Flexible Stretchable, Dry-Resistant MXene Nanocomposite Conductive Hydrogel for Human Motion Monitoring
Abstract
:1. Introduction
2. Materials and Methods
3. Results
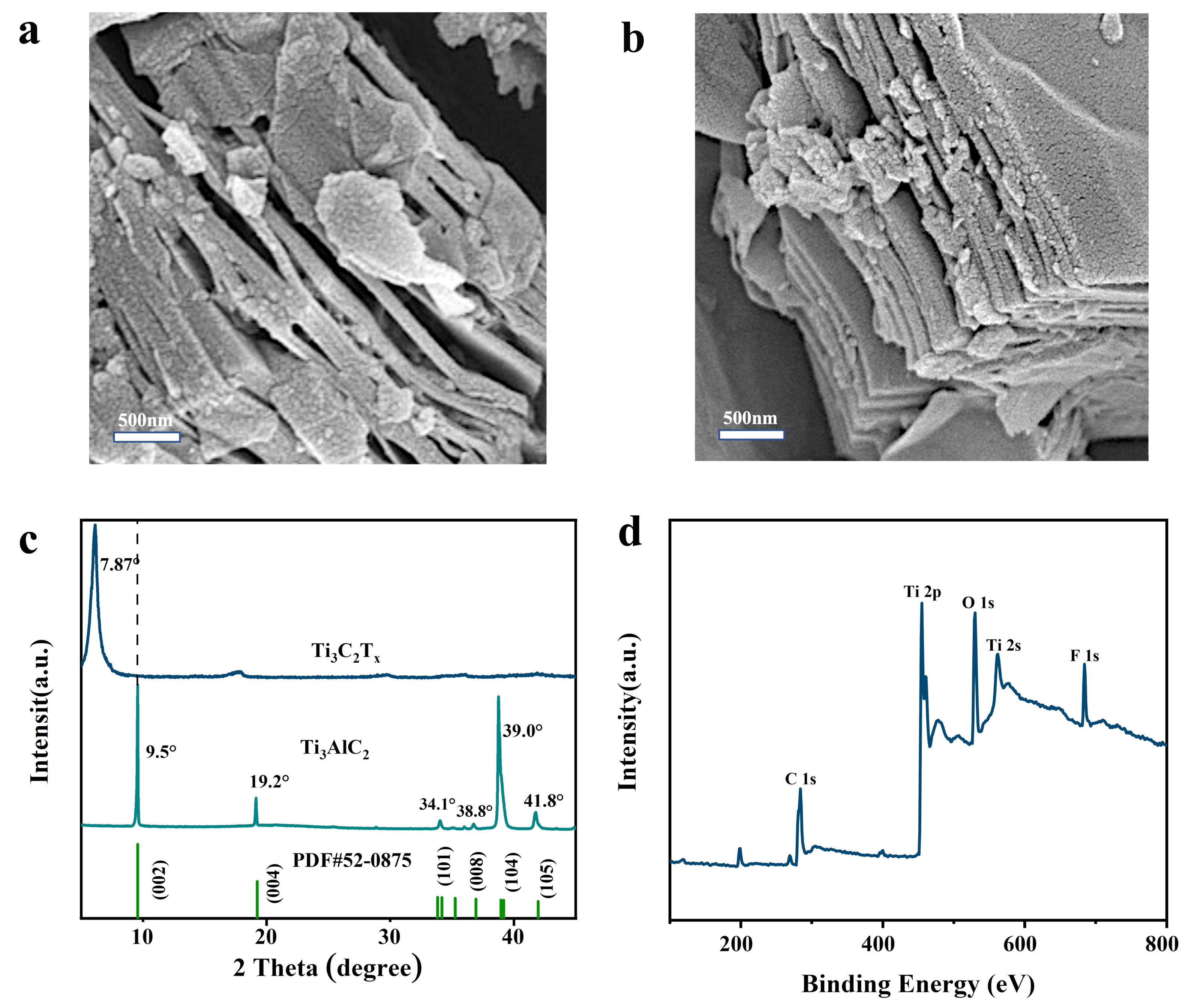
3.1. Preparation and Characterization of MXene Nanomaterials
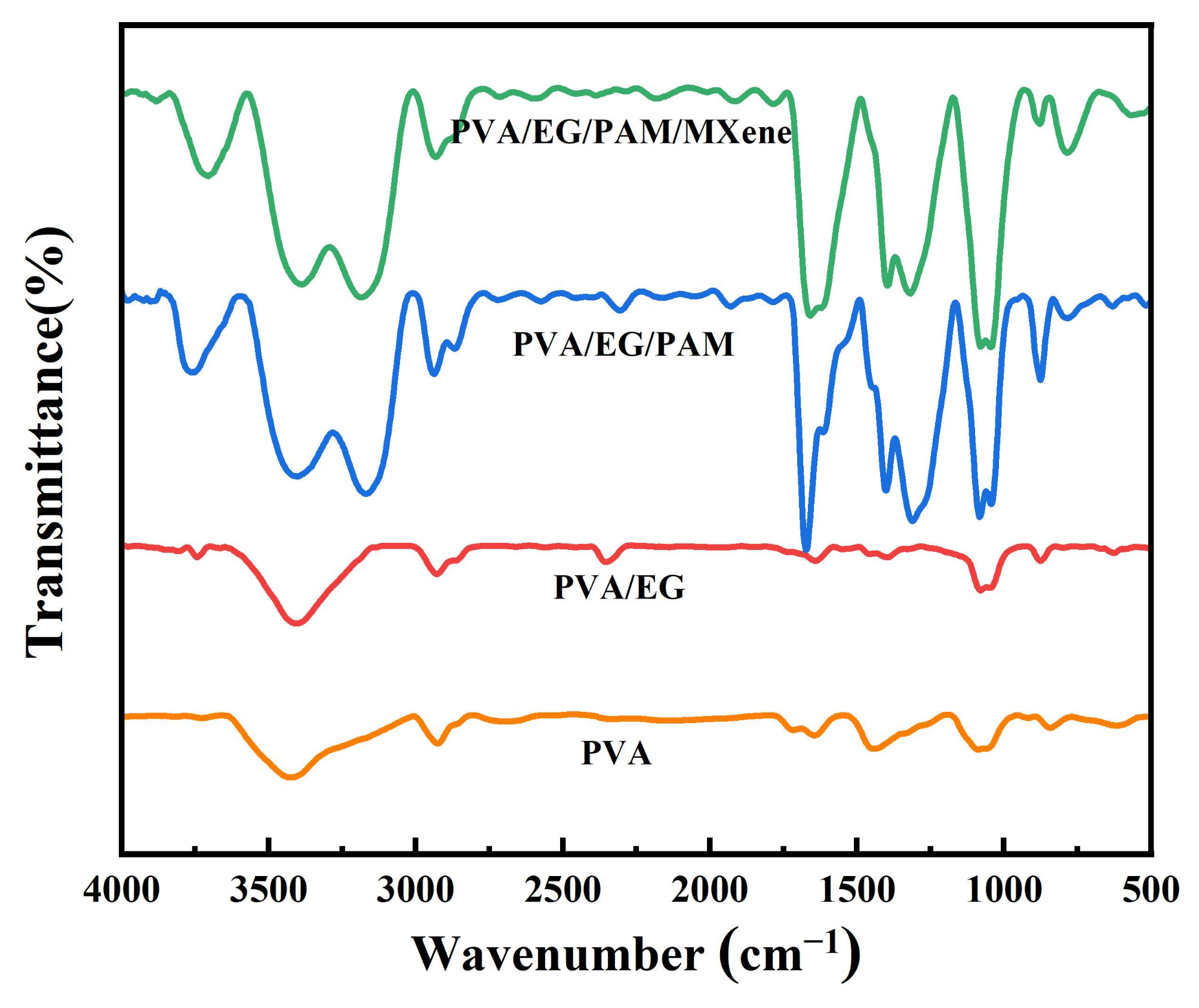
3.2. Design, Synthesis, and Structural Characterization of the PAEM Hydrogels
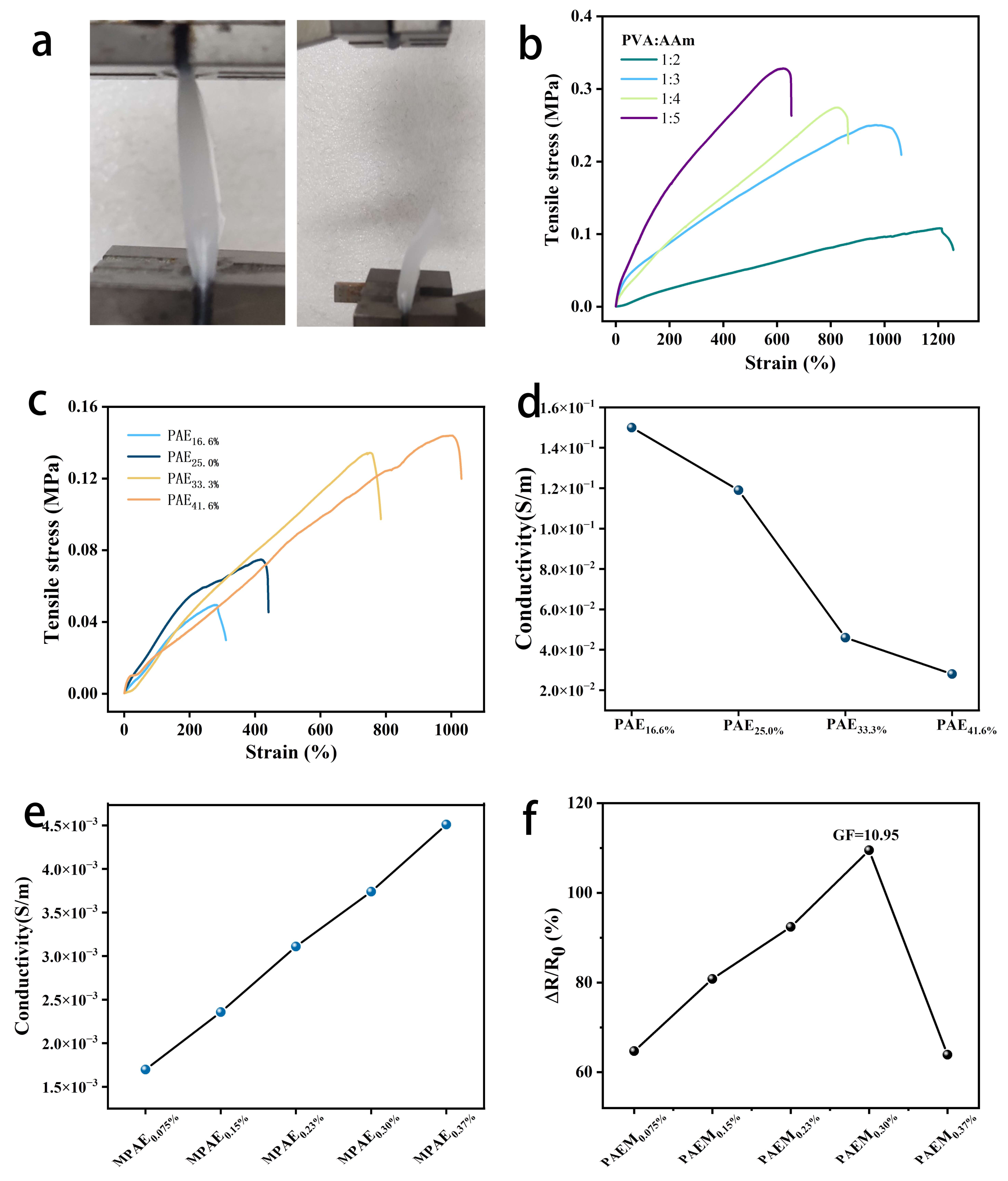
3.3. Exploration of Variables in PAEM
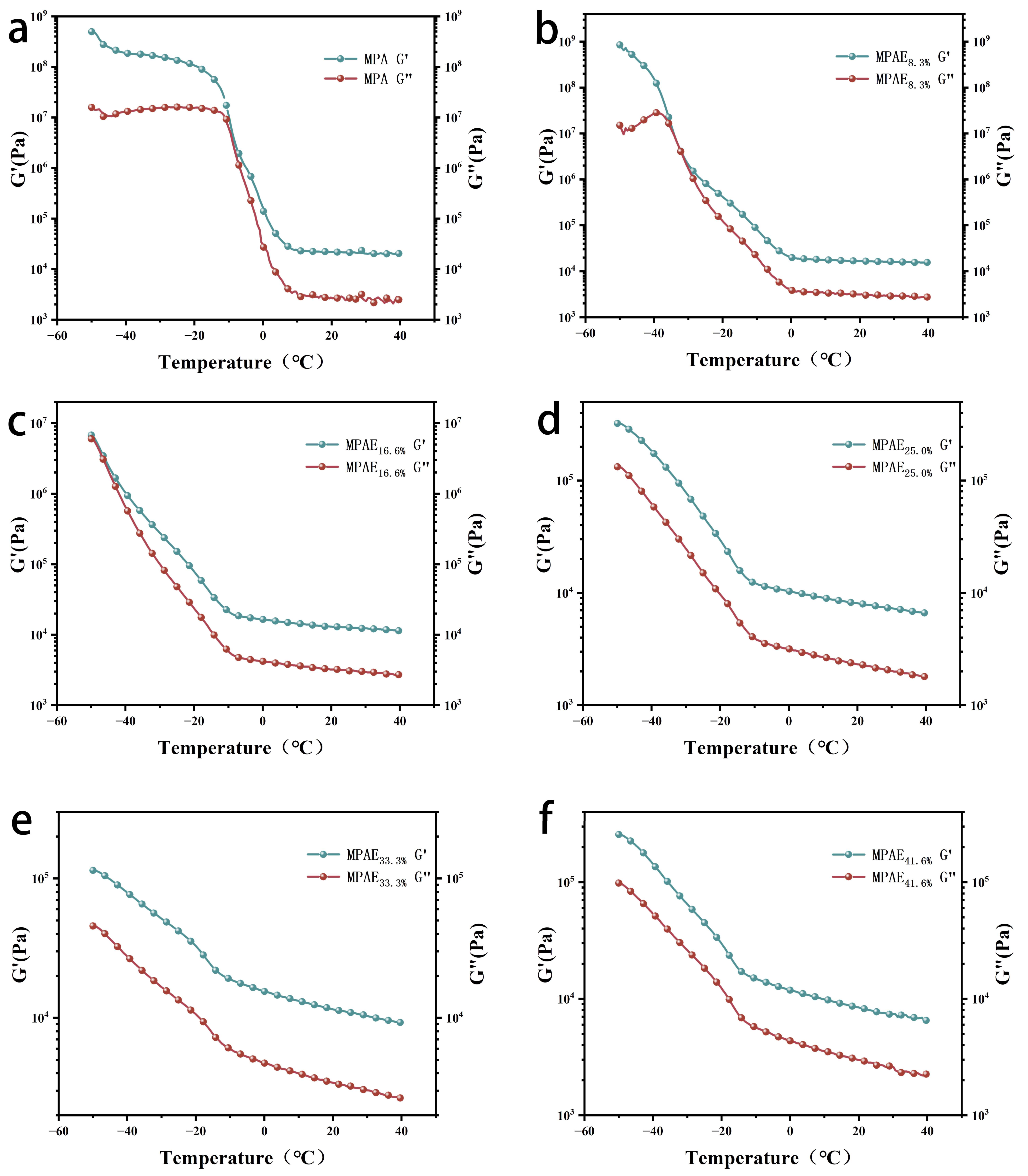
3.4. Temperature Tolerance
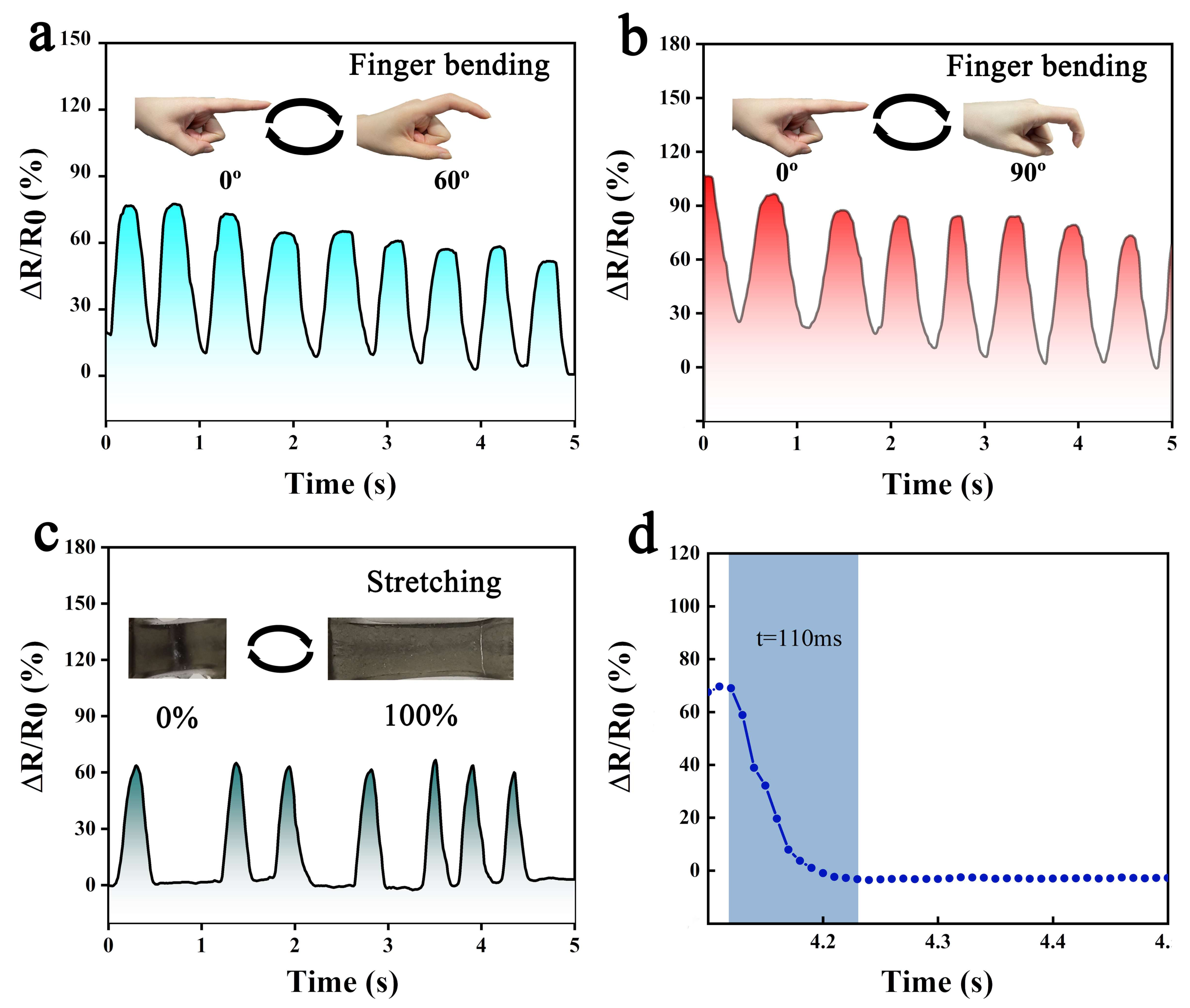
3.5. Electromechanical Performance
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Zhou, D.; Wang, J.; Li, T.; Zhou, X.; Gan, T.; Handschuh-Wang, S.; Zhou, X. Rational Fabrication of Anti-Freezing, Non-Drying Tough Organohydrogels by One-Pot Solvent Displacement. Angew. Chem. Int. Ed. Engl. 2018, 57, 6568–6571. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Liu, J.; Gu, J.; Zhu, J.; Huang, B.; Bai, J.; Guo, J.; Yang, X.; Guan, L. High toughness multifunctional organic hydrogels for flexible strain and temperature sensor. J. Mater. Chem. A 2021, 9, 23243–23255. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Y.; Ma, S.; Ren, J.; Yang, X.; Wang, Y.; Lü, S. Superstretching MXene Composite Hydrogel as a Bidirectional Stress Response Thixotropic Sensor. ACS Appl. Mater. Interfaces 2021, 13, 13629–13636. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-Stimuli-Responsive and Cellulose Conductive Ionic Hydrogel for Smart Wearable Devices and Thermal Actuators. ACS Appl. Mater. Interfaces 2021, 13, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Long, Y.; Jiang, B.; Guo, W.; Sha, W.; Wang, J.; Cong, Z.; Chen, J.; Wang, B.; Hu, W. Ultra-antifreeze, ultra-stretchable, transparent, and conductive hydrogel for multi-functional flexible electronics as strain sensor and triboelectric nanogenerator. Nano Res. 2022, 15, 5461–5468. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, J.; Gao, F.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Self-healable and recyclable polyurethane-polyaniline hydrogel toward flexible strain sensor. Compos. Part B Eng. 2021, 219, 108965. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, Y.; Shao, J.; Wang, W.; Si, W.; Huang, W.; Dong, X. Stretchable, Transparent, and Self-Patterned Hydrogel-Based Pressure Sensor for Human Motions Detection. Adv. Funct. Mater. 2018, 28, 1802576. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, H.; Ma, C.; Sun, H.; Liu, C.; Dai, K.; Zhang, J.; Wei, R.; Ding, T.; Guo, Z. Smart strain sensing organic–inorganic hybrid hydrogels with nano barium ferrite as the cross-linker. J. Mater. Chem. C 2019, 7, 2353–2360. [Google Scholar] [CrossRef]
- Han, L.; Liu, M.; Yan, B.; Li, Y.; Lan, J.; Shi, L.; Ran, R. Polydopamine/polystyrene nanocomposite double-layer strain sensor hydrogel with mechanical, self-healing, adhesive and conductive properties. Mater. Sci. Eng. C 2020, 109, 110567. [Google Scholar] [CrossRef]
