A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs
Abstract
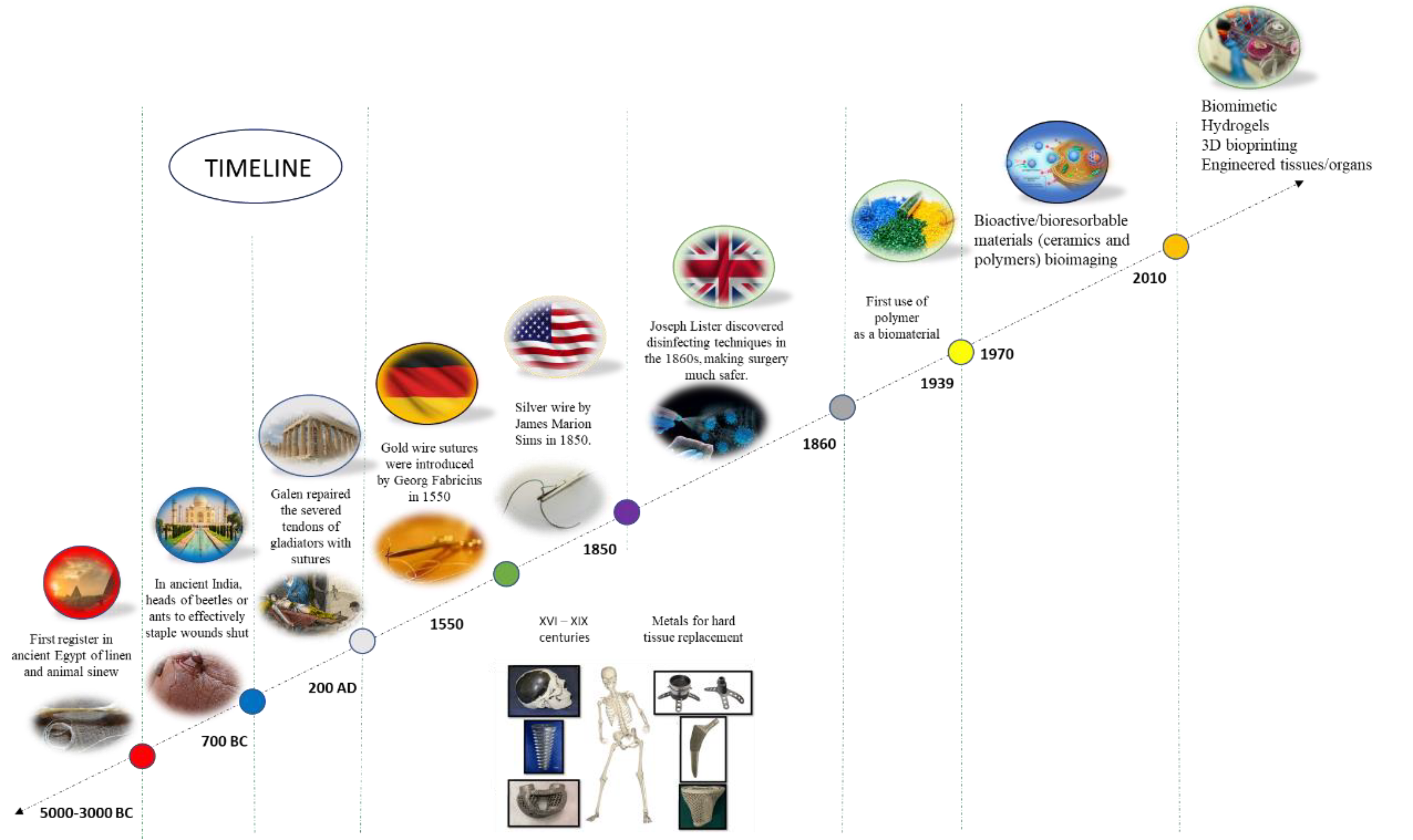
1. Introduction
| Classification | Material Type | Modulus (GPa) | Tensile Strength (MPa) |
|---|---|---|---|
| Hard Tissue | Cortical bone (longitudinal direction) | 10.0–30.0 | 100.0–150.0 |
| Cortical bone (transverse direction) | 10.0–30.0 | 1–50.0 | |
| Cancellous bone | 0.1–5.0 | 5.0–20.0 | |
| Enamel | 60.0–90.0 | 8.0–10.0 | |
| Dentine | 10.0–20.0 | 30.0–40.0 | |
| Soft Tissue | Articular cartilage | 0.5–10.5 | 0.5–27.0 |
| Fibrocartilage | 1.0–10.0 | 2.0–12.0 | |
| Ligament | 0.1–1.0 | 20.0–60.0 | |
| Tendon | 0.4–1.5 | 46.0–100.0 | |
| Skin | 1 × 10−4–1.0 | 10.0–20.0 | |
| Arterial tissue (longitudinal direction) | – | 0.1–0.5 | |
| Arterial tissue (transverse direction) | – | 1.0–5.0 | |
| Intraocular lens | 5 × 10−3–3.0 | 2.0–40.0 | |
| Metal alloys | Stainless steel | 1 × 10−2–10.0 | 500.0–2000.0 |
| Co-Cr alloy | 0.4–0.6 | 900.0–1100.0 | |
| Ti-alloy | 2.0–3.0 | 900.0–1100.0 | |
| Amalgam | 30 | 50.0–300.0 | |
| Ceramic | Alumina | 300–400 | 300.0–500.0 |
| Zirconia | 200–300 | 800.0–1200.0 | |
| Bioglass | 30–50 | 40.0–200.0 | |
| Hydroxyapatite | 90–100 | 50.0–130.0 | |
| Polymer | Polyethylene (PE) | 0.1–1.5 | 10.0–50.0 |
| Polyurethane (PU) | 1 × 10−2–10.0 | 20.0–70.0 | |
| Polytetrafluorethylene (PTFE) | 0.4–0.6 | 20.0–40.0 | |
| Polyacetal (PA) | 2.0–3.0 | 50.0–90.0 | |
| Polymethylmethacrylate (PMMA) | 2.0–3.0 | 50.0–100.0 | |
| Polyethylene terephthalate (PET) | 2.0–3.0 | 50.0–100.0 | |
| Polyetheretherketone (PEEK) | 3.0–8.0 | 90.0–140.0 | |
| Silicone rubber (SR) | 8 × 10−3–0.5 | 5.0–20.0 | |
| Polysulfone (PS) | 2.0–3.0 | 60.0–90.0 | |
| Polycaprolactone (PCL) | 0.1–1.0 | 10.0–40.0 | |
| Poly(lactic acid) (PLA) | 2.0–5.0 | 40.0–80.0 | |
| Poly(glycolic acid) (PGA) | 3.0–6.0 | 50.0–100.0 | |
| Poly(lactic-co-glycolic acid) (PLGA) | 2.0–8.0 | 30.0–80.0 | |
| Polydioxanone (PDO) | 1.0–5.0 | 40.0–70.0 | |
| Polypropylene (PP) | 1.0–2.0 | 20.0 | |
| Polycarbonate (PC) | 2.0–3.0 | 60.0–80.0 | |
