Physicochemical Pretreatment of Vietnamosasa pusilla for Bioethanol and Xylitol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analytical Processes
2.3. Alkaline-Catalyzed Steam Pretreatment of V. pusilla Feedstock
2.4. Enzymatic Hydrolysis
2.5. Structure and Crystallinity Analysis of Feedstock
2.6. Microorganisms
2.7. Preparation of Seed Culture
2.8. Preparing Biomass Hydrolysate for Ethanol Production
2.9. Preparing Biomass Hydrolysate for Xylitol Production
2.10. Medium for Bioethanol and Xylitol Production
2.11. Ethanol Fermentation
2.12. Xylitol Fermentation
2.13. Determination Growth of Microorganisms
3. Results
3.1. Composition of Raw Biomass
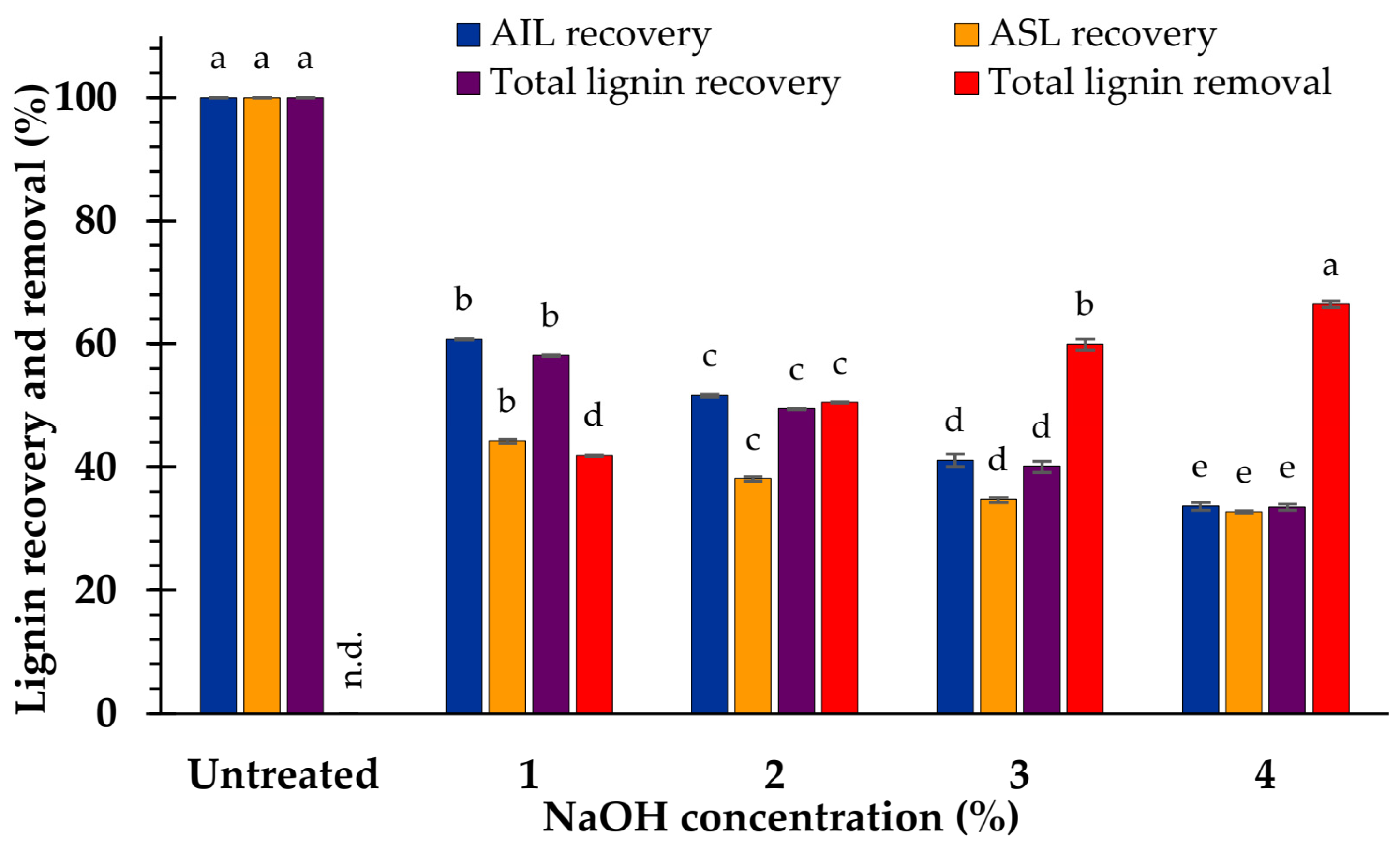