- Han, S.; Liu, C.; Lin, X.; Zheng, J.; Wu, J.; Liu, C. Dual Conductive Network Hydrogel for a Highly Conductive, Self-Healing, Anti-Freezing, and Non-Drying Strain Sensor. ACS Appl. Polym. Mater. 2020, 2, 996–1005. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Yang, Q.; Zhou, Q.; Hui, L.; Liu, Z.; Xu, F.; Ding, D. One-pot freezing-thawing preparation of cellulose nanofibrils reinforced polyvinyl alcohol based ionic hydrogel strain sensor for human motion monitoring. Carbohydr. Polym. 2022, 275, 118697. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lu, Y.; Lu, K.; Yue, Y.; Xu, X.; Xiao, H.; Li, J.; Han, J. Highly stretchable and self-healing cellulose nanofiber-mediated conductive hydrogel towards strain sensing appli-cation. J. Colloid Interface Sci. 2021, 597, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wu, P. A supramolecular biomimetic skin combining a wide spectrum of mechanical properties and multiple sensory capabilities. Nat. Commun. 2018, 9, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Chen, D.; Sun, X.; Xu, Z.; Yang, Y.; Song, Y.; Jiang, F. An environmentally tolerant, highly stable, cellulose nanofiber-reinforced, conductive hydrogel multifunctional sensor. Carbohydr. Polym. 2022, 284, 119199. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tu, Q.; Long, X.; Zhang, Q.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Flexible conductive hydrogel fabricated with polyvinyl alcohol, carboxymethyl chitosan, cellulose nanofibrils, and lignin-based carbon applied as strain and pressure sensor. Int. J. Biol. Macromol. 2021, 166, 1526–1534. [Google Scholar] [CrossRef]