| Polysaccharides (e.g., chitosan) | 0.1–1.0 | 5.0–20.0 | |
| Hydrogels (e.g., alginate, gelatin) | 0.01–1.0 | 0.1–10.0 | |
| Poly(ε-caprolactone-co-lactide) (PCLA) | 0.5–5.0 | 5.0–30.0 |
2. Hard Tissue Applications
2.1. Bone Fracture Repair
2.1.1. Porous Materials
2.1.2. Polymer-Based Scaffolds
2.1.3. Polymer-Based Kirschner-Wires (K-Wires)

2.1.4. Polymer-Based Screws
2.1.5. Other Applications
2.2. Dental Applications
3. Soft Tissue Applications
| Trade Name/Product Name | Materials | Company/Institution | Applications |
|---|---|---|---|
| Chongshu® composite hernia patch | Fibrinogen; poly (lactide-co-epsilon-caprolactone) | Shanghai Pine and Power Technology Co., LTD | Hernia repair |
| Haiao® oral repair membrane | Collagen | Yantai Zhenghai Biotechnology o. LTD | Peridontal tissue repair |
| GenossDESTM | Cobalt-chromium platform scaffolds containing sirolimus biodegradable polymers | Genoss Company Limited, Suwon, Korea | Coronary stent implantation |
| BEGO® collagen membrane | Collagen membrane | BEGO Implant Systems | Tissue engineering |
| Mucograft | Collagen types I and III | Geistlich Pharma AG, Wolhusen, Switzerland | Gingival recession |
| Collagen Graft and Collagen Membrane | Collagen Membrane, Collagen Graf | Genoss Company Limited, Suwon, Korea | Cleft palate repair |
| PACCG-GelMA Hydrogels | Poly (N-acryloyl 2-glycine)/methacrylated gelatin hydrogels | Tianjin Key Laboratory of Composite and Functional Materials | Osteochondal regeneration |
| PEG silk composite hydrogel | Silk | Research Institute of Agriculture and Life Sciences, Seoul National University, Seoul, South Korea | Articular cartilage repair |
| Elastin-silk fibroin double raschel knitted vascular graft | Silk | Tokyo University of Agriculture and Technology, Fuchu, Japan | Artificial blood vessel |
| Chondrotissue® | PGA, HA | Chondrotissue, BioTissue, AG, Zurich, Switzerland | Cartilage tissue engineering |
| IC scaffold | PLGA, COL | Tissue Engineering Research Center, AIST Kansai, Amagasaki Site | Cartilage tissue engineering |