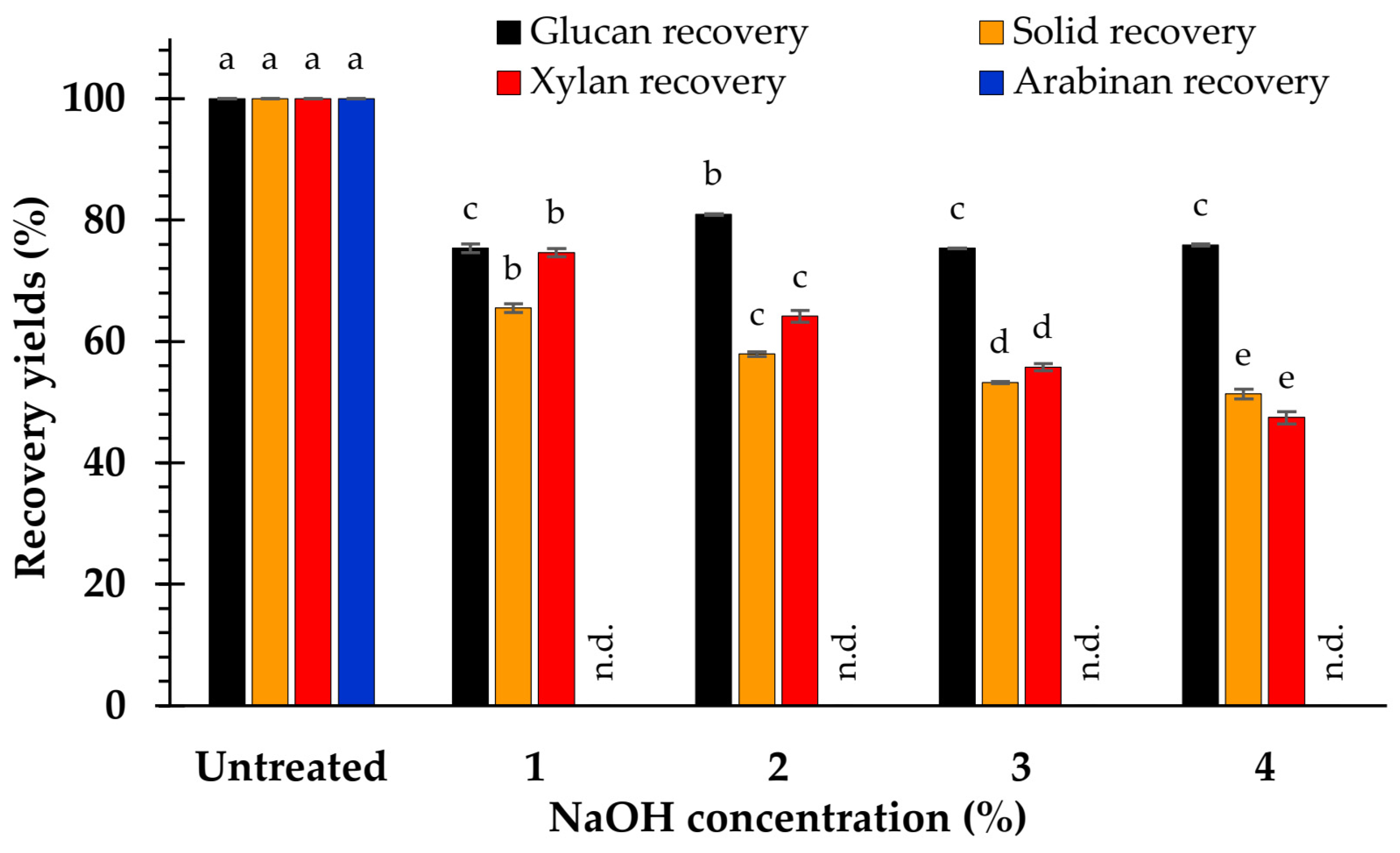
3.2. Effect of NaOH Concentration on Composition
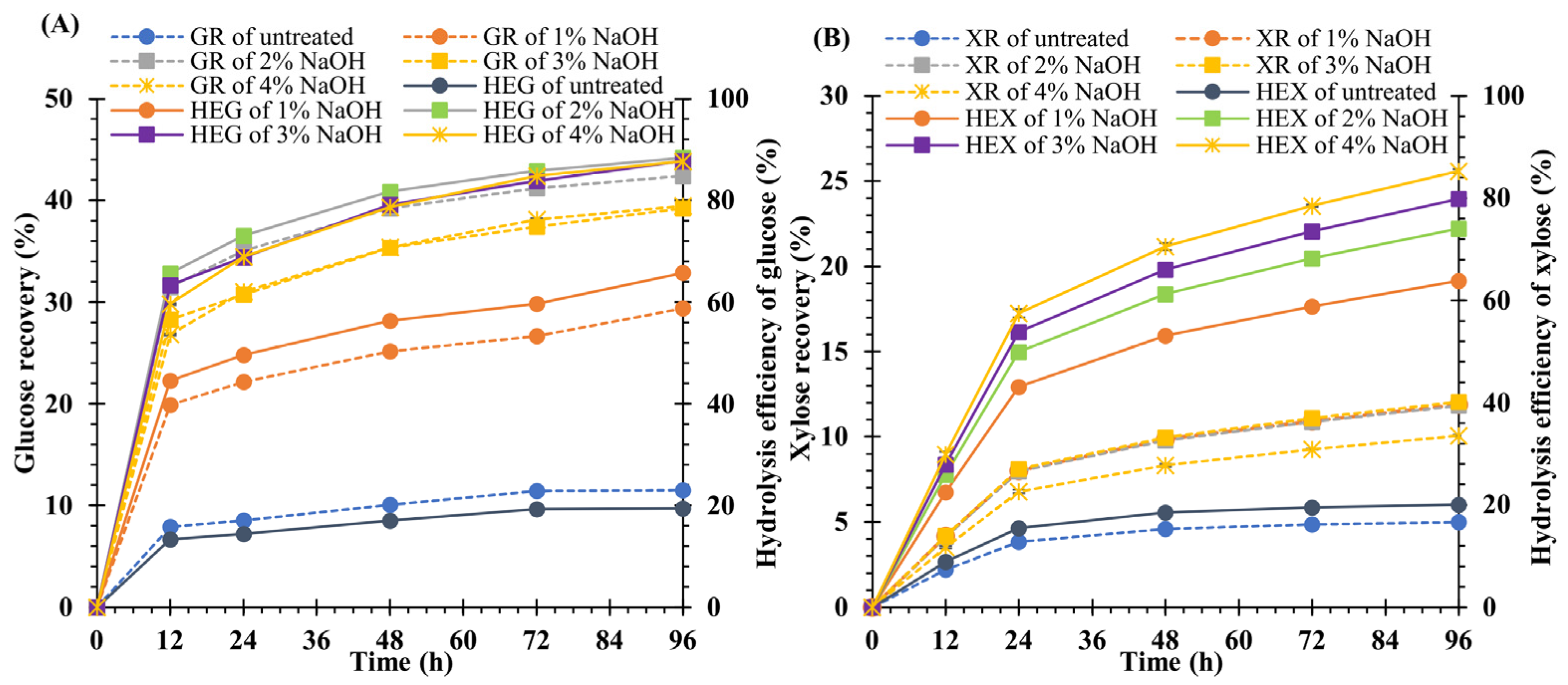
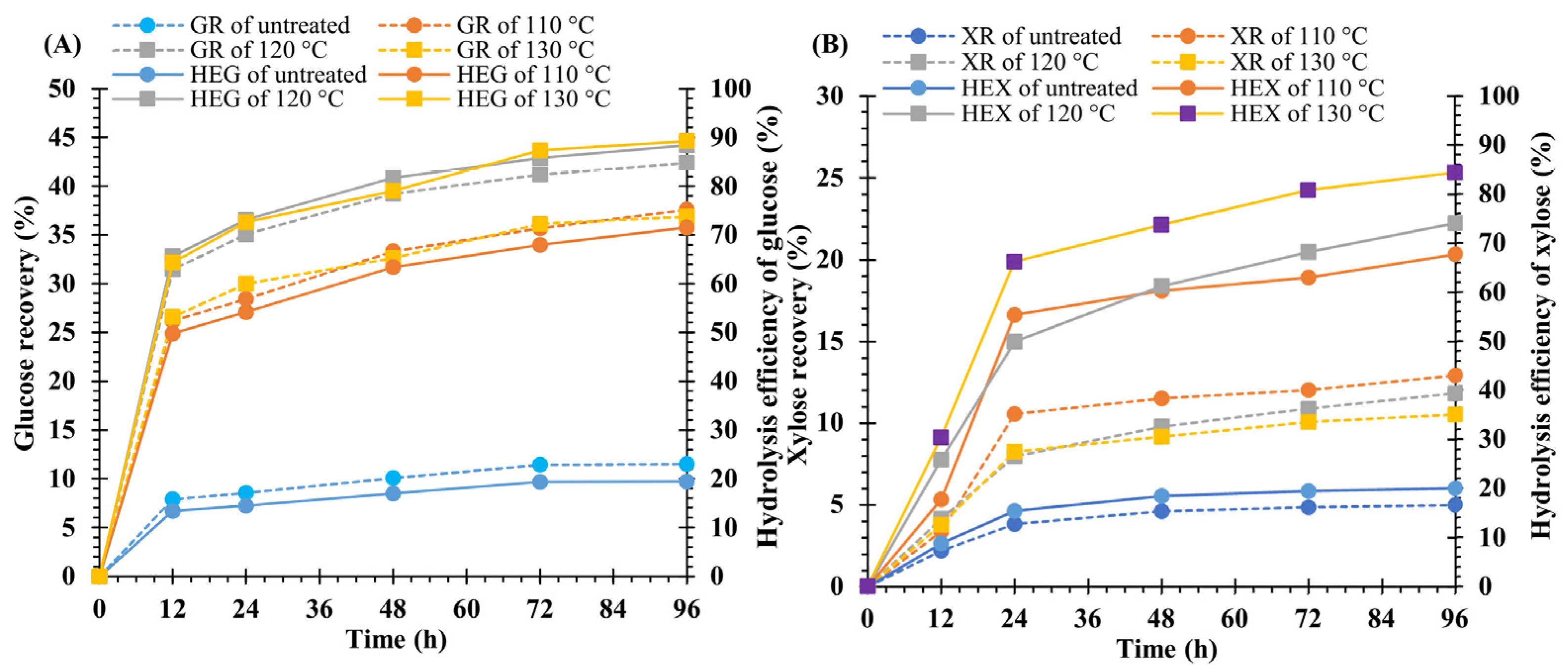
3.3. Effect of NaOH Concentration on Hydrolysis Yield
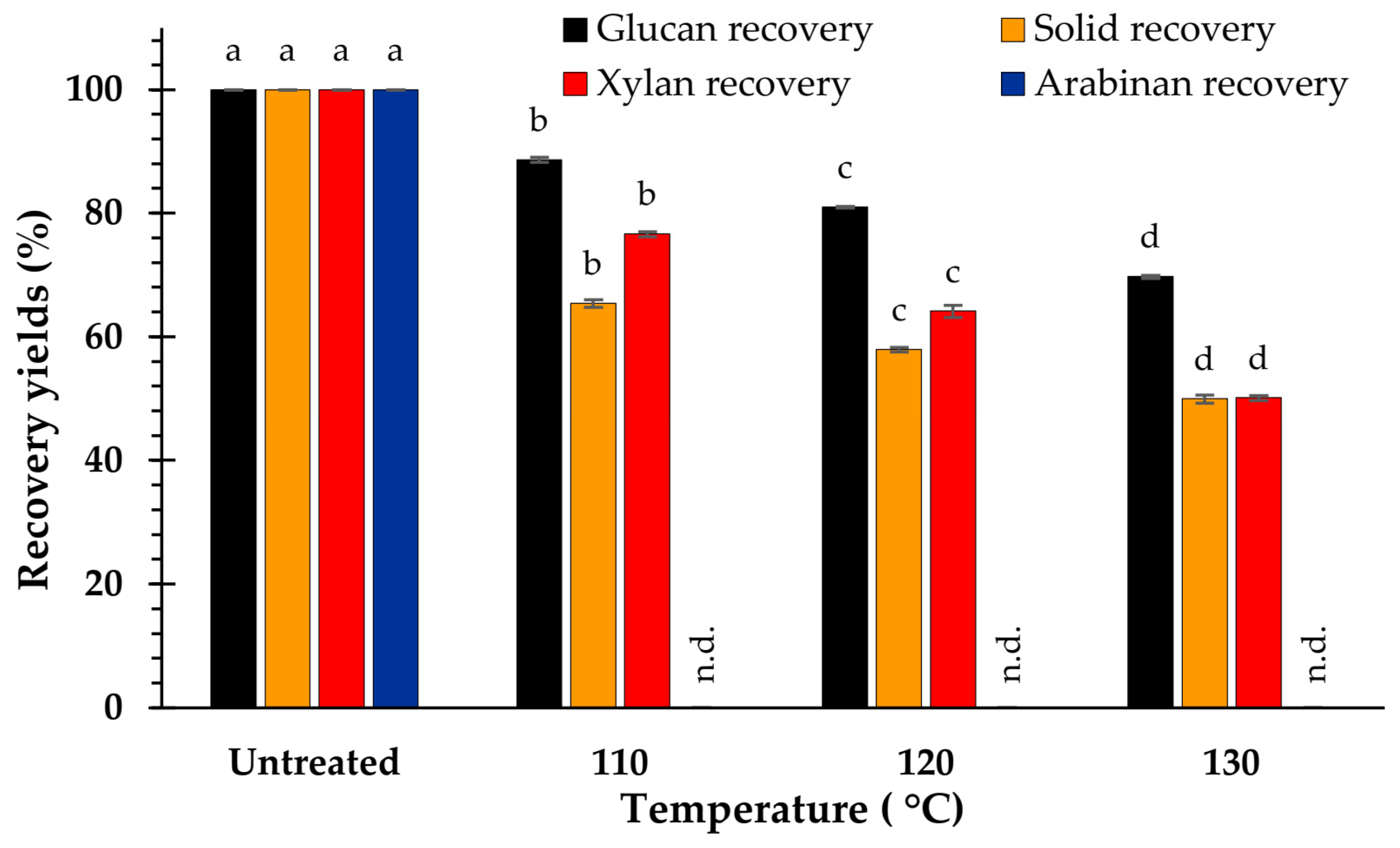
3.4. Effect of Temperature on Composition
3.5. Effect of Temperature on Hydrolysis Yield
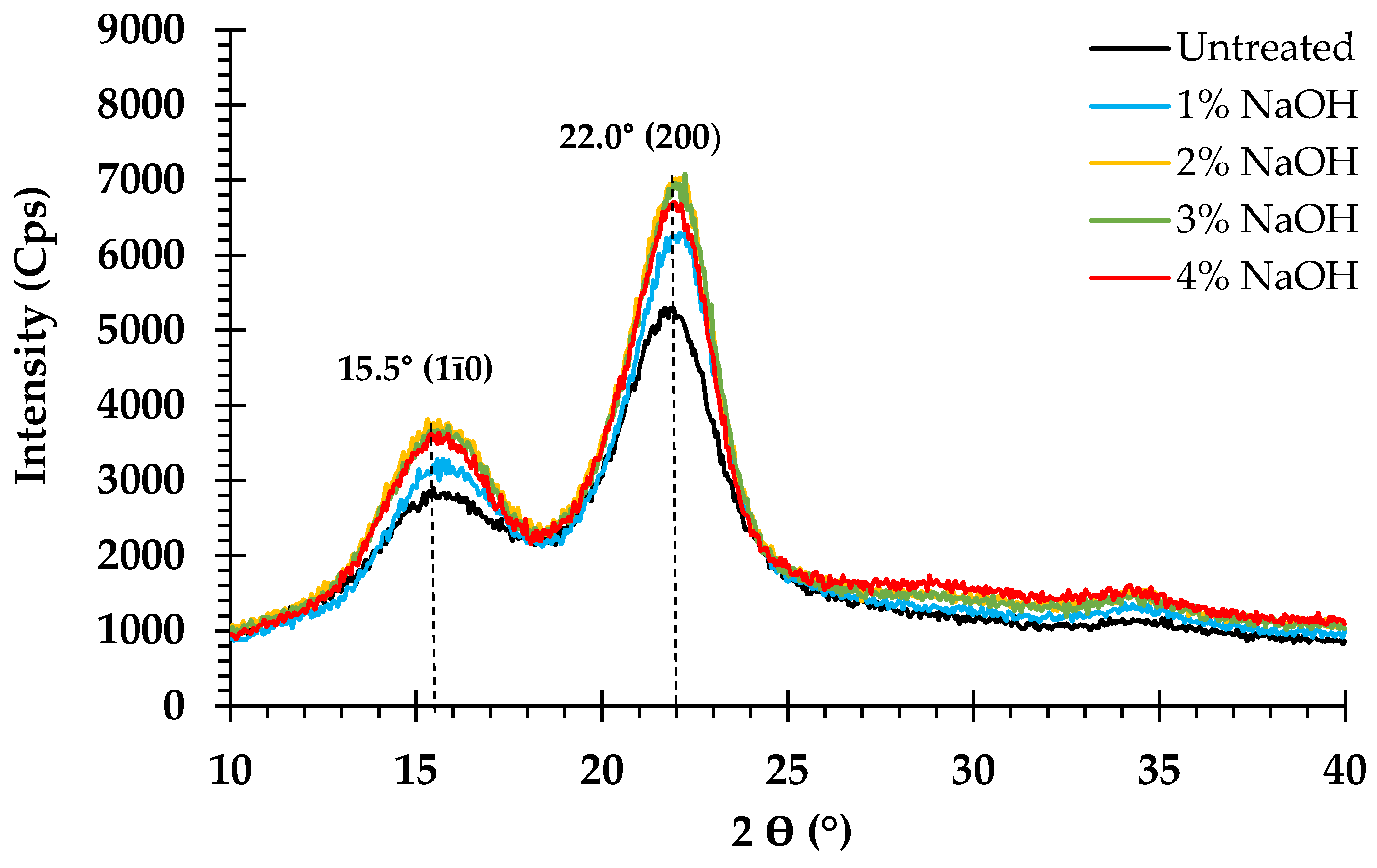
3.6. Effect of Pretreatment on Crystalline Structure
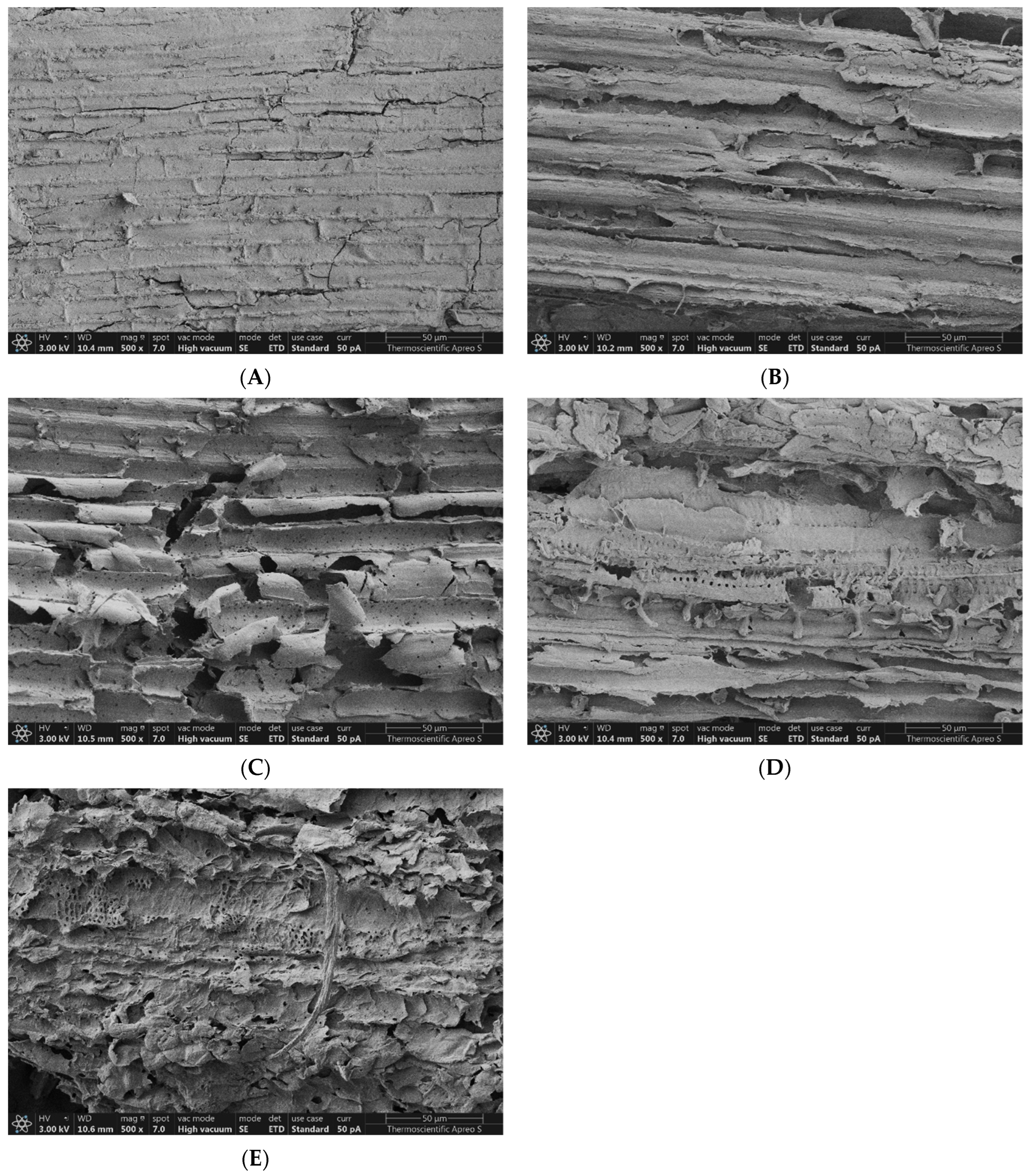
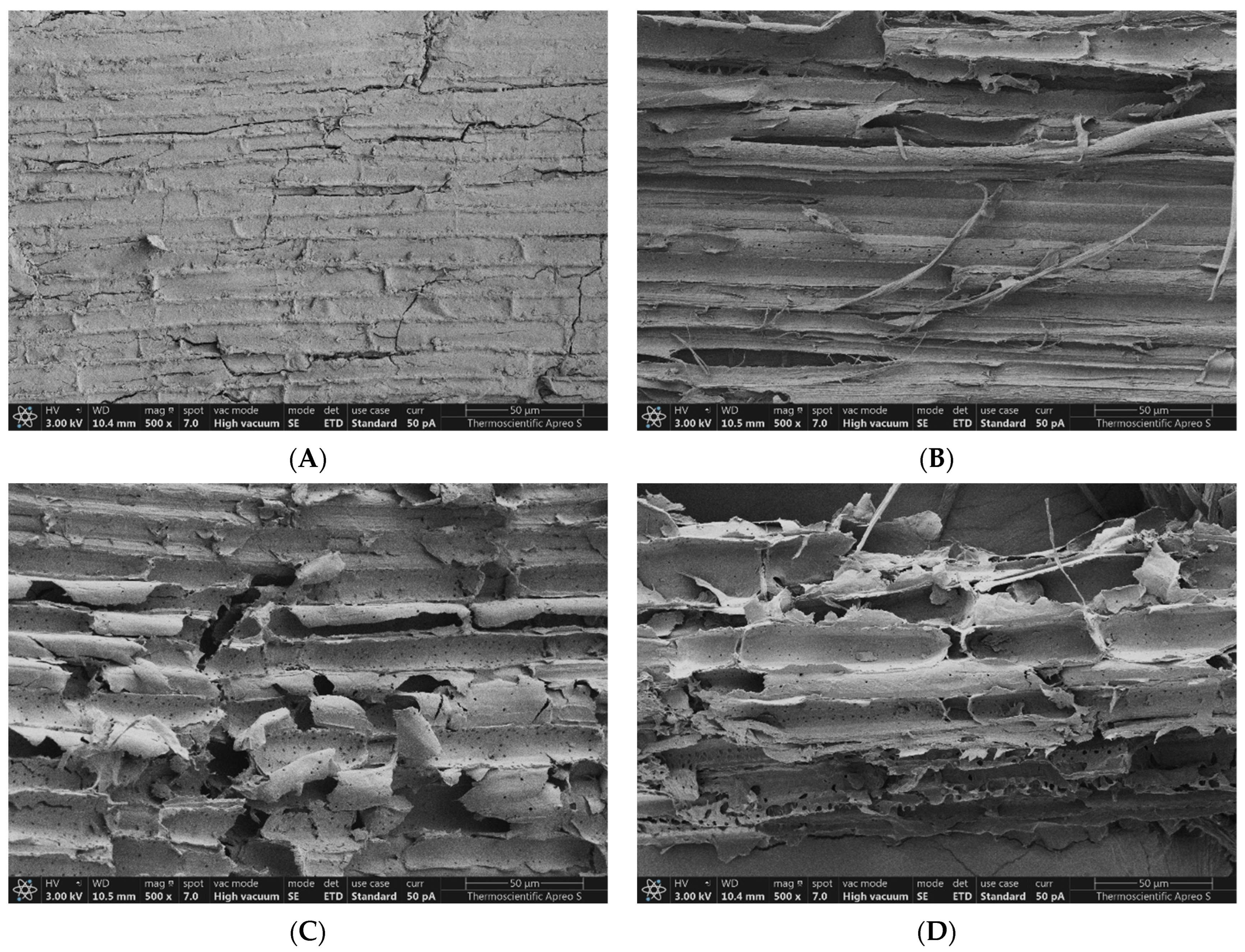
3.7. Effect on Morphological Structure
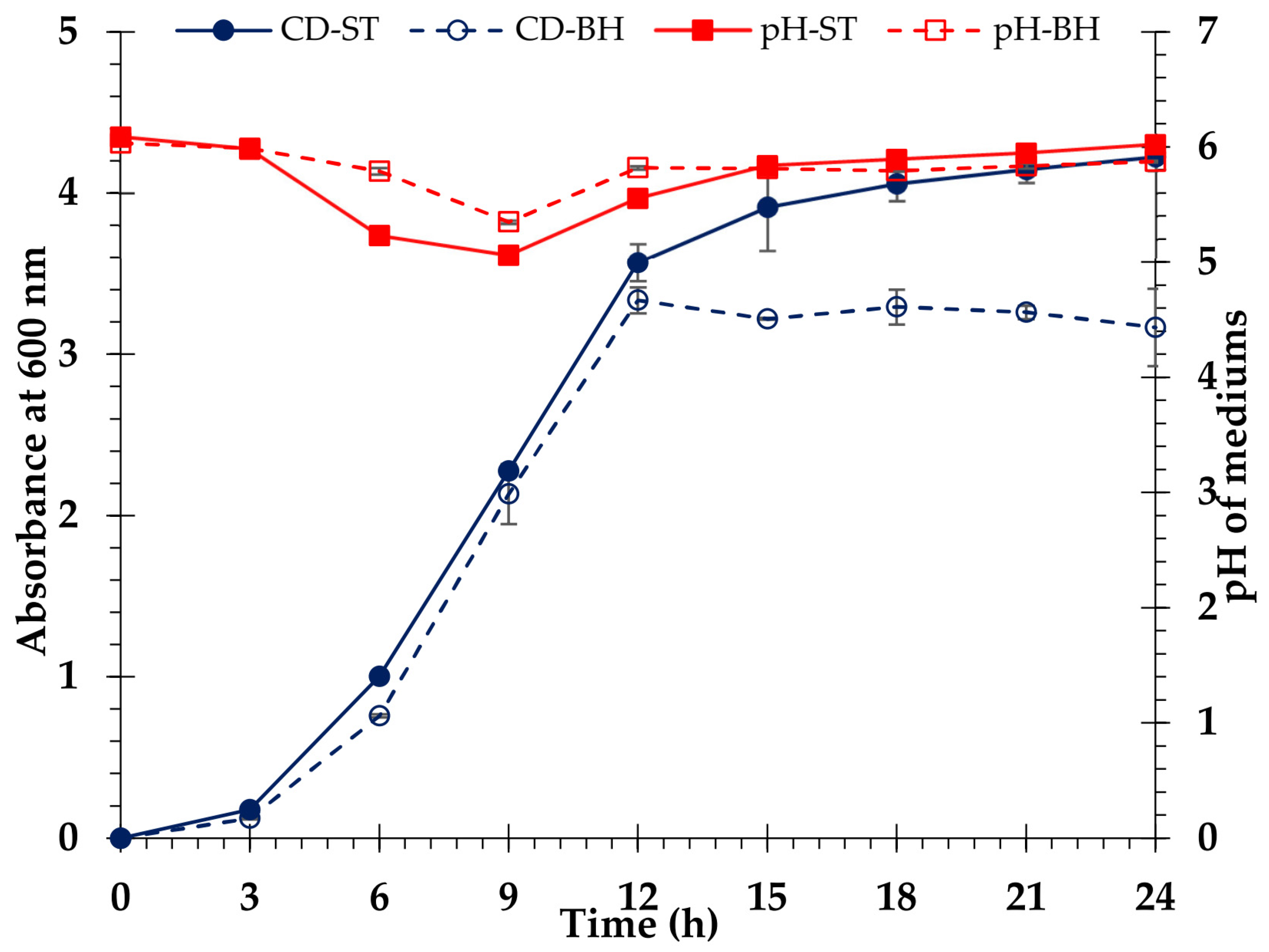
3.8. Ethanol Fermentation
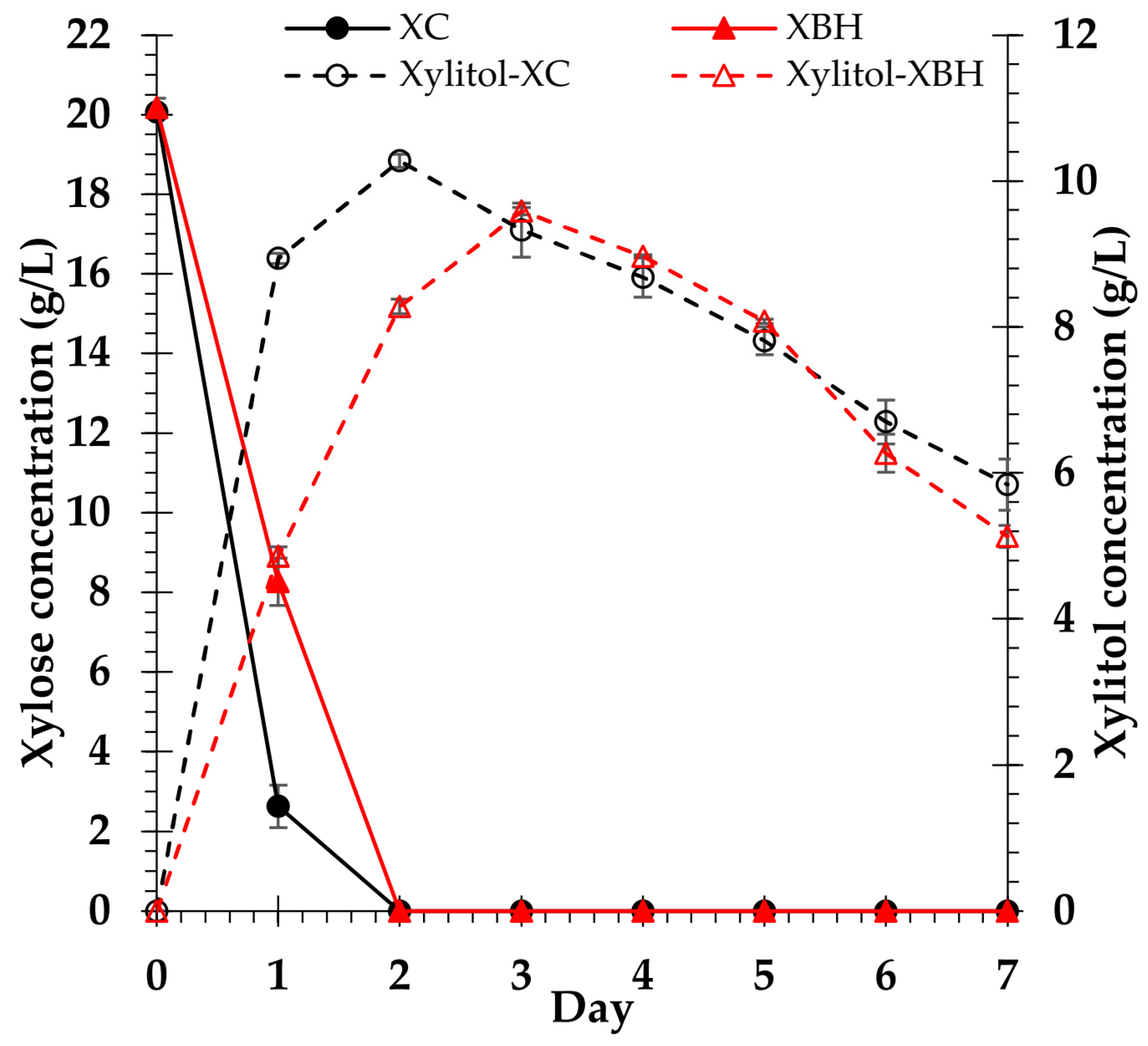
3.9. Xylitol Production
4. Discussion