- Li, S.-N.; Yu, Z.-R.; Guo, B.-F.; Guo, K.-Y.; Li, Y.; Gong, L.-X.; Zhao, L.; Bae, J.; Tang, L.-C. Environmentally stable, mechanically flexible, self-adhesive, and electrically conductive Ti3C2TX MXene hydrogels for wide-temperature strain sensing. Nano Energy 2021, 90, 106502. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Li, Y.; Chao, M.; Li, M.; Wan, P.; Zhang, L. Healable, Degradable, and Conductive MXene Nanocomposite Hydrogel for Multifunctional Epidermal Sensors. ACS Nano 2021, 15, 7765–7773. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, K.; Li, J.; Wang, H.; Xie, X.Q.; Cui, Y.; Zhang, Y.; Wang, M.; Liu, C.S. Low-Molecular-Weight Supramolecular-Polymer Double-Network Eutectogels for Self-Adhesive and Bidirectional Sensors. Adv. Funct. Mater. 2021, 31, 2104963. [Google Scholar] [CrossRef]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, Healable, and Adhesive Epidermal Sensors Assembled from Mussel-Inspired Conductive Hybrid Hydrogel Framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Li, Y.; Shi, G. High-Performance Strain Sensors with Fish-Scale-Like Graphene-Sensing Layers for Full-Range Detection of Human Motions. ACS Nano 2016, 10, 7901–7906. [Google Scholar] [CrossRef]
- Luo, J.; Yang, J.; Zheng, X.; Ke, X.; Chen, Y.; Tan, H.; Li, J. A Highly Stretchable, Real-Time Self-Healable Hydrogel Adhesive Matrix for Tissue Patches and Flexible Electronics. Adv. Healthc. Mater. 2020, 9, e1901423. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, J.; Yang, X.; Fan, D.; Cao, J.; Luo, Y.