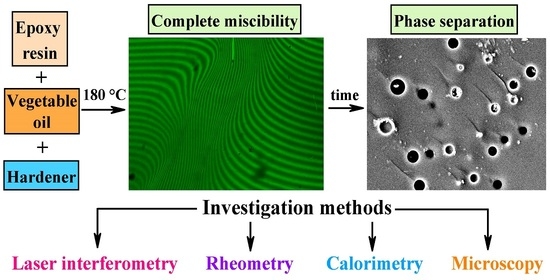
Self-Lubricating and Shape-Stable Phase-Change Materials Based on Epoxy Resin and Vegetable Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
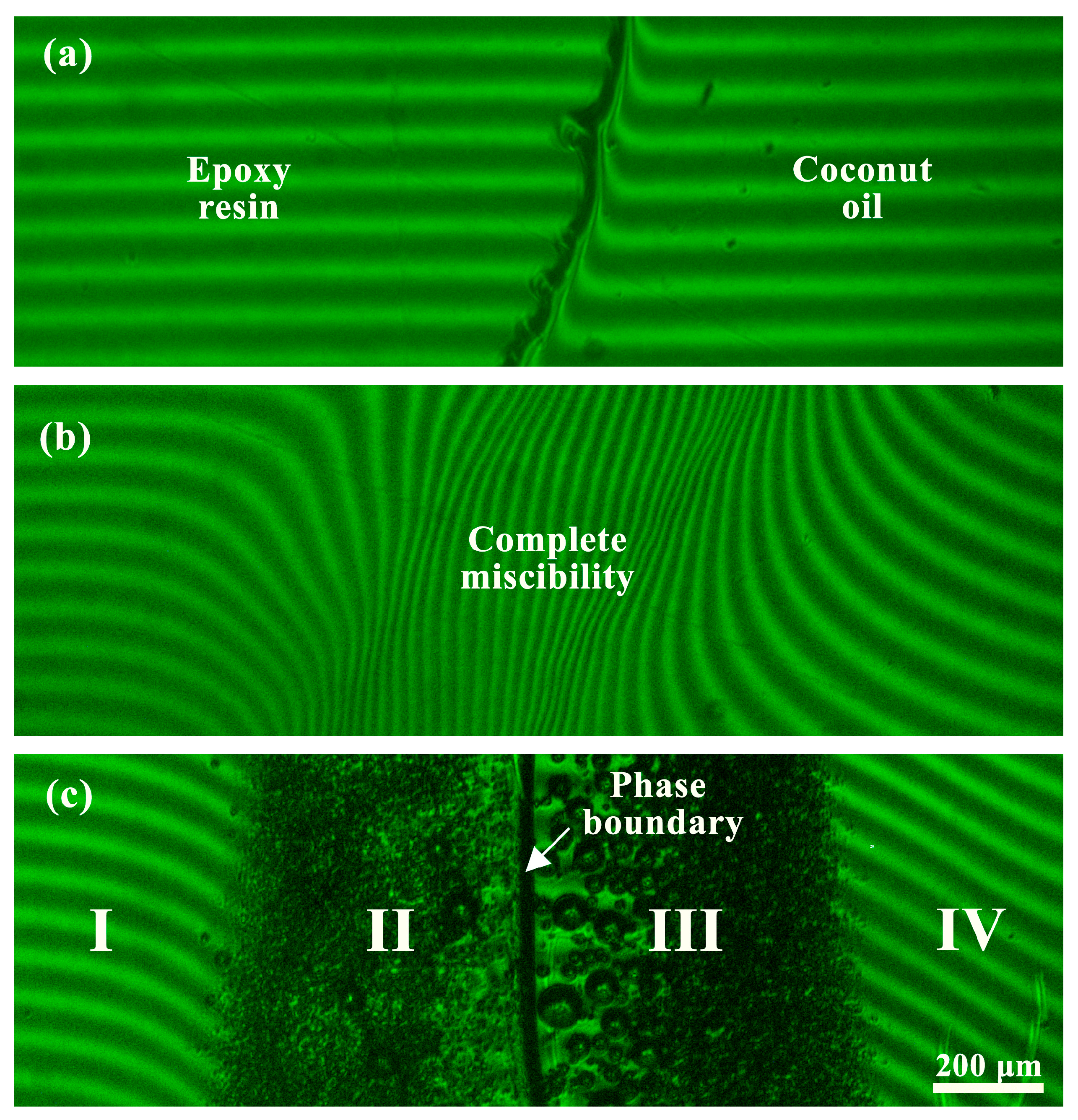
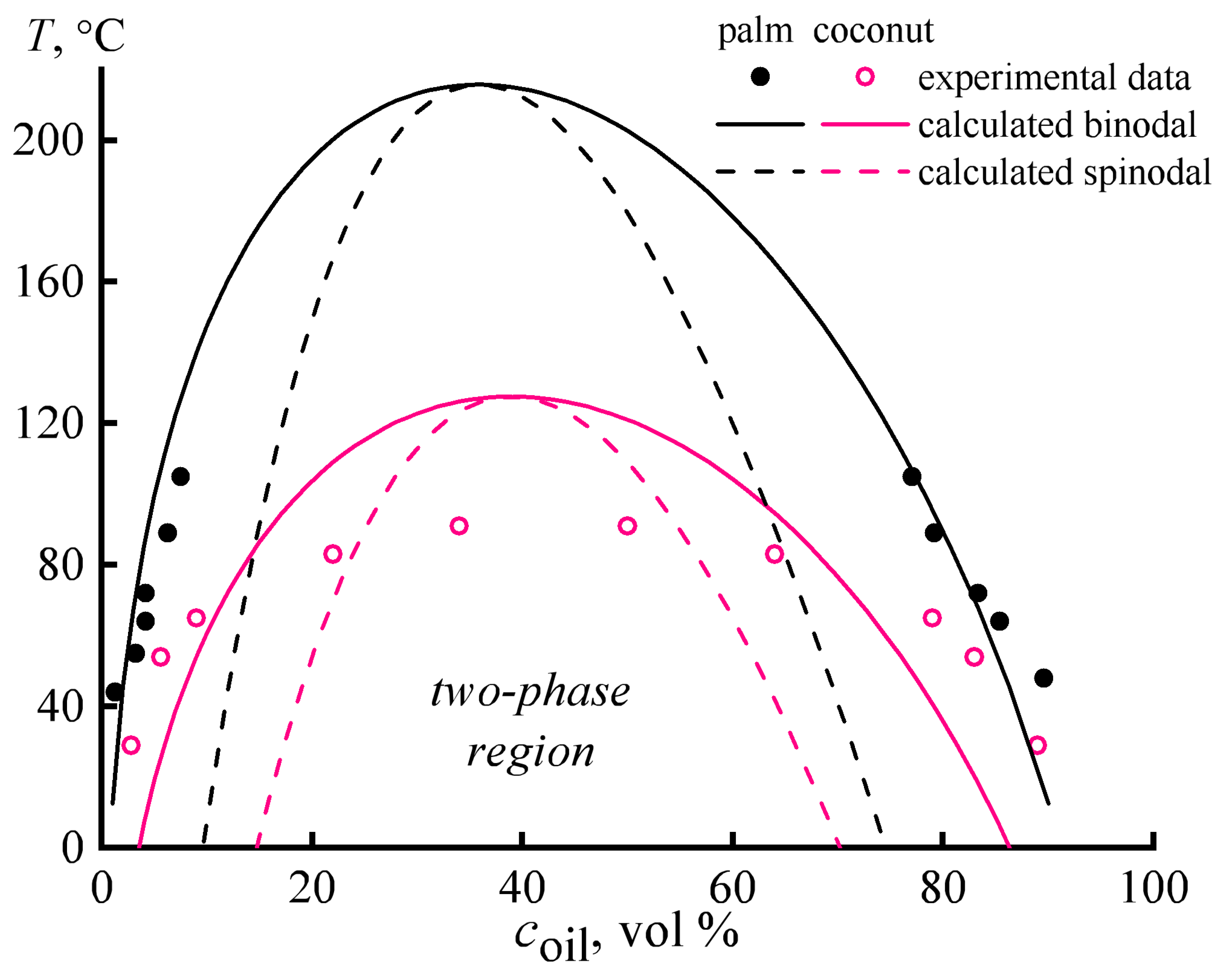
3.1. Miscibility of Epoxy Resin and Vegetable Oils
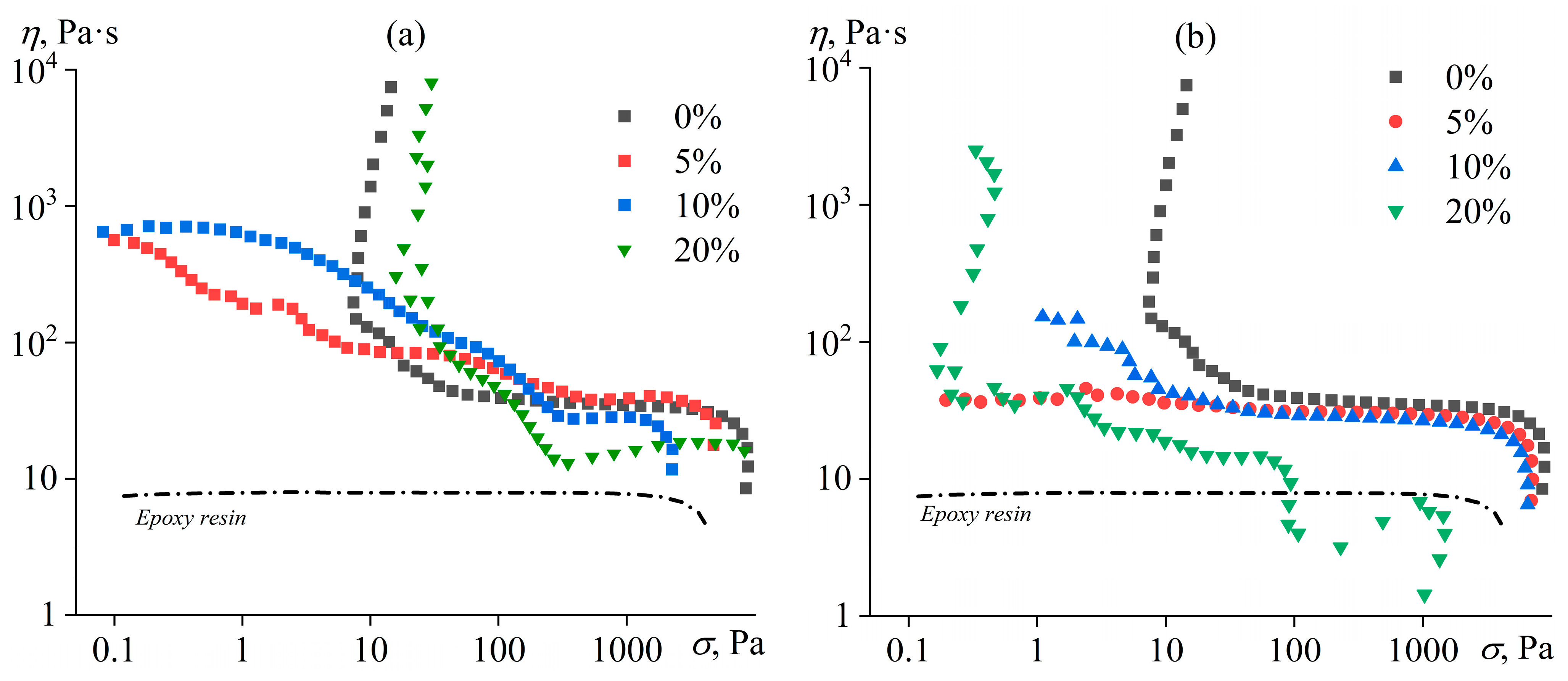
3.2. Rheology of Uncured Blends
3.3. Effect of Oil Addition on Resin Curing
3.4. Characteristics of Cured Epoxy Compositions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Solomon, B.D.; Krishna, K. The coming sustainable energy transition: History, strategies, and outlook. Energy Policy 2011, 39, 7422–7431. [Google Scholar] [CrossRef]
- Navarro, M.E.; Martínez, M.; Gil, A.; Fernández, A.I.; Cabeza, L.F.; Olives, R.; Py, X. Selection and characterization of recycled materials for sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 131–135. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Raoux, S. Phase Change Materials. Annu. Rev. Mater. Res. 2009, 39, 25–48. [Google Scholar] [CrossRef]
- Demirbas, M.F. Thermal Energy Storage and Phase Change Materials: An Overview. Energy Sources Part B Econ. Plan. Policy 2006, 1, 85–95. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Kalnæs, S.E.; Jelle, B.P. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic nanosilica-stabilized graphite particles for improving thermal conductivity of paraffin wax-based phase-change materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z.W. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Makarova, V.V.; Gorbacheva, S.N.; Antonov, S.V.; Ilyin, S.O. On the Possibility of a Radical Increase in Thermal Conductivity by Dispersed Particles. Russ. J. Appl. Chem. 2020, 93, 1796–1814. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, rheology and possible application of water-in-oil emulsions stabilized by asphaltenes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Prediction of the properties of eutectic fatty acid phase change materials. Thermochim. Acta 2018, 660, 94–100. [Google Scholar] [CrossRef]