4.1. Effect of NaOH Concentration on Biomass Composition
4.2. Effect of NaOH Concentration on Hydrolysis Yield
4.3. Effect of Temperature on Biomass Composition
4.4. Effect of Temperature on Hydrolysis Yield
4.5. Effect of Pretreatment on Crystalline Structure
4.6. Effect of Pretreatment on Morphological Structure
4.7. Ethanol Fermentation
4.8. Xylitol Production
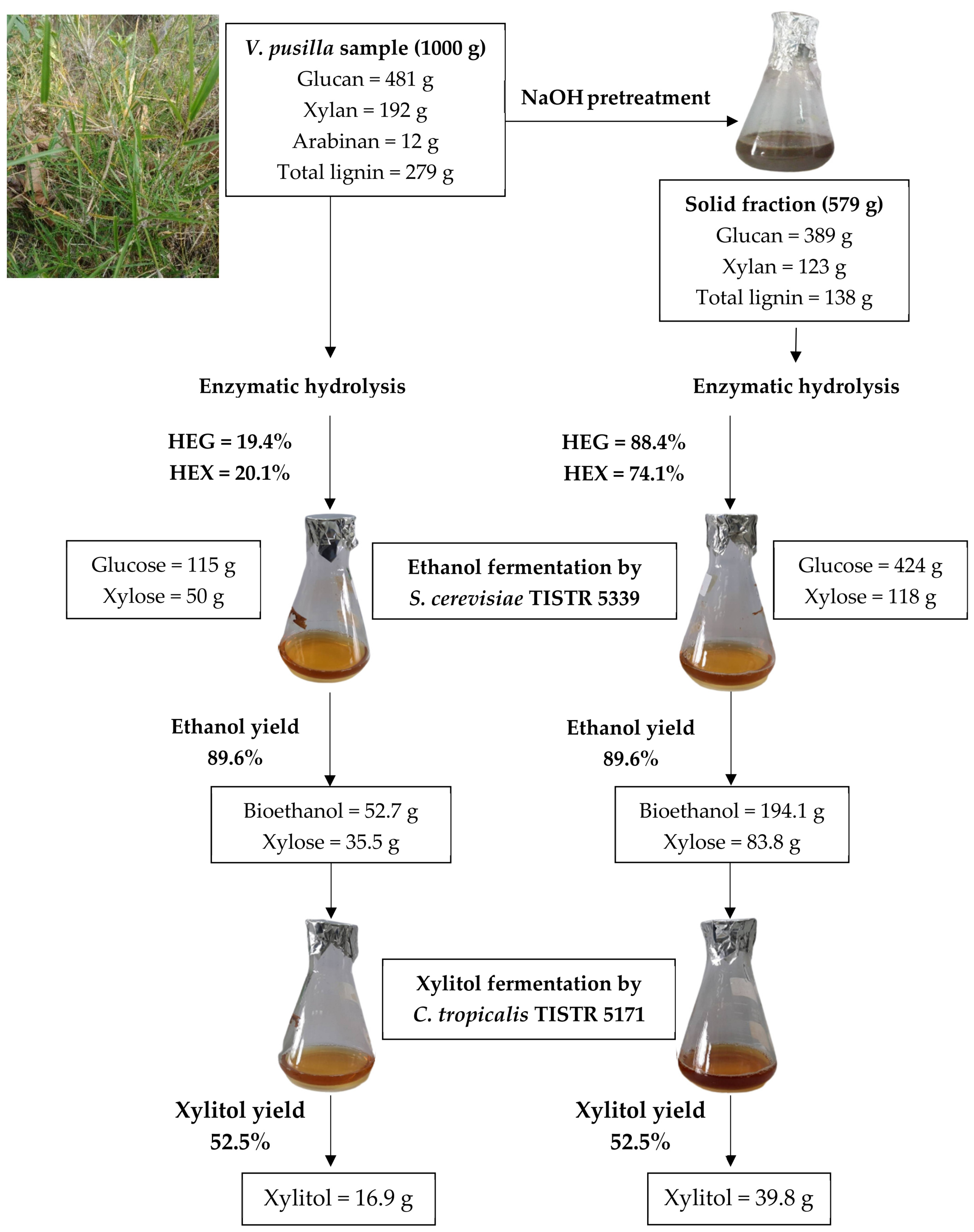
4.9. Material Balance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tusher, T.R.; Chang, J.J.; Saunivalu, M.I.; Wakasa, S.; Li, W.-H.; Huang, C.C.; Inoue, C.; Chien, M.F. Second-generation bioethanol production from phytomass after phytoremediation using recombinant bacteria-yeast co-culture. Fuel 2022, 326, 124975. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Bonifacio, S.; Clowes, J.; Foulds, A.; Holland, R.; Matthews, J.C.; Percival, C.J.; Shallcross, D.E. Investigation of biofuel as a potential renewable energy source. Atmosphere 2021, 12, 1289. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Qian, J. Pretreatment with a combination of steam explosion and NaOH increases butanol production of enzymatically hydrolyzed corn stover. Renew. Energy 2022, 203, 301–311. [Google Scholar] [CrossRef]
- Casau, M.; Dias, M.F.; Matias, J.C.O.; Nunes, L.J.R. Residual biomass: A comprehensive review on the importance, uses and potential in a circular bioeconomy approach. Resources 2022, 11, 35. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Zhao, J.; Qin, Y. Pretreatment strategies to enhance enzymatic hydrolysis and cellulosic ethanol production for biorefinery of corn stover. Int. J. Mol. Sci. 2022, 23, 13163. [Google Scholar] [CrossRef] [PubMed]
- Wongleang, S.; Premjet, D.; Premjet, S. Cellulosic ethanol production from weed biomass hydrolysate of Vietnamosasa pusilla. Polymers 2023, 15, 1103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Messaoudi, Y.; Smichi, N.; Moujahed, N.; Gargouri, M. Hydrothermal and chemical pretreatment process for bioethanol production from agricultural and forest lignocellulosic wastes: Design and modeling. Chem. Afr. 2022, 6, 1–18. [Google Scholar] [CrossRef]
- Thuy, V.T.; Huyen, N.T.; Tu, L.H.; Loi, N.K. Status of bamboos in Binh Duong province, Vietnam: Distribution, species diversity, conservation and utilization. Trees For. People 2021, 6, 100137. [Google Scholar] [CrossRef]
- Pagad, S. Bamboos and Invasiveness-Identifying Which Bamboo Species Pose a Risk to the Natural Environment and What Can Be Done to Reduce This Risk; The International Bamboo and Rattan Organization (INBAR): Beijing, China, 2016; Available online: https://www.researchgate.net/publication/315720069 (accessed on 15 March 2023).
- The International Plant Names Index and World Checklist of Vascular Plants. Available online: https://powo.science.kew.org/ (accessed on 20 September 2023).