| C2C1H scaffold | PLA, COL, CH | BioMediTech, Institute of Biosciences and Medical Technology, Tampere, Finland | Cartilage tissue engineering |
| Chitosan-modified PLCL scaffold | PLCL, CH | Tissue Engineering Program, Life Sciences Institute, National University of Singapore, Singapore | Cartilage tissue formation |
| CSMA/PECA/GO (S2) scaffold | CSMA, MPEG-PCL-AC (PECA), GO | State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University | Cartilage tissue engineering |
| Hyalofast® | Benzyl ester of hyaluronic acid | Anika Therapeutics Inc., Bedford, Massachusetts, United States | Osteochondral Injury |
| ChondroGide® | Type I/III collagen | Geistlich Biomaterials, Wolhusen, Switzerland | Cartilage defects of the knee joint |
| Cartipatch® | Agarose and alginate | Tissue Bank of France, TBF, Lyon, France | Knee cartilage injury |
| Silk Voice® | Silk | Sofregen, United States | Wound healing |
| NOVOCART® 3D | Type I collagen, chondroitin sulfate | TETEC, Reutlingen, Germany | Isolated retro patellar cartilage defects |
4. Prosthetic Limbs
5. Future Trends
6. Limitations and Gaps
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agrawal, R.; Kumar, A.; Mohammed, M.K.A.; Singh, S. Biomaterial types, properties, medical applications, and other factors: A recent review. J. Zhejiang Univ. Sci. A 2023. [Google Scholar] [CrossRef]
- Espessante E-2-Modificador de Reologia Uretenico. Available online: https://www.nokxeller.com.br/br/produtos/interna/espessante-e-2. (accessed on 20 July 2023).
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Medical Polymer Market Size, Share & Trends Analysis Report by Product, by Application (Medical Device Packaging, Tooth Implants, Wound Care, Mobility Aids, Denture-Based Materials), by Region, and Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/medical-polymers-market (accessed on 15 July 2023).
- RESOMER® Bioresorbable Polymers for Medical Devices. Available online: https://healthcare.evonik.com/en/medical-devices/bioresorbable-polymers/standard-polymers (accessed on 20 July 2023).
