | F-Value | p-Value | F Value | p-Value | F Value | p-Value | F Value | p-Value |
| Model | 37.46 | <0.0001 | 23.38 | 0.0014 | 11.19 | 0.0022 | 12.85 | 0.006 |
| A (1) | 72.56 | <0.0001 | 72.46 | 0.0004 | 9.28 | 0.0111 | ||
| B (2) | 52.03 | <0.0001 | 0.0292 | 0.8711 | 1.19 | 0.2995 | 4.65 | 0.0541 |
| C (3) | 70.03 | <0.0001 | 0.5828 | 0.4797 | 19.18 | 0.0011 | 24.61 | 0.0004 |
| AB | 1.51 | 0.2547 | 12.86 | 0.0158 | ||||
| AC | 16.12 | 0.0039 | 12.65 | 0.0163 | ||||
| BC | 12.54 | 0.0076 | 36.09 | 0.0018 | ||||
| A² | 20.11 | 0.0065 | ||||||
| B² | 14.60 | 0.0124 | ||||||
| C² | 51.08 | 0.0008 | ||||||
| Lack of Fit | 2.49 | 0.314 | 1.14 | 0.4984 | 1.37 | 0.4918 | 25.58 | 0.1676 |
| Acetic Acid | HMF | Furfural | TPC | |||||
| Source | F-value | p-value | F value | p-value | F value | p-value | F value | p-value |
| Model | 67.14 | <0.0001 | 7.36 | 0.0056 | 6.39 | 0.0091 | 1.27 | 0.3686 |
| A | 81.91 | 0.0001 | 1.89 | 0.1966 | 12.55 | 0.0046 | 0.6425 | 0.446 |
| B | 8.08 | 0.0295 | 5.72 | 0.0357 | 4.17 | 0.0658 | 0.3256 | 0.5839 |
| C | 256.01 | <0.0001 | 14.48 | 0.0029 | 2.45 | 0.1459 | 2.29 | 0.1686 |
| AB | 2.64 | 0.1552 | 0.798 | 0.3978 | ||||
| AC | 7.68 | 0.0323 | 0.1287 | 0.7291 | ||||
| BC | 3.41 | 0.1021 | ||||||
| A² | 42.54 | 0.0006 | ||||||
| B² | 47.81 | 0.0005 | ||||||
| C² | ||||||||
| Lack of Fit | 5.71 | 0.1545 | 3.12 | 0.2622 | ||||
| Glucose | Cellobiose | Arabinose | Xylose | Acetic Acid | HMF | Furfural | |
|---|---|---|---|---|---|---|---|
| R² | 0.97 | 0.98 | 0.67 | 0.78 | 0.99 | 0.67 | 0.64 |
| Adjusted R2 | 0.94 | 0.94 | 0.61 | 0.72 | 0.97 | 0.58 | 0.54 |
| Predicted R2 | 0.90 | 0.75 | 0.42 | 0.61 | 0.90 | 0.30 | 0.30 |
| Adeq Precision | 18.92 | 14.12 | 9.09 | 10.97 | 25.00 | 8.49 | 7.65 |
| Goal | Importance | |
|---|---|---|
| Sulfuric acid | minimize | 3 |
| Time | minimize | 3 |
| Temperature | In range | |
| Glucose | maximize | 5 |
| Cellobiose | maximize | 2 |
| Arabinose | maximize | 2 |
| Acetic acid | minimize | 3 |
| Xylose | maximize | 2 |
| Furfural | minimize | 2 |
| HMF | minimize | 2 |
| TPC | maximize | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, M.; Puertas, G.; Cazón, P. Processing of Grape Bagasse and Potato Wastes for the Co-Production of Bacterial Cellulose and Gluconic Acid in an Airlift Bioreactor. Polymers 2023, 15, 3944. https://doi.org/10.3390/polym15193944
Vázquez M, Puertas G, Cazón P. Processing of Grape Bagasse and Potato Wastes for the Co-Production of Bacterial Cellulose and Gluconic Acid in an Airlift Bioreactor. Polymers. 2023; 15(19):3944. https://doi.org/10.3390/polym15193944
Chicago/Turabian StyleVázquez, Manuel, Gema Puertas, and Patricia Cazón. 2023. "Processing of Grape Bagasse and Potato Wastes for the Co-Production of Bacterial Cellulose and Gluconic Acid in an Airlift Bioreactor" Polymers 15, no. 19: 3944. https://doi.org/10.3390/polym15193944
APA StyleVázquez, M., Puertas, G., & Cazón, P. (2023). Processing of Grape Bagasse and Potato Wastes for the Co-Production of Bacterial Cellulose and Gluconic Acid in an Airlift Bioreactor. Polymers, 15(19), 3944. https://doi.org/10.3390/polym15193944