- Vasilyev, G.; Koifman, N.; Shuster, M.; Gishvoliner, M.; Cohen, Y.; Zussman, E. Synergistic Effect of Two Organogelators for the Creation of Bio-Based, Shape-Stable Phase-Change Materials. Langmuir 2020, 36, 15572–15582. [Google Scholar] [CrossRef] [PubMed]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wang, Z.; Peng, K.; Pan, W.; Xie, Q. Novel form stable phase change materials based on the composites of polyethylene glycol/polymeric solid-solid phase change material. Sol. Energy Mater. Sol. Cells 2015, 134, 80–88. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Liu, J.; Yang, Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer 2008, 49, 2903–2910. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Wei, C.; Liang, S.; Luo, X.; Wang, J.; Zhang, L. Nanoencapsulated phase change materials with polymer-SiO2 hybrid shell materials: Compositions, morphologies, and properties. Energy Convers. Manag. 2018, 164, 83–92. [Google Scholar] [CrossRef]
- Tahan Latibari, S.; Mehrali, M.; Mehrali, M.; Indra Mahlia, T.M.; Cornelis Metselaar, H.S. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci. Technol. 2019, 91, 116–128. [Google Scholar] [CrossRef]
- Fang, Y.; Kuang, S.; Gao, X.; Zhang, Z. Preparation and characterization of novel nanoencapsulated phase change materials. Energy Convers. Manag. 2008, 49, 3704–3707. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Lian, Q.; Li, K.; Sayyed, A.A.S.; Cheng, J.; Zhang, J. Study on a reliable epoxy-based phase change material: Facile preparation, tunable properties, and phase/microphase separation behavior. J. Mater. Chem. A 2017, 5, 14562–14574. [Google Scholar] [CrossRef]
- Weingrill, H.M.; Resch-Fauster, K.; Zauner, C. Applicability of Polymeric Materials as Phase Change Materials. Macromol. Mater. Eng. 2018, 303, 1800355. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Morphology and Rheology of Heavy Crude Oil/Water Emulsions Stabilized by Microfibrillated Cellulose. Energy Fuels 2021, 35, 6527–6540. [Google Scholar] [CrossRef]
- Vinogradov, G.V. Ultimate regimes of deformation of linear flexible chain fluid polymers. Polymer 1977, 18, 1275–1285. [Google Scholar] [CrossRef]
- Vinogradov, G.V.; Yanovsky, Y.G.; Titkova, L.V.; Barancheeva, V.V.; Sergeenkov, S.I.; Borisenkova, E.K. Viscoelastic properties of linear polymers in the fluid state and their transition to the high-elastic state. Polym. Eng. Sci. 1980, 20, 1138–1146. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Two-functional phase-change pressure-sensitive adhesives based on polyisobutylene matrix filled with paraffin wax. J. Energy Storage 2022, 52, 104797. [Google Scholar] [CrossRef]