-Y.; Zhang, X. Naturally Derived Wearable Strain Sensors with Enhanced Mechanical Properties and High Sensitivity. ACS Appl. Mater. Interfaces 2020, 12, 22163–22169. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shuai, X.; Hu, Y.; Liang, X.; Zhu, P.; Sun, R.; Wong, C.-P. A highly sensitive and flexible capacitive pressure sensor based on a micro-arrayed polydimethylsiloxane dielectric layer. J. Mater. Chem. C 2018, 6, 13232–13240. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W.; Yang, W.; Chen, J.; Peng, Q.; Wang, T.; Yang, D.; Wang, J.; Zhang, H.; Zeng, H. Stretchable, compressible, and conductive hydrogel for sensitive wearable soft sensors. J. Colloid Interface Sci. 2022, 618, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Hu, M.; Li, X.; Yan, L.; Wu, C.; Liu, J.; Gao, H.; Shan, G.; Huang, W. A highly sensitive piezoresistive sensor based on MXenes and polyvinyl butyral with a wide detection limit and low power consumption. Nanoscale 2020, 12, 17715–17724. [Google Scholar] [CrossRef] [PubMed]
- Robby, A.I.; Lee, G.; Lee, K.D.; Jang, Y.C.; Park, S.Y. GSH-responsive self-healable conductive hydrogel of highly sensitive strain-pressure sensor for cancer cell de-tection. Nano Today 2021, 39, 101178. [Google Scholar] [CrossRef]
- Yin, B.-S.; Zhang, S.-W.; Ren, Q.-Q.; Liu, C.; Ke, K.; Wang, Z.-B. Elastic soft hydrogel supercapacitor for energy storage. J. Mater. Chem. A 2017, 5, 24942–24950. [Google Scholar] [CrossRef]