- Ramírez-Torres, A.; Penta, R.; Rodríguez-Ramos, R.; Merodio, J.; Sabina, F.J.; Bravo-Castillero, J.; Guinovart-Díaz, R.; Preziosi, L.; Grillo, A. Three scales asymptotic homogenization and its application to layered hierarchical hard tissues. Int. J. Solids Struct. 2018, 130, 190–198. [Google Scholar] [CrossRef]
- Benazzi, S.; Nguyen, H.N.; Kullmer, O.; Hublin, J.J. Unravelling the Functional Biomechanics of Dental Features and Tooth Wear. PLoS ONE 2013, 8, e69990. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.; Sherratt, M.J.; Cruickshank, K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]
- Senra, M.R.; Marques, M.D.F.V. Synthetic polymeric materials for bone replacement. J. Compos. Sci. 2020, 4, 191. [Google Scholar] [CrossRef]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Sebastiammal, S.; Fathima, A.S.L.; Devanesan, S.; AlSalhi, M.S.; Henry, J.; Govindarajan, M.; Vaseeharan, B. Curcumin-encased hydroxyapatite nanoparticles as novel biomaterials for antimicrobial, antioxidant and anticancer applications: A perspective of nano-based drug delivery. J. Drug Deliv. Sci. Technol. 2020, 57, 101752. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Isa, I.L.M.; Zaman, W.S.W.K.; Tabata, Y.; Fauzi, M.B. The effect of nanoparticle-incorporated natural-based biomaterials towards cells on activated pathways: A systematic review. Polymers 2022, 14, 476. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Bilingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Nakamura, R.; Kuroda, K.; Takahashi, M.; Katsuki, Y. Open wedge high tibial osteotomy with pes anserinus preservation and insertion of bone substitutes. Arthrosc. Tech. 2022, 11, e69–e78. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Liu, Y.; Zhang, Z.; Li, Z. Current advances in the roles of doped bioactive metal in biodegradable polymer composite scaffolds for bone repair: A mini review. Adv. Eng. Mater. 2022, 24, 2101510. [Google Scholar] [CrossRef]
- Le, Q.V.; Lee, J.; Lee, H.; Shim, G.; Oh, Y.K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B 2021, 11, 2096–2113. [Google Scholar] [CrossRef] [PubMed]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Siraj, E.A. Targeted drug delivery—From magic bullet to nanomedicine: Principles, challenges, and future perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Lett, J.A.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Castillo-Henriquez, L.; Castro-Alpizar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of bioengineered scaffolds composed of thermo-responsive polymers for drug delivery in wound healing. Int. J. Mol. Sci. 2021, 22, 1408. [Google Scholar] [CrossRef] [PubMed]
- Agueda, J.R.H.S.; Chen, Q.; Maalihan, R.D.; Ren, J.; da Silva, I.G.M.; Dugos, N.P.; Caldona, E.B.; Advincula, R.C. 3D printing of biomedically relevant polymer materials and biocompatibility. MRS Commun. 2021, 11, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Sheng, Y.; Huang, L.; Chow, D.H.-K.; Chau, W.H.; Tang, N.; Ngai, T.; Wu, C.; Lu, J.; Qin, L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials 2018, 180, 173–183. [Google Scholar] [CrossRef]
- Yi, H.; Rehman, F.U.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.D.; Cooper, L.; Craddock, H.; Hyde, T.P.; Nattress, B.; Pavitt, S.H.; Seymour, D.W. Removable partial dentures: The clinical need for innovation. J. Prosthet. Dent. 2017, 118, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2020, 29, 509–527. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Zhou, R.; Zhou, F.Z.; Qiao, Y.; Qiu, D.Q. Bioactive pore-forming bone adhesives facilitating cell ingrowth for fracture healing. Adv. Mater. 2020, 32, e1907491. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, Y.-Z.; Zhang, H.; Gao, R.-N. Development of functionally graded porous titanium/silk fibroin composite scaffold for bone repair. Mater. Lett. 2021, 282, 128670. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, X.; Chen, X.; Zeng, X.; Luo, K. Programmable, biodegradable composite scaffolds with variable pore morphology for minimal invasive bone repair. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107130. [Google Scholar] [CrossRef]