- Wang, Z.; Situ, W.; Li, X.; Zhang, G.; Huang, Z.; Yuan, W.; Yang, C. Novel shape stabilized phase change material based on epoxy matrix with ultrahigh cycle life for thermal energy storage. Appl. Therm. Eng. 2017, 123, 1006–1012. [Google Scholar] [CrossRef]
- Ma, T.; Li, L.; Wang, Q.; Guo, C. High-performance flame retarded paraffin/epoxy resin form-stable phase change material. J. Mater. Sci. 2019, 54, 875–885. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Ilyin, S.O.; Kostyuk, A.V.; Bondarenko, G.N.; Antonov, S.V. Acceleration of epoxy resin curing by using a combination of aliphatic and aromatic amines. Polym. Bull. 2020, 77, 1519–1540. [Google Scholar] [CrossRef]
- Amberkar, T.; Mahanwar, P. Thermal Energy Management in Buildings and Constructions with Phase Change Material-Epoxy Composites: A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 727–761. [Google Scholar] [CrossRef]
- Ren, X.; Shen, H.; Yang, Y.; Yang, J. Study on the Properties of a Novel Shape-Stable Epoxy Resin Sealed Expanded Graphite/Paraffin Composite PCM and Its Application in Buildings. Phase Transit. 2019, 92, 581–594. [Google Scholar] [CrossRef]
- Luo, W.; Hu, X.; Che, Y.; Zu, S.; Li, Q.; Jiang, X.; Liu, D. Form-Stable Phase Change Materials Enhanced Photothermic Conversion and Thermal Conductivity by Ag-Expanded Graphite. J. Energy Storage 2022, 52, 105060. [Google Scholar] [CrossRef]
- Ilyina, S.O.; Vlasova, A.V.; Gorbunova, I.Y.; Lukashov, N.I.; Kerber, M.L.; Ilyin, S.O. Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes. Polymers 2023, 15, 3243. [Google Scholar] [CrossRef]
- Ohayon-Lavi, A.; Lavi, A.; Alatawna, A.; Ruse, E.; Ziskind, G.; Regev, O. Graphite-Based Shape-Stabilized Composites for Phase Change Material Applications. Renew. Energy 2021, 167, 580–590. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, Y.; Wang, Y.; Zhou, C.; Zhang, X.; Lei, J. Study on a PEG/Epoxy Shape-Stabilized Phase Change Material: Preparation, Thermal Properties and Thermal Storage Performance. Int. J. Heat Mass Transf. 2018, 126, 1134–1142. [Google Scholar] [CrossRef]
- Lian, Q.; Li, Y.; Sayyed, A.A.S.; Cheng, J.; Zhang, J. Facile Strategy in Designing Epoxy/Paraffin Multiple Phase Change Materials for Thermal Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 3375–3384. [Google Scholar] [CrossRef]
- Arinina, M.P.; Ilyin, S.O.; Makarova, V.V.; Gorbunova, I.Y.; Kerber, M.L.; Kulichikhin, V.G. Miscibility and rheological properties of epoxy resin blends with aromatic polyethers. Polym. Sci. Ser. A 2015, 57, 177–185. [Google Scholar] [CrossRef]