- Yao, B.; Peng, H.; Zhang, H.; Kang, J.; Zhu, C.; Delgado, G.; Byrne, D.; Faulkner, S.; Freyman, M.; Lu, X.; et al. Printing Porous Carbon Aerogels for Low Temperature Supercapacitors. Nano Lett. 2021, 21, 3731–3737. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, C.; Sun, Y.; Gan, S.; Dong, L.; Zhao, J.; Rong, J. Flexible, all-hydrogel supercapacitor with self-healing ability. Chem. Eng. J. 2021, 418, 128616. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, Conductive Self-healing Organohydrogels with Stable Strain-Sensitivity at Subzero Temperatures. Angew. Chem. Int. Ed. Engl. 2017, 56, 14159–14163. [Google Scholar] [CrossRef]
- Shit, A.; Kim, S.G.; In, I.; Park, S.Y. Self-repairable and recyclable self-powered human motion sensor with NIR/pH-responsive amplified Stretchable, Conductive, and Self-Healable hydrogel. Chem. Eng. J. 2021, 426, 131846. [Google Scholar] [CrossRef]
- Sun, F.; Huang, X.; Wang, X.; Liu, H.; Wu, Y.; Du, F.; Zhang, Y. Highly transparent, adhesive, stretchable and conductive PEDOT:PSS/polyacrylamide hydrogels for flexible strain sensors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126897. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Li, Z.; Yue, M.; Qing, X.; Zhang, P.; Liao, X.; Fan, Z.; Yang, S. PVA/SA/MXene dual-network conductive hydrogel for wearable sensor to monitor human motions. J. Appl. Polym. Sci. 2021, 139, 51627. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Jiang, C.; Zhu, P.; Sun, B.; Gao, Q.; Gao, C.; Liu, R. Highly adhesive and self-healing γ-PGA/PEDOT:PSS conductive hydrogels enabled by multiple hydrogen bonding for wearable electronics. Nano Energy 2022, 95, 106991. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Li, B.; Zhang, P.; Kan, L.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. One-Step Preparation of a Highly Stretchable, Conductive, and Transparent Poly(vinyl alcohol)-Phytic Acid Hydrogel for Casual Writing Circuits. ACS Appl. Mater. Interfaces 2019, 11, 32441–32448. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Xie, H.; Mao, J.; Wang, S.; Hu, Y.; Guo, Z. Conductive PNIPAM/CMCS/MWCNT/PANI hydrogel with temperature, pressure and pH sensitivity. ChemistrySelect 2021, 6, 4229–4237. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, X.; Xiong, Y.; Tang, Y.; Ma, X.; Tao, Q.; Sun, C.; Xu, W. A review of etching methods of MXene and applications of MXene conductive hydrogels. Eur. Polym. J. 2022, 167, 111063. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, H.; Fan, R.; Ji, P.; Fu, X.; Li, H. High Concentration of Ti3C2Tx MXene in Organic Solvent. ACS Nano 2021, 15, 5249–5262. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, S.; Guo, C.; Lu, D.; Geng, Z.; Pei, D. Mxene Reinforced Supramolecular Hydrogels with High Strength, Stretchability, and Reliable Conductivity for Sensitive Strain Sensors. Macromol. Rapid Commun. 2022, 43, e2200103. [Google Scholar] [CrossRef]
- Liao, H.; Guo, X.; Wan, P.; Yu, G. Conductive MXene Nanocomposite Organohydrogel for Flexible, Healable, Low-Temperature Tolerant Strain Sensors. Adv. Funct. Mater. 2019, 29, 1904507. [Google Scholar] [CrossRef]