- Wu, X.; Huo, Y.; Ci, Z.; Wang, Y.; Xu, W.; Bai, B.; Hao, J.; Hu, G.; Yu, M.; Ren, W.; et al. Biomimetic porous hydrogel scaffolds enabled vascular ingrowth and osteogenic differentiation for vascularized tissue-engineered bone regeneration. Appl. Mater. Today 2022, 27, 101478. [Google Scholar] [CrossRef]
- Kuang, T.; Chen, S.; Gu, Z.; Shen, Z.; Hejna, A.; Saeb, M.R.; Chen, F.; Zhong, M.; Liu, T. A facile approach to fabricate load-bearing porous polymer scaffolds for bone tissue engineering. Adv. Compos. Hybrid. Mater. 2022, 5, 1376–1384. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and prospects of polymer-based drug delivery systems for bone tissue regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Elhattab, K.; Bhaduri, S.B.; Lawrence, J.G.; Sikder, P. Fused filament fabrication (three-dimensional printing) of amorphous magnesium phosphate/polylactic acid macroporous biocomposite scaffolds. ACS Appl. Bio Mater. 2021, 4, 3276–3286. [Google Scholar] [CrossRef] [PubMed]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, D.; Zhong, J.; Ma, M.; Zhou, J.; Peng, X.; Ye, Y.; Sun, G.; He, D. Electrospun nanofibrous P (DLLA–CL) balloons as calcium phosphate cement filled containers for bone repair: In vitro and in vivo studies. ACS Appl. Mater. Interfaces 2015, 7, 18540–18552. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Hatt, L.P.; Rosenberg, G.S.; Thompson, K.; Stoddart, M.J. Functional biomaterials for bone regeneration: A lesson in complex biology. Adv. Funct. Mater. 2020, 30, 1909874. [Google Scholar] [CrossRef]
- Shokri, M.; Dalili, F.; Kharaziha, M.; Eslaminejad, M.B.; Tafti, H.A. Strong and bioactive bioinspired biomaterials, next generation of bone adhesives. Adv. Colloid Interface Sci. 2022, 305, 102706. [Google Scholar] [CrossRef]
- Chakladar, N.D.; Harper, L.T.; Parsons, A.J. Optimisation of composite bone plates for ulnar transverse fractures. J. Mech. Behav. Biomed. Mater. 2016, 57, 334–346. [Google Scholar] [CrossRef]
- Schliemann, B.; Seifert, R.; Theisen, C.; Gehweiler, D.; Wahnert, D.; Schulze, M.; Raschke, M.J.; Weimann, A. PEEK versus titanium locking plates for proximal humerus fracture fixation: A comparative biomechanical study in two-and three-part fractures. Arch. Orthop. Trauma Surg. 2017, 137, 63–71. [Google Scholar] [CrossRef]
- Morawska-Chochol, A.; Chlopek, J.; Szaraniec, B.; Domalik-Pyzik, P.; Balacha, E.; Bogun, M.; Kucharski, R. Influence of the intramedullary nail preparation method on nail’s mechanical properties and degradation rate. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 99–106. [Google Scholar] [CrossRef]
- Takashima, K.; Nakahara, I.; Uemura, K.; Hamada, H.; Ando, W.; Takao, M.; Sugano, N. Clinical outcomes of proximal femoral fractures treated with a novel carbon fiber-reinforced polyetheretherketone intramedullary nail. Injury 2020, 51, 678–682. [Google Scholar] [CrossRef]
- Saikku-Backstrom, A.; Tulamo, R.M.; Raiha, J.E.; Pohjonen, T.; Toivonen, T.; Tormala, P.; Rokkanen, P. Intramedullary fixation of femoral cortical osteotomies with interlocked biodegradable self-reinforced poly-96L/4D-lactide (SR-PLA96) nails. Biomaterials 2004, 25, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Kassai, T.; Varga, M.; Jozsa, G. Pediatric medial humeral epicondyle fracture in children: Are biodegradable pins with tension band absorbable sutures efficient? Medicine 2022, 101, e29817. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Nan, G. Treatment of medial humeral epicondyle fractures in children using absorbable self-reinforced polylactide pins. Medicine 2020, 99, e19861. [Google Scholar] [CrossRef]
- Asgari, M.; Hang, R.; Wang, C.; Yu, Z.; Li, Z.; Xiao, Y. Biodegradable metallic wires in dental and orthopedic applications: A review. Metals 2018, 8, 212. [Google Scholar] [CrossRef]
- Heye, P.; Matissek, C.; Seidl, C.; Varga, M.; Kassai, T.; Jozsa, G.; Krebs, T. Making Hardware Removal Unnecessary by Using Resorbable Implants for Osteosynthesis in Children. Children 2022, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, H.; Jiang, Y.; Wang, J.; Zhang, S. A high-strength biodegradable thermoset polymer for internal fixation bone screws: Preparation, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2019, 183, 110445. [Google Scholar] [CrossRef]