- Shapagin, A.V.; Budylin, N.Y.; Chalykh, A.E.; Solodilov, V.I.; Korokhin, R.A.; Poteryaev, A.A. Phase Equilibrium, Morphology, and Physico-Mechanics in Epoxy-Thermoplastic Mixtures with Upper and Lower Critical Solution Temperatures. Polymers 2021, 13, 35. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Kostina, J.V.; Bakhtin, D.S.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Heavy crude oil asphaltenes as a nanofiller for epoxy resin. Polym. Eng. Sci. 2020, 60, 1530–1545. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Volkov, V.P.; Roginskaya, G.F.; Chalykh, A.E.; Rozenberg, B.A. Phase Structure of Epoxide–Rubber Systems. Russ. Chem. Rev. 1982, 51, 994–1006. [Google Scholar] [CrossRef]
- Chalykh, A.E.; Gerasimov, V.K. Phase equilibria and phase structures of polymer blends. Russ. Chem. Rev. 2004, 73, 59–74. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase state and rheology of polyisobutylene blends with silicone resin. Rheol. Acta 2020, 59, 375–386. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase behavior and rheology of miscible and immiscible blends of linear and hyperbranched siloxane macromolecules. Mater. Today Commun. 2020, 22, 100833. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, Q.; Sui, X.; Wang, Z.; Li, Y.; Jiang, L. Differential scanning calorimetry study–assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016, 194, 601–607. [Google Scholar] [CrossRef]
- Kratzeisen, M.; Müller, J. Influence of free fatty acid content of coconut oil on deposit and performance of plant oil pressure stoves. Fuel 2010, 89, 1583–1589. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Yadykova, A.Y.; Makarova, V.V.; Yashchenko, V.S.; Matveenko, Y.V. Sulfonated polyoxadiazole synthesis and processing into ion-conducting films. Polym. Int. 2020, 69, 1243–1255. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G. Rheology and Miscibility of Linear/Hyperbranched Polydimethylsiloxane Blends and an Abnormal Decrease in Their Viscosity. Macromolecules 2023, 56, 6818–6833. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry, 2nd ed.; Thermo Electron: Karlsruhe, Germany, 2004. [Google Scholar]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and Adhesive Properties of Nanocomposite Bitumen Binders Based on Hydrophilic or Hydrophobic Silica and Modified with Bio-Oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Scott, R.L. The thermodynamics of high polymer solutions. IV. Phase equilibria in the ternary system: Polymer–liquid 1–liquid 2. J. Chem. Phys. 1949, 17, 268–279. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Anokhina, T.S.; Ignatenko, V.Y.; Brantseva, T.V.; Volkov, A.V.; Antonov, S.V. Diffusion and phase separation at the morphology formation of cellulose membranes by regeneration from N-methylmorpholine N-oxide solutions. Cellulose 2018, 25, 2515–2530. [Google Scholar] [CrossRef]
- Minton, A.P. Simple Calculation of Phase Diagrams for Liquid-Liquid Phase Separation in Solutions of Two Macromolecular Solute Species. J. Phys. Chem. B 2020, 124, 2363–2370. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hoy, K.L. Solubility Parameter as a Design Parameter for Water Borne Polymers and Coatings. J. Coat. Fabr. 1989, 19, 53–67. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters; CRC: Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Cepeda, E.A.; Bravo, R.; Lomas, J.M. Solubilities of Fatty Acids and Triglycerides in 1-Bromopropane. J. Chem. Eng. Data 2012, 57, 1160–1164. [Google Scholar] [CrossRef]
- MacKnight, W.J.; Karasz, F.E. Polymer Blends. In Comprehensive Polymer Science and Supplements; Pergamon: Oxford, UK, 1989; Volume 7. [Google Scholar] [CrossRef]
- Chung, T.S. The limitations of using Flory-Huggins equation for the states of solutions during asymmetric hollow-fiber formation. J. Membr. Sci. 1997, 126, 19–34. [Google Scholar] [CrossRef]
- Vinogradov, G.V.; Insarova, N.I.; Boiko, B.B.; Borisenkova, E.K. Critical regimes of shear in linear polymers. Polym. Eng. Sci. 1972, 12, 323–334. [Google Scholar] [CrossRef]
- Borisenkova, E.K.; Dreval, V.E.; Vinogradov, G.V.; Kurbanaliev, M.K.; Moiseyev, V.V.; Shalganova, V.G. Transition of polymers from the fluid to the forced high-elastic and leathery states at temperatures above the glass transition temperature. Polymer 1982, 23, 91–99. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Strelets, L.A.; Ilyin, S.O. Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization. Molecules 2023, 28, 2065. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Ignatenko, V.Y.; Kostyuk, A.V.; Levin, I.S.; Bondarenko, G.N. Deasphalting of heavy crude oil by hexamethyldisiloxane: The effect of a solvent/oil ratio on the structure, composition, and properties of precipitated asphaltenes. J. Pet. Sci. Eng. 2022, 208, 109329. [Google Scholar] [CrossRef]