- Hu, Z.-C.; Lu, J.-Q.; Zhang, T.-W.; Liang, H.-F.; Yuan, H.; Su, D.-H.; Ding, W.; Lian, R.-X.; Ge, Y.-X.; Liang, B.; et al. Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment. Bioact. Mater. 2023, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Peng, K.; Lin, L.; Yang, J.; Cheng, Z.; Gan, Q.; Chen, Y.; Feng, C. A conductive hydrogel based on nature polymer agar with self-healing ability and stretchability for flexible sensors. Chem. Eng. J. 2023, 454, 139843. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Feng, H.; Gui, Y.; Chen, T.; Xu, H.; Huang, X.; Ma, X. Flexible Stretchable, Dry-Resistant MXene Nanocomposite Conductive Hydrogel for Human Motion Monitoring. Polymers 2023, 15, 250. https://doi.org/10.3390/polym15020250
Liu Y, Feng H, Gui Y, Chen T, Xu H, Huang X, Ma X. Flexible Stretchable, Dry-Resistant MXene Nanocomposite Conductive Hydrogel for Human Motion Monitoring. Polymers. 2023; 15(2):250. https://doi.org/10.3390/polym15020250
Chicago/Turabian StyleLiu, Yafei, Huixia Feng, Yujie Gui, Ting Chen, Haidong Xu, Xiaoxue Huang, and Xuemei Ma. 2023. "Flexible Stretchable, Dry-Resistant MXene Nanocomposite Conductive Hydrogel for Human Motion Monitoring" Polymers 15, no. 2: 250. https://doi.org/10.3390/polym15020250
APA StyleLiu, Y., Feng, H., Gui, Y., Chen, T., Xu, H., Huang, X., & Ma, X. (2023). Flexible Stretchable, Dry-Resistant MXene Nanocomposite Conductive Hydrogel for Human Motion Monitoring. Polymers, 15(2), 250. https://doi.org/10.3390/polym15020250