- Wichelhaus, A.; Emmerich, J.; Mittlmeier, T. A case of implant failure in partial wrist fusion applying magnesium-based headless bone screws. Case Rep. Orthop. 2016, 2016, 7049130. [Google Scholar] [CrossRef]
- Agarwal, R.; Mehtani, H.K.; Singh, J.; Gupta, V. Post-yielding fracture mechanics of 3D printed polymer-based orthopedic cortical screws. Polym. Compos. 2022, 43, 6829–6837. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Ngai, T. Polymer coatings on magnesium-based implants for orthopedic applications. J. Polym. Sci. 2021, 60, 32–51. [Google Scholar] [CrossRef]
- Tooth-The Anatomy of a Tooth. Available online: https://www.mouthhealthy.org/all-topics-a-z/ (accessed on 20 July 2023).
- Dental Implant Tooth Replacement for a Healthy, Confident Smile. Available online: https://sunriseperio.com/dental-implants-massapequa-park-ny/ (accessed on 15 July 2023).
- Enhancing Health through Comprehensive Dental Care. Available online: https://www.healthyrootstulsa.com/services/mouth-body-connection (accessed on 20 July 2023).
- Raszweski, Z.; Chojnacka, K.; Mikulewicz, M. Preparation and characterization of acrylic resins with bioactive glasses. Nature 2022, 12, 16624. [Google Scholar] [CrossRef] [PubMed]
- Tiskaya, M.; Shahid, S.; Gillam, D.; Hill, R. The use of bioactive glass (BAG) in dental composites: A critical review. Dent. Mater. 2021, 37, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Semisch-Dieter, O.K.; Choi, A.H.; Ben-Nissan, B.; Stewart, M.P. Modifying an Implant: A Mini-review of Dental Implant Biomaterials. BIO Integration 2021, 2, 12–21. [Google Scholar] [CrossRef]
- Henderson, E. Review: Using Biomaterials in Dental Implants. 2021. Available online: https://www.news-medical.net/news/20210322/Review-Using-biomaterials-in-dental-implants.aspx (accessed on 15 July 2023).
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Han, C.-M.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H.; Kim, K.N.; Ha, Y.; Kuh, S.-U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, R.; Mohapatra, A.; Das, S.S.; Nanda, K.; Bharadwaj, S. Polymers Used in Dentistry: An Overview of Literature. Indian J. Forensic Med. Tox. 2020, 14, 8883–8887. [Google Scholar]
- Carlsson, G.E.; Omar, R. Trends in prosthodontics. Med. Princ. Pract. 2006, 15, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Pithon, M.M.; Oliveira, M.V.; Ruellas, A.C.O.; Bolognese, A.M.; Romano, F.L. Shear bond strength of orthodontic brackets to enamel under different surface treatment conditions. J. Appl. Oral Sci. 2007, 15, 127–130. [Google Scholar] [CrossRef]
- Littlewood, S.J.; Mitchell, L. An Introduction to Orthodontics, 5th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Umer, F.; Habib, S. Critical analysis of artificial intelligence in endodontics: A scoping review. J. Endod. 2022, 48, 152–160. [Google Scholar] [CrossRef]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied. Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef]
- Mummery, C.; Roelen, B.A.J.; van de Stolpe, A.; Clevers, H. Chapter 10—Adult Stem Cells: Generation of Self-Organizing Mini-Organs in a Dish; Academic Press: Cambridge, MA, USA, 2014; pp. 279–290. [Google Scholar]
- Chen, M.; Jiang, R.; Deng, N.; Zhao, X.; Li, X.; Guo, C. Natural polymer-based scaffolds for soft tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 954699. [Google Scholar] [CrossRef] [PubMed]
- Arbade, G.K.; Srivastava, J.; Tripathi, V.; Lenka, N.; Patro, T.U. Enhancement of hydrophilicity, biocompatibility and biodegradability of poly(ε-caprolactone) electrospun nanofiber scaffolds using poly(ethylene glycol) and poly(L-lactide-co-ε-caprolactone-co-glycolide) as additives for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 1648–1670. [Google Scholar] [CrossRef] [PubMed]
- Understanding the Transplant Waitlist. Available online: https://www.kidney.org/ (accessed on 20 July 2023).