- Brantseva, T.V.; Ilyin, S.O.; Gorbunova, I.Y.; Antonov, S.V.; Korolev, Y.M.; Kerber, M.L. Epoxy reinforcement with silicate particles: Rheological and adhesive properties-Part II: Characterization of composites with halloysite. Int. J. Adhes. Adhes. 2016, 68, 248–255. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Arinina, M.P.; Malkin, A.Y.; Kulichikhin, V.G. Sol–gel transition and rheological properties of silica nanoparticle dispersions. Colloid J. 2016, 78, 608–615. [Google Scholar] [CrossRef]
- Chow, M.C.; Ho, C.C. Surface active properties of palm oil with respect to the processing of palm oil. J. Oil Palm Res. 2000, 12, 107–116. [Google Scholar]
- Blijdenstein, T.B.; van der Linden, E.; van Vliet, T.; van Aken, G.A. Scaling behavior of delayed demixing, rheology, and microstructure of emulsions flocculated by depletion and bridging. Langmuir 2004, 20, 11321–11328. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.S.; Gerrard, D.D.; Rhodes, T.S.; Mason, T.G.; Weitz, D.A. Rheology of attractive emulsions. Phys. Rev. E 2011, 84, 041404. [Google Scholar] [CrossRef] [PubMed]
- Mironova, M.V.; Ilyin, S.O. Effect of silica and clay minerals on rheology of heavy crude oil emulsions. Fuel 2018, 232, 290–298. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Rheological, thermophysical, and morphological features of original and hydrogenated bio-oils. Sustain. Energy Fuels 2021, 5, 4425–4433. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Valencia, C.; Franco, J.M.; Gallegos, C. Wall slip phenomena in oil-in-water emulsions: Effect of some structural parameters. J. Colloid Interface Sci. 2001, 241, 226–232. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological properties of polyethylene/metaboric acid thermoplastic blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Zhang, X.; Lorenceau, E.; Bourouina, T.; Basset, P.; Oerther, T.; Ferrari, M.; Coussot, P. Wall slip mechanisms in direct and inverse emulsions. J. Rheol. 2018, 62, 1495–1513. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G.; Malkin, A.Y. Rheological properties of emulsions formed by polymer solutions and modified by nanoparticles. Colloid Polym. Sci. 2015, 293, 1647–1654. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Ignatenko, V.Y.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Polyethylene Wax as an Alternative to Mineral Fillers for Preparation of Reinforced Pressure-Sensitive Adhesives. Int. J. Adhes. Adhes. 2020, 102, 102689. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G. The rheological state of suspensions in varying the surface area of nano-silica particles and molecular weight of the poly(ethylene oxide) matrix. Colloid Polym. Sci. 2017, 295, 555–563. [Google Scholar] [CrossRef]