- Obeng, A.K.; Premjet, D.; Premjet, S. Combining autoclaving with mild alkaline solution as a pretreatment technique to enhance glucose recovery from the invasive weed Chloris barbata. Biomolecules 2019, 9, 120. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- An, H.-E.; Lee, K.H.; Jang, Y.W.; Kim, C.-B.; Yoo, H.Y. Improved glucose recovery from Sicyos angulatus by NaOH pretreatment and application to bioethanol production. Processes 2021, 9, 245. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M.; Ahmed, N.A.; Ogunkunle, O. Influence of alkali pretreatment on morphological structure and methane yield of Arachis hypogea shells. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Improved glucose recovery from durian peel by alkaline-catalyzed steam pretreatment. PeerJ 2021, 9, 12026. [Google Scholar] [CrossRef]
- Kumar, N.; Yadav, A.; Singh, G.; Singh, A.; Kumar, P.; Aggarwal, N.K. Comparative study of ethanol production from sodium hydroxide pretreated rice straw residue using Saccharomyces cerevisiae and Zymomonas mobilis. Arch. Microbiol. 2023, 205, 146. [Google Scholar] [CrossRef]
- Chinwatpaiboon, P.; Savarajara, A.; Luengnaruemitchai, A. Enzymatic hydrolysate of water hyacinth with NaOH pretreatment for biobutanol production via ABE fermentation by Clostridium beijerinckii JCM 8026. Biomass Bioenergy 2023, 173, 106782. [Google Scholar] [CrossRef]
- Sarmiento-Vásquez, Z.; Vandenberghe, L.P.d.S.; Karp, S.G.; Soccol, C.R. Production of polyhydroxyalkanoates through soybean hull and waste glycerol valorization: Subsequent alkaline pretreatment and enzymatic hydrolysis. Fermentation 2022, 8, 433. [Google Scholar] [CrossRef]
- Papathoti, N.K.; Mendam, K.; Thepbandit, W.; Burgula, N.; Sangpueak, R.; Saengchan, C.; Hoang, N.H.; Keshav, P.K.; Le Thanh, T.; Buensanteai, N. Bioethanol production from alkali-pretreated cassava stem waste via consolidated bioprocessing by ethanol-tolerant Clostridium thermocellum ATCC 31,924. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Papathoti, N.K.; Laemchiab, K.; Megavath, V.S.; Keshav, P.K.; Numparditsub, P.; Le Thanh, T.; Buensanteai, N. Augmented ethanol production from alkali-assisted hydrothermal pretreated cassava peel waste. Energy Sources A Recovery Util. Environ. Eff. 2021, 1–11. [Google Scholar] [CrossRef]
- Melesse, G.T.; Hone, F.G.; Mekonnen, M.A. Extraction of cellulose from sugarcane bagasse optimization and characterization. Adv. Mater. Sci. Eng. 2022, 2022, 1712207. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Thermochemical pretreatment enhanced bioconversion of elephant grass (Pennisetum purpureum): Insight on the production of sugars and lignin. Biomass Convers. Biorefinery 2022, 12, 1125–1138. [Google Scholar] [CrossRef]
- Manokhoon, P.; Rangseesuriyachai, T. Effect of two-stage sodium hydroxide pretreatment on the composition and structure of Napier grass (Pakchong 1) (Pennisetum purpureum). Int. J. Green Energy 2020, 17, 864–871. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Fan, C.; Zheng, X.; Zhang, W.; Wu, D.; Wang, X.; Kong, H. Optimisation of enzymatic saccharification of wheat straw pre-treated with sodium hydroxide. Sci. Rep. 2021, 11, 23234. [Google Scholar] [CrossRef]
- Jung, W.; Savithri, D.; Sharma-Shivappa, R.; Kolar, P. Effect of sodium hydroxide pretreatment on lignin monomeric components of Miscanthus × giganteus and enzymatic hydrolysis. Waste Biomass Valorization 2020, 11, 5891–5900. [Google Scholar] [CrossRef]
- Premjet, S.; Dana, S.; Obeng, A.K.; Premjet, D. Enzymatic response to structural and chemical transformations in Hibiscus sabdariffa var. altissima bark and core during phosphoric acid pretreatment. BioResources 2018, 13, 6778–6789. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012; pp. 1–15. [Google Scholar]
- Premjet, D.; Wongleang, S.; Premjet, S. Enhancing glucose recovery from Hibiscus cannabinus L. through phosphoric acid pretreatment. Energies 2022, 15, 7573. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Premjet, S.; Premjet, D.; Yoo, H.Y.; Kim, S.W. Improvement of sugar recovery from Sida acuta (Thailand Weed) by NaOH pretreatment and application to bioethanol production. Korean J. Chem. Eng. 2018, 35, 2413–2420. [Google Scholar] [CrossRef]
- Ngamsirisomsakul, M.; Reungsang, A.; Kongkeitkajorn, M.B. Assessing oleaginous yeasts for their potentials on microbial lipid production from sugarcane bagasse and the effects of physical changes on lipid production. Bioresour. Technol. Rep. 2021, 14, 100650. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S. Effects of sodium hydroxide pretreatment on structural components of biomass. Trans ASABE 2014, 57, 1187–1198. [Google Scholar] [CrossRef]
- Wongsiridetchai, C.; Chiangkham, W.; Khlaihiran, N.; Sawangwan, T.; Wongwathanarat, P.; Charoenrat, T.; Chantorn, S. Alkaline pretreatment of spent coffee grounds for oligosaccharides production by mannanase from Bacillus sp. GA2(1). Agric. Nat. Resour. 2018, 52, 222–227. [Google Scholar] [CrossRef]
- Ashoor, S.; Sukumaran, R.K. Mild alkaline pretreatment can achieve high hydrolytic and fermentation efficiencies for rice straw conversion to bioethanol. Prep. Biochem. Biotechnol. 2020, 50, 814–819. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, J.-R.; Wang, Y.-T.; Zhu, M.-J. Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresour. Technol. 2020, 301, 122756. [Google Scholar] [CrossRef]
- Sato, A.; Soeprijanto, S.; Widjaja, A. Influence of alkaline hydrothermal pretreatment of rice straw on biomass composition. Int. Energy J. 2019, 19, 115–124. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, Y.; Yu, Q.; Tan, X.; Zhuang, X.; Yuan, Z. Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind. Crops Prod. 2020, 145, 112145. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Bhattacharya, A.; Rashamuse, K.; Pletschke, B.I. The effects of alkaline pretreatment on agricultural biomasses (corn cob and sweet sorghum bagasse) and their hydrolysis by a termite-derived enzyme cocktail. Agronomy 2020, 10, 1211. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Siti, S.; Rahman, R.A.; Rosli, M.I.; Harisun, Y. Optimization of alkaline pretreatment conditions of oil palm fronds in improving the lignocelluloses contents for reducing sugar production. Rom. Biotechnol. Lett. 2014, 19, 9006–9018. [Google Scholar]
- Fernández-Delgado, M.; Plaza, P.E.; Coca, M.; García-Cubero, M.T.; González-Benito, G.; Lucas, S. Comparison of mild alkaline and oxidative pretreatment methods for biobutanol production from brewer’s spent grains. Ind. Crops Prod. 2019, 130, 409–419. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Li, C.; Fan, M.; Xie, J.; Zhang, H. Effect of NaOH-catalyzed organosolv pretreatment on the co-production of ethanol and xylose from poplar. Ind. Crops Prod. 2023, 200, 116774. [Google Scholar] [CrossRef]
- Hoşgün, E.Z.; Bozan, B. Effect of different types of thermochemical pretreatment on the enzymatic hydrolysis and the composition of hazelnut shells. Waste Biomass Valorization 2020, 11, 3739–3748. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuels 2010, 24, 2113–2119. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Lee, J.H.; Kim, D.S.; Lee, J.H.; Lee, S.K.; Lee, S.J.; Park, C.; Kim, S.W. Enhancement of glucose yield from canola agricultural residue by alkali pretreatment based on multi-regression models. J. Ind. Eng. Chem. 2017, 51, 303–311. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Rehman, M.S.U.; Terán-Hilares, R.; Khalid, S.; Han, J.-I. Optimization of twin gear-based pretreatment of rice straw for bioethanol production. Energy Convers. Manag. 2017, 141, 120–125. [Google Scholar] [CrossRef]