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent progress in biopolymer-based hydrogel materials for biomedical applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Seidi, F.; Azarfam, M.Y.; Yazdi, M.K.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- COVVI-Partners in Prosthetics. Available online: https://www.covvi.com/ (accessed on 15 July 2023).
- Williams, W. A Complete Guide to Assistive Robotic Gloves. 2022. Available online: https://bionicsforeveryone.com/robotic-gloves/ (accessed on 20 July 2023).
- Frossard, L.; Lloyd, D. The Future of Bionic Limbs. 2021. Available online: https://researchfeatures.com/future-bionic-limbs/ (accessed on 15 July 2023).
- Williams, W. A Complete Guide to Bionic Arms & Hands. 2020. Available online: https://bionicsforeveryone.com/bionic-arms-hands/ (accessed on 15 July 2023).
- Prosthetic Technology—Devices, Materials and Other Options to Maximize Your Rehabilitation Potential. Available online: https://www.armdynamics.com/research-and-technology/prosthetic-technology (accessed on 20 July 2023).
- Henson, A. Introduction to Myoelectric Prostheses. 2021. Available online: https://www.armdynamics.com/upper-limb-library/introduction-to-myoelectric-prostheses (accessed on 20 July 2023).
- Adams, D.S.D.; Schwartz-Fernandes, F.A. Myoelectric prosthesis: A potential emerging therapeutic in restoring function post-arm amputation complicated by necrotizing fasciitis. J. Surg. Case Rep. 2020, 2020, rjaa381. [Google Scholar] [CrossRef] [PubMed]
- Fougner, A.; Stavdahl, O.; Kyberd, P.J.; Losier, Y.G.; Parker, P.A. Myoelectric Control for Upper Limb Prostheses. IEEE Trans. Neural. Syst. Rehabil. Eng. 2012, 20, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.J.; Fergason, J.R.; Pierrie, S.N. Technological Advances in Prosthesis Design and Rehabilitation Following Upper Extremity Limb Loss. Curr. Rev. Musculoskelet. Med. 2020, 13, 485–493. [Google Scholar] [CrossRef]
- Mereu, F.; Leone, F.; Gentile, C.; Cordella, F.; Gruppioni, E.; Zollo, L. Control strategies and performance assessment of upper-limb TMR prostheses: A review. Sensors 2021, 21, 1953. [Google Scholar] [CrossRef]
- Colachis, S.C.T.; Bockbrader, M.A.; Zhang, M.; Friedenberg, D.A.; Annetta, N.V.; Schwemmer, M.A.; Skomrock, N.D.; Mysiw, W.J.; Rezai, A.R.; Bresler, H.S.; et al. Dexterous control of seven functional hand movements using cortically-controlled transcutaneous muscle stimulation in a person with tetraplegia. Front. Neurosci. 2018, 12, 208. [Google Scholar] [CrossRef]
- Scudellari, M. A Prosthetic That Feels Pain—Electronic Receptors Mimic the Ability of Human Skin to Sense Pain and Pressure. 2018. Available online: https://spectrum.ieee.org/a-prosthetic-that-feels-pain#toggle-gdpr (accessed on 20 July 2023).