- Arinina, M.P.; Kostenko, V.A.; Gorbunova, I.Y.; Il’in, S.O.; Malkin, A.Y. Kinetics of curing of epoxy resin by diaminodiphenyl sulfone: Rheology and calorimetry. Polym. Sci. Ser. A 2018, 60, 683–690. [Google Scholar] [CrossRef]
- Galy, J.; Sabra, A.; Pascault, J.P. Characterization of epoxy thermosetting systems by differential scanning calorimetry. Polym. Eng. Sci. 1986, 26, 1514–1523. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; López-Beltrán, F.; Valencia, C.; Franco, J.M. Effect of an Alkali Treatment on the Development of Cellulose Pulp-Based Gel-like Dispersions in Vegetable Oil for Use as Lubricants. Tribol. Int. 2018, 123, 329–336. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and Tribology of Nanocellulose-Based Biodegradable Greases: Wear and Friction Protection Mechanisms of Cellulose Microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Hamnas, A.; Unnikrishnan, G. Bio-Lubricants from Vegetable Oils: Characterization, Modifications, Applications and Challenges—Review. Renew. Sustain. Energy Rev. 2023, 182, 113413. [Google Scholar] [CrossRef]
- Scott, D.; Blackwell, J.; McCullagh, P.J.; Mills, G.H. Composite Materials for Rolling Bearing Cages. Wear 1970, 15, 257–269. [Google Scholar] [CrossRef]
- Feyzullahoglu, E.; Saffak, Z. The Tribological Behaviour of Different Engineering Plastics under Dry Friction Conditions. Mater. Des. 2008, 29, 205–211. [Google Scholar] [CrossRef]
- Friedrich, K. Polymer Composites for Tribological Applications. Adv. Ind. Eng. Polym. Res. 2018, 1, 3–39. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, M.; Liang, H.; Gao, K.; Zhang, Y.; Chen, B.; Min, C.; Hua, X.; Fu, Y. Fabrication of Low Friction and Wear Carbon/Epoxy Nanocomposites Using the Confinement and Self-Lubricating Function of Carbon Nanocage Fillers. Appl. Surf. Sci. 2021, 538, 148109. [Google Scholar] [CrossRef]


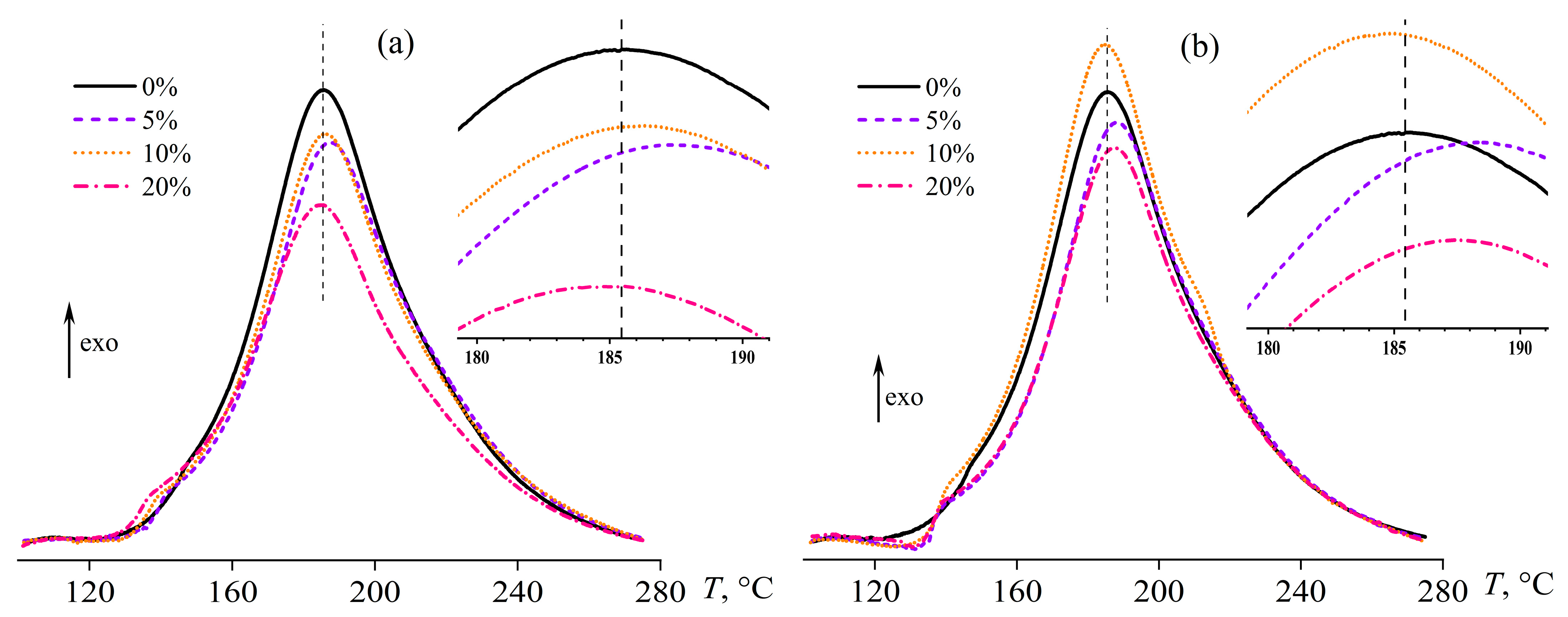

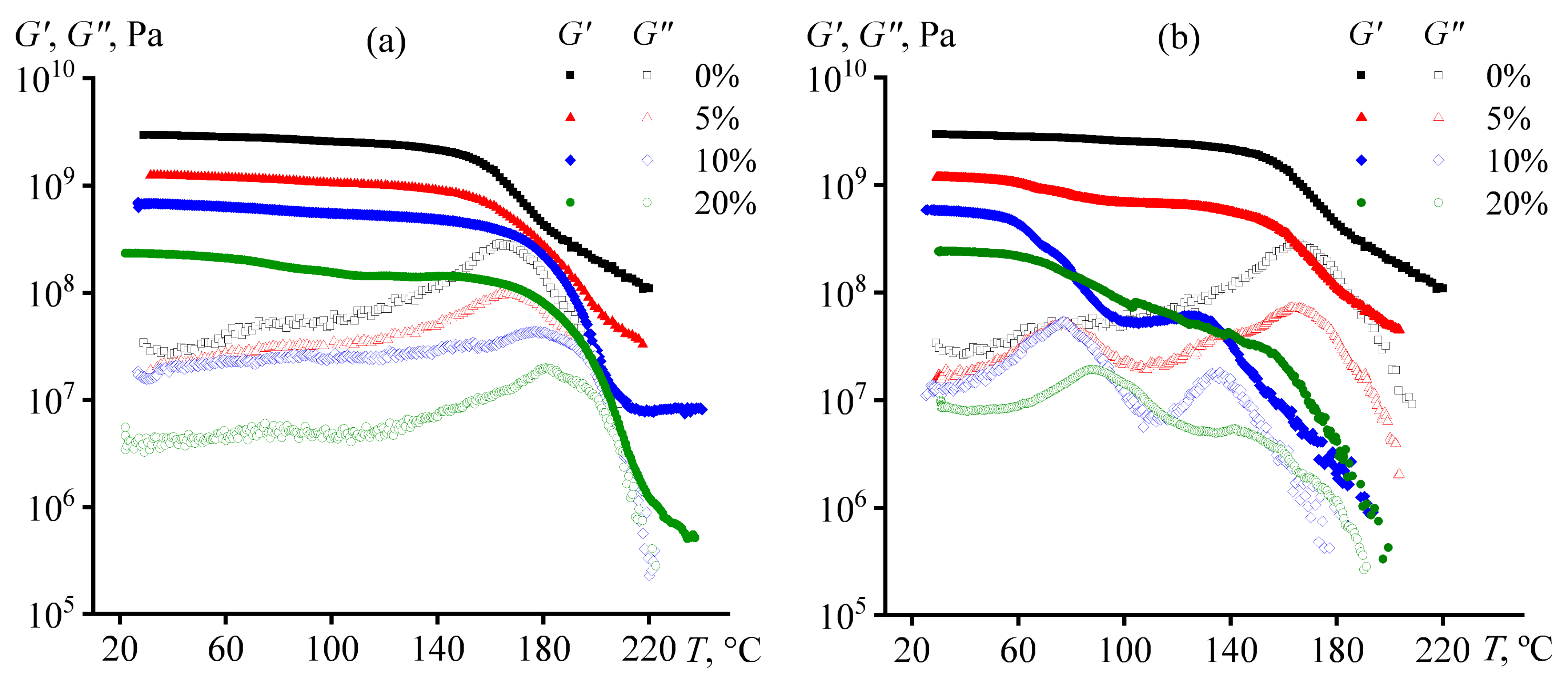

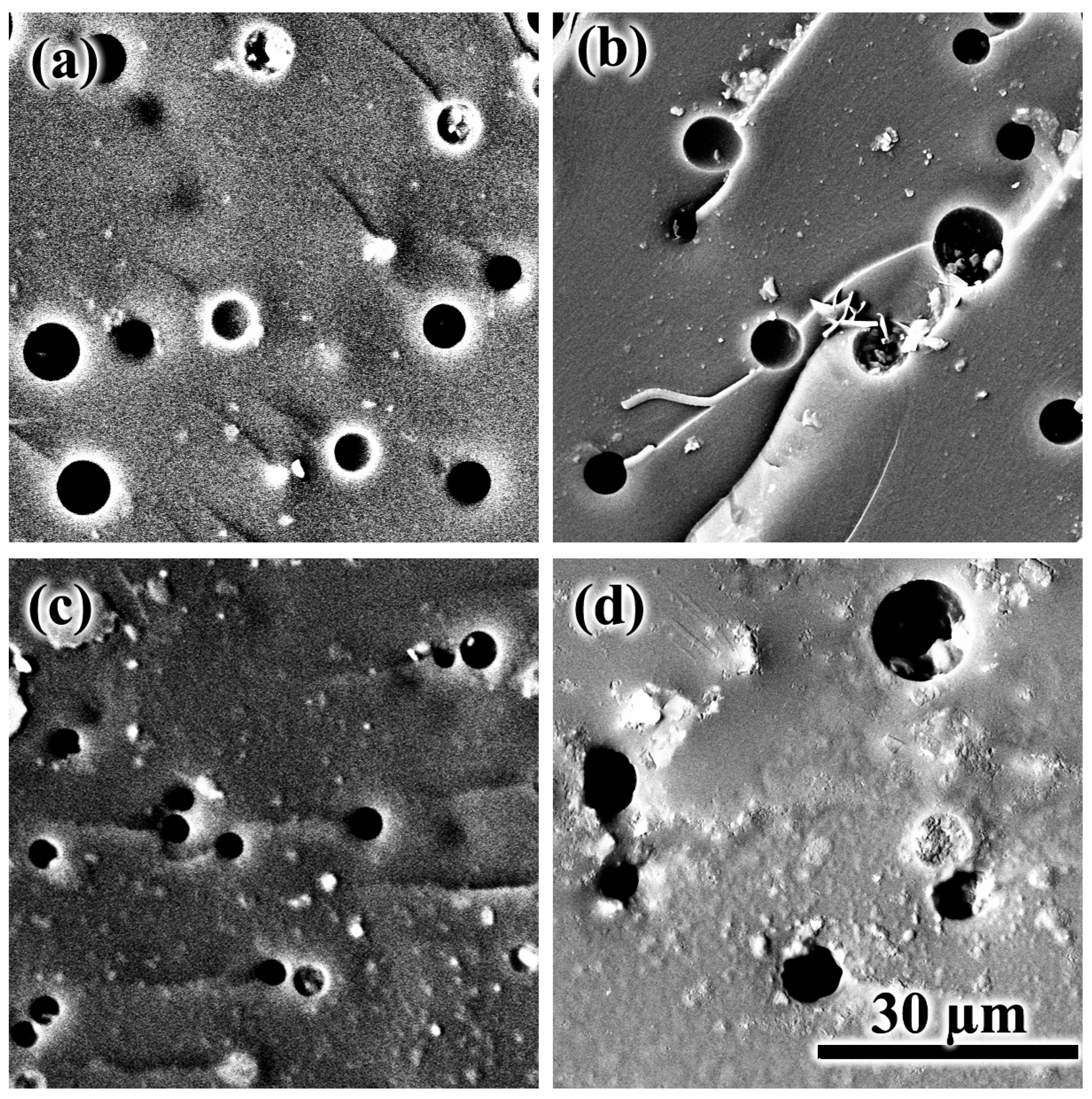
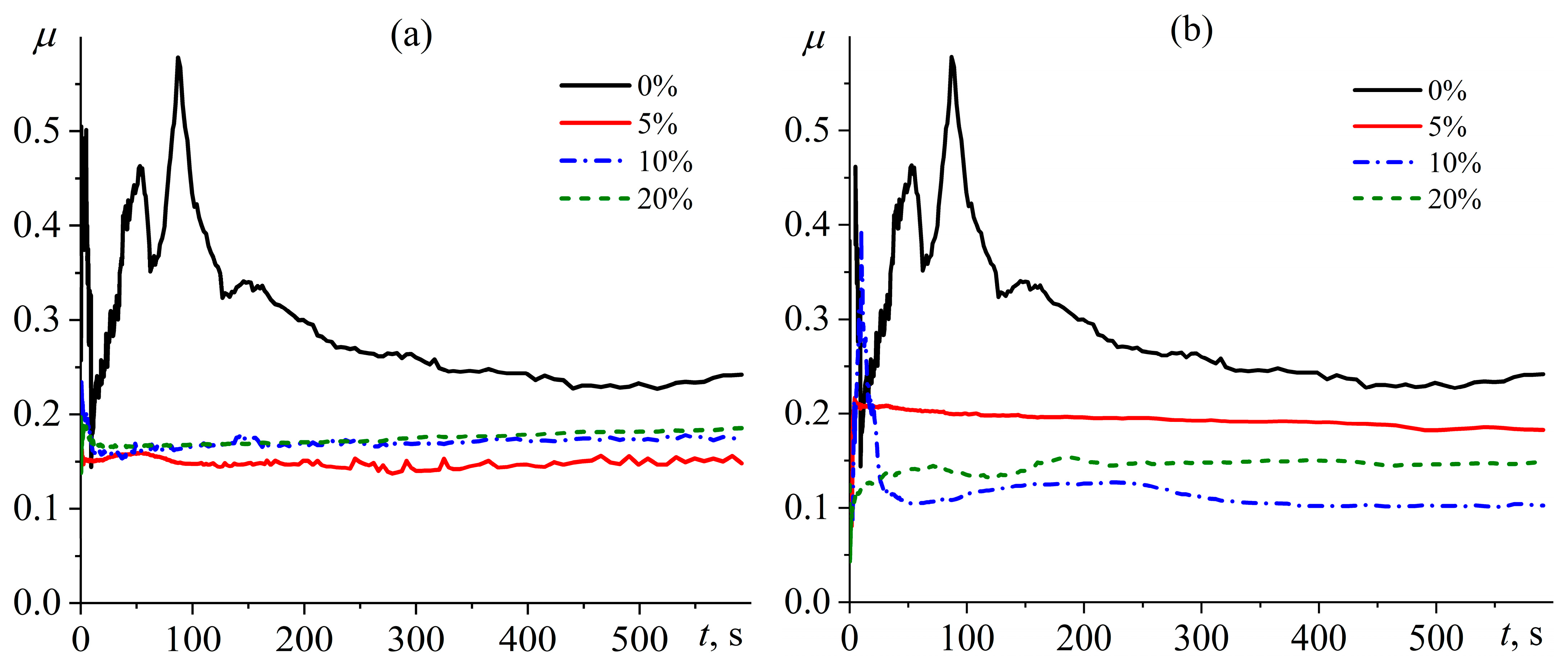

| Oil Type | coil, wt.% | Tpeak, °C | ΔH, J/g | ΔHr, J/g |