- Debiagi, F.; Madeira, T.B.; Nixdorf, S.L.; Mali, S. Pretreatment efficiency using autoclave high-pressure steam and ultrasonication in sugar production from liquid hydrolysates and access to the residual solid fractions of wheat bran and oat hulls. Appl. Biochem. Biotechnol. 2020, 190, 166–181. [Google Scholar] [CrossRef]
- Kontogianni, N.; Barampouti, E.M.; Mai, S.; Malamis, D.; Loizidou, M. Effect of alkaline pretreatments on the enzymatic hydrolysis of wheat straw. Environ. Sci. Pollut. Res. 2019, 26, 35648–35656. [Google Scholar] [CrossRef] [PubMed]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, T.; Huang, S.; Xie, J. Enhancement of enzymatic hydrolysis of sugarcane bagasse by the combination of delignification pretreatment and polysorbate 80. Fermentation 2023, 9, 371. [Google Scholar] [CrossRef]
- Xu, H.; Li, B.; Mu, X. Review of alkali-based pretreatment to enhance enzymatic saccharification for lignocellulosic biomass conversion. Ind. Eng. Chem. Res. 2016, 55, 8691–8705. [Google Scholar] [CrossRef]
- Shahabazuddin, M.; Sarat Chandra, T.; Meena, S.; Sukumaran, R.K.; Shetty, N.P.; Mudliar, S.N. Thermal assisted alkaline pretreatment of rice husk for enhanced biomass deconstruction and enzymatic saccharification: Physico-chemical and structural characterization. Bioresour. Technol. 2018, 263, 199–206. [Google Scholar] [CrossRef]
- Mardawati, E.; Hartono, A.T.; Nurhadi, B.; Fitriana, H.N.; Hermiati, E.; Ermawar, R.A. Xylitol production from pineapple cores (Ananas comosus (L.) Merr) by enzymatic and acid hydrolysis using microorganisms Debaryomyces hansenii and Candida tropicalis. Fermentation 2022, 8, 694. [Google Scholar] [CrossRef]
- Thamsee, T.; Choojit, S.; Cheirsilp, B.; Yamseangsung, R.; Ruengpeerakul, T.; Sangwichien, C. Combination of superheated steam explosion and alkaline autoclaving pretreatment for improvement of enzymatic digestibility of the oil palm tree residues as alternative sugar sources. Waste Biomass Valorization 2019, 10, 3009–3023. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Zhang, P.; Jin, S.; Tao, X.; Tang, X.; Ye, J.; Nabi, M.; Wang, H. Ultrasound assisted alkaline pretreatment to enhance enzymatic saccharification of grass clipping. Energy Conv. Manag. 2017, 149, 409–415. [Google Scholar] [CrossRef]
- Huang, W.; Wang, E.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F. Effect of physicochemical pretreatments and enzymatic hydrolysis on corn straw degradation and reducing sugar yield. Bioresources 2017, 12, 7002–7015. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Ladeira-Ázar, R.I.S.; Morgan, T.; Maitan-Alfenas, G.P.; Guimarães, V.M. Inhibitors compounds on sugarcane bagasse saccharification: Effects of pretreatment methods and alternatives to decrease inhibition. Appl. Biochem. Biotechnol. 2019, 188, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Geng, A.; Yao, C.; Lu, Y.; Li, Q. Effects of lignin-derived phenolic compounds on xylitol production and key enzyme activities by a xylose utilizing yeast Candida athensensis SB18. Bioresour. Technol. 2012, 121, 369–378. [Google Scholar] [CrossRef]
- Łukajtis, R.; Rybarczyk, P.; Kucharska, K.; Konopacka-Łyskawa, D.; Słupek, E.; Wychodnik, K.; Kamiński, M. Optimization of saccharification conditions of lignocellulosic biomass under alkaline pre-treatment and enzymatic hydrolysis. Energies 2018, 11, 886. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting non-conventional yeasts for ethanol production from rice straw hydrolysate and their inhibitor tolerance. Renew. Energy 2020, 147, 1694–1703. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef]
- Vargas, A.C.G.; Dresch, A.P.; Schmidt, A.R.; Tadioto, V.; Giehl, A.; Fogolari, O.; Mibielli, G.M.; Alves, S.L.; Bender, J.P. Batch fermentation of lignocellulosic elephant grass biomass for 2G ethanol and xylitol production. BioEnergy Res. 2023, 1–10. [Google Scholar] [CrossRef]
- Patiño, M.A.; Ortiz, J.P.; Velásquez, M.; Stambuk, B.U. d-Xylose consumption by nonrecombinant Saccharomyces cerevisiae: A review. Yeast 2019, 36, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Zininga, J.T.; Puri, A.K.; Dlangamandla, N.; Wang, Z.; Singh, S.; Permaul, K. Integrated biorefinery of Mucor circinelloides biomass and sugarcane bagasse for application of high-value biopolymers. Biomass Convers. Biorefinery 2023, 1–12. [Google Scholar] [CrossRef]
- Vardhan, H.; Sasamal, S.; Mohanty, K. Xylitol production by Candida tropicalis from areca nut husk enzymatic hydrolysate and crystallization. Appl. Biochem. Biotechnol. 2023, 1–24. [Google Scholar] [CrossRef]
- Bianchini, I.d.A.; Jofre, F.M.; Queiroz, S.d.S.; Lacerda, T.M.; Felipe, M.d.G.d.A. Relation of xylitol formation and lignocellulose degradation in yeast. Appl. Microbiol. Biotechnol. 2023, 107, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xing, X.; Xie, Y.; Sun, Y.; Bian, S.; Liu, L.; Chen, G.; Wang, X.; Yu, X.; Su, Y. Evaluation of preparation and detoxification of hemicellulose hydrolysate for improved xylitol production from quinoa straw. Int. J. Mol. Sci. 2023, 24, 516. [Google Scholar] [CrossRef]
- Misra, S.; Raghuwanshi, S.; Saxena, R.K. Evaluation of corncob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydr. Polym. 2013, 92, 1596–1601. [Google Scholar] [CrossRef] [PubMed]




| Composition (% DW) | Untreated Sample | Concentration of NaOH (%) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Glucan | 48.1 ± 0.3 e | 55.3 ± 0.5 d | 67.2 ± 0.1 c | 68.0 ± 0.0 b | 71.0 ± 0.2 a |
| Xylan | 19.2 ± 0.4 c | 21.9 ± 0.2 a | 21.3 ± 0.3 a | 20.1 ± 0.2 b | 17.8 ± 0.4 d |
| Arabinan | 1.2 ± 0.1 | n.d. | n.d. | n.d. | n.d. |
| AIL | 23.5 ± 0.1 a | 21.8 ± 0.0 b | 20.9 ± 0.1 c | 18.1 ± 0.5 d | 15.4 ± 0.3 e |
| ASL | 4.4 ± 0.1 a | 3.0 ± 0.0 b | 2.9 ± 0.0 b | 2.9 ± 0.0 b | 2.9 ± 0.0 b |
| Total lignin | 27.9 ± 0.2 a | 24.8 ± 0.1 b | 23.9 ± 0.1 c | 21.0 ± 0.5 d | 18.3 ± 0.3 e |
| Composition (% DW) | Untreated Sample | Temperatures (°C) | ||
|---|---|---|---|---|
| 110 | 120 | 130 | ||
| Glucan | 48.1 ± 0.3 c | 65.1 ± 0.3 b | 67.2 ± 0.1 a | 67.1 ± 0.3 a |
| Xylan | 19.2 ± 0.4 c | 22.5 ± 0.1 a | 21.3 ± 0.3 b | 19.3 ± 0.2 c |
| Arabinan | 1.2 ± 0.1 | n.d. | n.d. | n.d. |
| AIL | 23.5 ± 0.1 a | 23.0 ± 0.1 a | 20.9 ± 0.1 b | 16.1 ± 0.5 c |
| ASL | 4.4 ± 0.1 a | 3.2 ± 0.0 b | 2.9 ± 0.0 c | 2.9 ± 0.0 c |
| Total lignin | 27.9 ± 0.2 a | 26.1 ± 0.1 b | 23.9 ± 0.1 c | 19.0 ± 0.5 d |
| Concentration (%) | Temperature (°C) | CrI (%) |
|---|---|---|
| Untreated | - | 59.5 |
| 1% NaOH | 120 | 66.2 |
| 2% NaOH | 120 | 67.6 |
| 3% NaOH | 120 | 67.7 |
| 4% NaOH | 120 | 67.9 |
| 2% NaOH | 110 | 68.5 |
| 2% NaOH | 130 | 67.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongleang, S.; Premjet, D.; Premjet, S. Physicochemical Pretreatment of Vietnamosasa pusilla for Bioethanol and Xylitol Production. Polymers 2023, 15, 3990. https://doi.org/10.3390/polym15193990
Wongleang S, Premjet D, Premjet S. Physicochemical Pretreatment of Vietnamosasa pusilla for Bioethanol and Xylitol Production. Polymers. 2023; 15(19):3990. https://doi.org/10.3390/polym15193990
Chicago/Turabian StyleWongleang, Suwanan, Duangporn Premjet, and Siripong Premjet. 2023. "Physicochemical Pretreatment of Vietnamosasa pusilla for Bioethanol and Xylitol Production" Polymers 15, no. 19: 3990. https://doi.org/10.3390/polym15193990
APA StyleWongleang, S., Premjet, D., & Premjet, S. (2023). Physicochemical Pretreatment of Vietnamosasa pusilla for Bioethanol and Xylitol Production. Polymers, 15(19), 3990. https://doi.org/10.3390/polym15193990






