A Review of the Release Profiles and Efficacies of Chemotherapy Drug-Loaded Electrospun Membranes
Abstract
:1. Introduction
2. Release Profiles of the Chemotherapy Drug-Loaded Electrospun Membranes
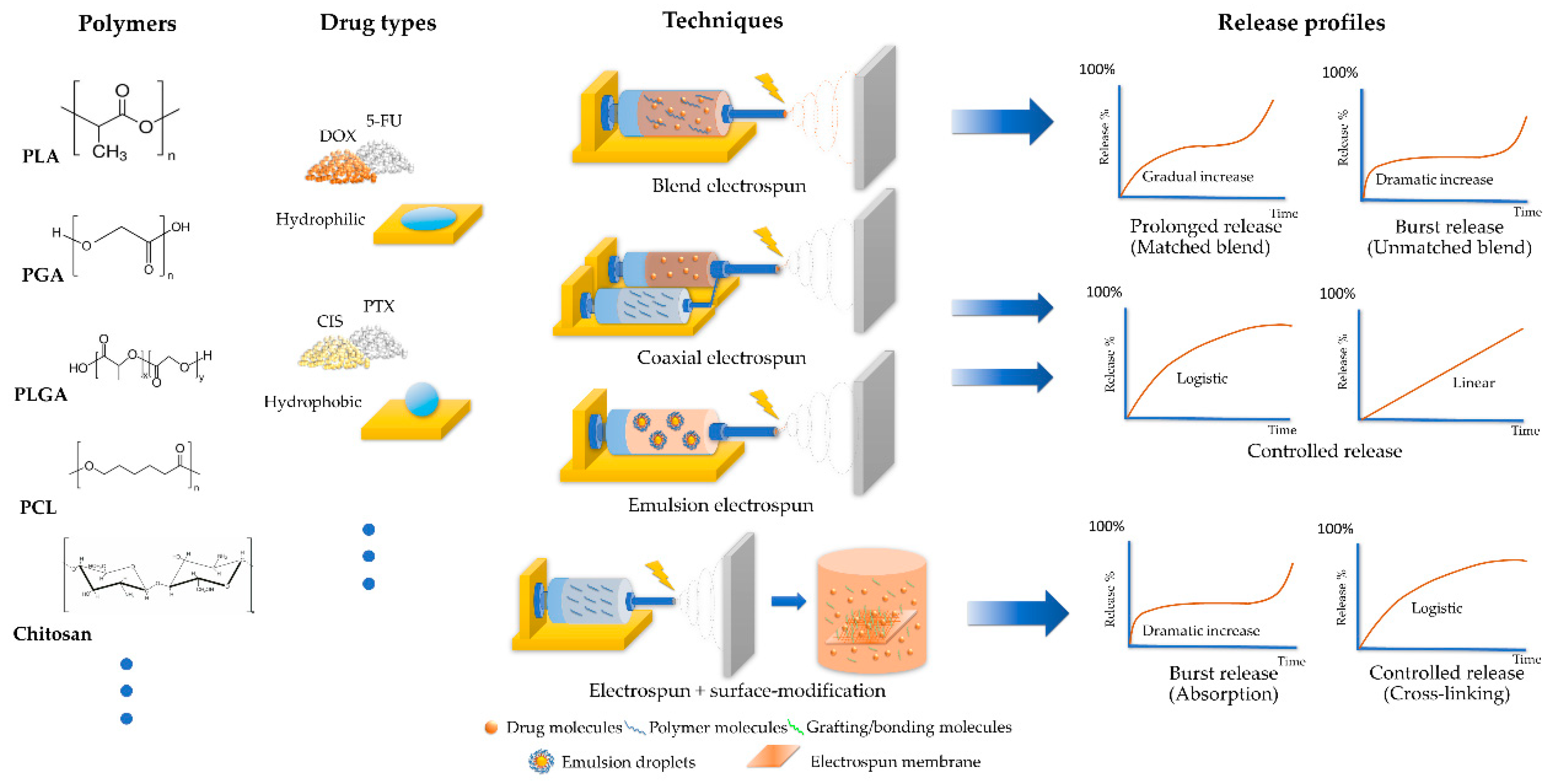
2.1. Key Factors Affecting the Release Profiles
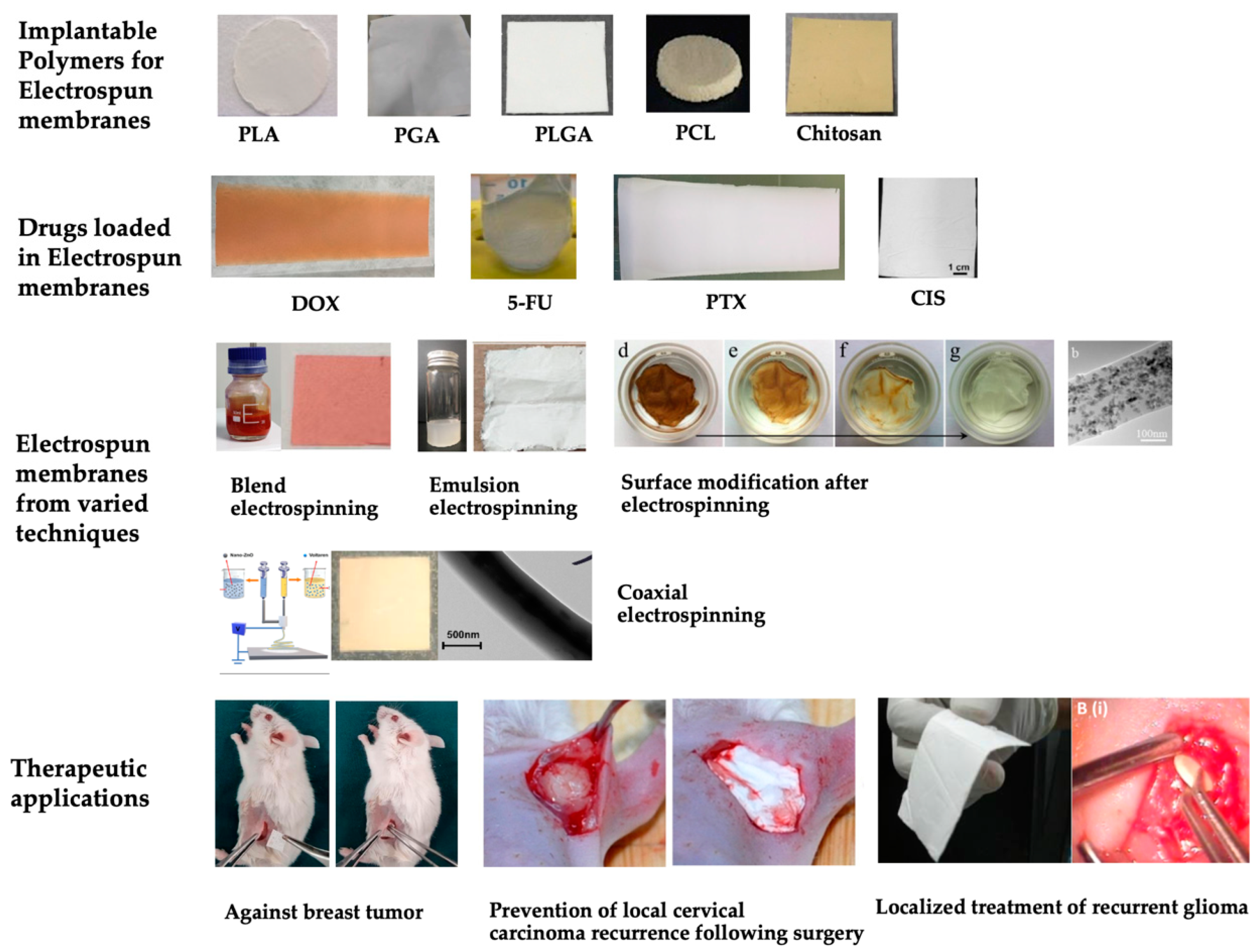
2.1.1. Polymers
2.1.2. Techniques of Electrospinning
2.2. Investigating the Release Profiles of Drug-Loaded Electrospun Membranes
2.2.1. Challenges in the Drug Release of Chemotherapy Drug-Loaded Electrospun Membranes
2.2.2. Strategies to Enhance the Release Profiles of Hydrophilic Chemotherapy Drug-Loaded Electrospun Membranes
2.2.3. Strategies to Enhance the Release Profiles of Hydrophobic Chemotherapy Drug-Loaded Electrospun Membranes
3. Therapeutic Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Compton, C. Local Therapy for Cancer. In Cancer: The Enemy from within: A Comprehensive Textbook of Cancer’s Causes, Complexities and Consequences; Springer International Publishing: Cham, Switzerland, 2020; pp. 197–222. [Google Scholar]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.-g.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Sun, B.; Taha, M.S.; Ramsey, B.; Torregrosa-Allen, S.; Elzey, B.D.; Yeo, Y. Intraperitoneal chemotherapy of ovarian cancer by hydrogel depot of paclitaxel nanocrystals. J. Control. Release 2016, 235, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Cheng, Y.; Chen, J.; Ding, J.; Li, M.; Li, C.; Wang, J.C.; Chen, X. Injectable Hydrogel-Microsphere Construct with Sequential