|---|---|---|---|---|
| w/o | 0 | 185.6 | 417 | 417 |
| Palm | 5 | 187.4 | 379 | 399 |
| Palm | 10 | 186.2 | 392 | 436 |
| Palm | 20 | 185.5 | 337 | 421 |
| Coconut | 5 | 188.3 | 396 | 417 |
| Coconut | 10 | 184.9 | 390 | 434 |
| Coconut | 20 | 185.5 | 337 | 421 |
| Standard deviation | ±0.01 wt.% | ±0.2 °C | ±21 J/g | ±21 J/g |
| Oil Type | coil, wt.% | Tg,DSC, °C | ∆Hm1, J/g | Tm1, °C | ∆Hm2, J/g | Tm2, °C | DCoil, % |
|---|---|---|---|---|---|---|---|
| w/o | 0 | 178.1 | – | – | – | – | – |
| Palm | 5 | 190.9 | 0.34 | 0.6 | 0.37 | 40.8 | 29.0 |
| Palm | 10 | 186.7 | 0.35 | 4.1 | 0.46 | 42.5 | 16.6 |
| Palm | 20 | 181.1 | 2.72 | 4.1 | 3.67 | 45.8 | 65.3 |
| Palm | 100 | – | 48.9 | 3.7 | 20.7 | 39.0 | 100 |
| Coconut | 5 | 190.9 | 0.48 | 1.9 | 0.31 | 38.8 | 15.8 |
| Coconut | 10 | 191.3 | 1.39 | 20.0 | – | – | 13.9 |
| Coconut | 20 | 185.0 | 1.45 | 21.1 | – | – | 7.3 |
| Coconut | 100 | – | 99.7 | 22.7 | – | – | 100 |
| Standard deviation | ±0.01 wt.% | ±0.2 °C | ±5% * | ±0.2 °C | ±5% * | ±0.2 | ±5% * |
| Oil Type | coil, wt.% | Tg,DMA, °C | G′25°C, GPa |
|---|---|---|---|
| w/o | 0 | 165 | 2.96 |
| Palm | 5 | 166 | 1.25 |
| Palm | 10 | 175 | 0.68 |
| Palm | 20 | 180 | 0.23 |
| Coconut | 5 | 77 and 163 | 1.18 |
| Coconut | 10 | 76 and 135 | 0.59 |
| Coconut | 20 | 88 and 140 | 0.24 |
| Standard deviation | ±0.01 wt.% | ±1 °C | ±5% * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyina, S.O.; Gorbunova, I.Y.; Makarova, V.V.; Kerber, M.L.; Ilyin, S.O. Self-Lubricating and Shape-Stable Phase-Change Materials Based on Epoxy Resin and Vegetable Oils. Polymers 2023, 15, 4026. https://doi.org/10.3390/polym15194026
Ilyina SO, Gorbunova IY, Makarova VV, Kerber ML, Ilyin SO. Self-Lubricating and Shape-Stable Phase-Change Materials Based on Epoxy Resin and Vegetable Oils. Polymers. 2023; 15(19):4026. https://doi.org/10.3390/polym15194026
Chicago/Turabian StyleIlyina, Svetlana O., Irina Y. Gorbunova, Veronika V. Makarova, Michael L. Kerber, and Sergey O. Ilyin. 2023. "Self-Lubricating and Shape-Stable Phase-Change Materials Based on Epoxy Resin and Vegetable Oils" Polymers 15, no. 19: 4026. https://doi.org/10.3390/polym15194026
APA StyleIlyina, S. O., Gorbunova, I. Y., Makarova, V. V., Kerber, M. L., & Ilyin, S. O. (2023). Self-Lubricating and Shape-Stable Phase-Change Materials Based on Epoxy Resin and Vegetable Oils. Polymers, 15(19), 4026. https://doi.org/10.3390/polym15194026