- Kumar, S.; Bhowmik, S. Potential use of natural fiber-reinforced polymer biocomposites in knee prostheses: A review on fair inclusion in amputees. Iran. Polym. J. 2022, 31, 1297–1319. [Google Scholar] [CrossRef]
- Biddiss, E.; Chau, T. Electroactive polymeric sensors in hand prostheses: Bending response of an ionic polymer metal composite. Med. Eng. Phys. 2006, 28, 568–578. [Google Scholar] [CrossRef]
- Khare, J.M.; Dahiya, S.; Gangil, B.; Ranakoti, L. Influence of different resins on Physico-Mechanical properties of hybrid fiber reinforced polymer composites used in human prosthetics. Mater. Today Proc. 2021, 38, 345–349. [Google Scholar] [CrossRef]
- Cheung, C. The future of bone healing. Clin. Podiatr. Med. Surg. 2005, 22, 631–641. [Google Scholar] [CrossRef]
- Covani, U.; Bortolaia, C.; Barone, A.; Sbordone, L. Bucco-lingual crestal bone changes after immediate and delayed implant placement. J. Periodontol. 2004, 75, 1605–1612. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Y.; Peng, B.; Chen, M.; Liu, Z.; Li, Z.; Kuang, H.; Gong, B.; Li, Z.; Sun, H. PEEK for oral applications: Recent advances in mechanical and adhesive properties. Polymers 2023, 15, 386. [Google Scholar] [CrossRef]
- Ashammakhi, N.; GhavamiNejad, A.; Tutar, R.; Fricker, A.; Roy, I.; Chatzistavrou, X.; Apu, E.H.; Nguyen, K.-L.; Ahsan, T.; Pountos, I.; et al. Highlights on advancing frontiers in tissue engineering. Tissue Eng. Part B Rev. 2022, 28, 633–664. [Google Scholar] [CrossRef] [PubMed]










| Implant Type | Study Type | Model Used | Results |
|---|---|---|---|
| pPEEK CFR-PPEK | In vivo | Dog femur | BIC: pPEEK < Ti; CFR-PEEK > Ti |
| pPEEK CFR-PEEK | In vivo | Dog mandible | BIC: pPEEK < Ti; CFR-PEEK < Ti |
| CFR-PEEK | In silico | FEA | Stress peaks: CFR-PEEK > Ti |
| CFR-PEEK GFR-PEEK | In vivo | ISO 14,801 protocol | Stress shielding effects: CFR-PEEK < Ti rods; GFR-PEEK < Ti rods |
| HAcCFR-PEEK CFR-PEEK | In vivo | Rabbit femur | Interfacial shear strength: HAcCFR-PEEK = grit blasted Ti allow with HA; HAcCFR-PEEK > CFR-PEEK |
| pPEEK | In vivo | MG-63 cells | Proliferation rate: pPEEK < Ti; mRNA processing: pPEEK < Ti |
| nTiO2-PEEK | In vitro and in vivo | MG-63 cells and beagle dog tibia | Bioactivity: nTiO2/PEEK > Ti |
| St-HAcCFR-PEEK | In vitro | MG-63 cells | Bioactivity: St-HAcCFR-PEEK > Ti |
| nHAcPEEK | In vivo | Rabbit femur | Osseointegration: nHAcPEEK > Ti Implant loss: nHAcPEEK > Ti |
| eTicPEEK | In vitro and in vivo | MC3T3-E1 cells and rabbit tibia | Cell proliferation: eTicPEEK > Ti BIC: eTicPEEK > Ti |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ornaghi, H.L., Jr.; Monticeli, F.M.; Agnol, L.D. A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs. Polymers 2023, 15, 4034. https://doi.org/10.3390/polym15194034
Ornaghi HL Jr., Monticeli FM, Agnol LD. A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs. Polymers. 2023; 15(19):4034. https://doi.org/10.3390/polym15194034
Chicago/Turabian StyleOrnaghi, Heitor Luiz, Jr., Francisco Maciel Monticeli, and Lucas Dall Agnol. 2023. "A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs" Polymers 15, no. 19: 4034. https://doi.org/10.3390/polym15194034
APA StyleOrnaghi, H. L., Jr., Monticeli, F. M., & Agnol, L. D. (2023). A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs. Polymers, 15(19), 4034. https://doi.org/10.3390/polym15194034