Degradation for Locally Synergistic Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Min, H.; Mujeeb, A.; Zhang, Y.; Han, X.; Zhao, X.; Anderson, G.J.; Zhao, Y.; Nie, G. Injectable Hexapeptide Hydrogel for Localized Chemotherapy Prevents Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2018, 10, 6972–6981. [Google Scholar] [CrossRef]
- Heidari, B.S.; Oliaei, E.; Shayesteh, H.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Bahrami, M.; Rashedi, H. Simulation of mechanical behavior and optimization of simulated injection molding process for PLA based antibacterial composite and nanocomposite bone screws using central composite design. J. Mech. Behav. Biomed. 2017, 65, 160–176. [Google Scholar] [CrossRef]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Rudd, C.D. Bioresorbable screws reinforced with phosphate glass fibre: Manufacturing and mechanical property characterisation. J. Mech. Behav. Biomed. 2013, 17, 76–88. [Google Scholar] [CrossRef]
- Abate, J.A.; Fadale, P.D.; Hulstyn, M.J.; Walsh, W.R. Initial fixation strength of polylactic acid interference screws in anterior cruciate ligament reconstruction. Arthroscopy 1998, 14, 278–284. [Google Scholar] [CrossRef]
- Felfel, R.M.; Ahmed, I.; Parsons, A.J.; Rudd, C.D. Bioresorbable composite screws manufactured via forging process: Pull-out, shear, flexural and degradation characteristics. J. Mech. Behav. Biomed. 2013, 18, 108–122. [Google Scholar] [CrossRef]
- Hirata, T.; Fukuse, T.; Mizuno, H.; Hitomi, S.; Wada, H. Clinical application of biodegradable rib connecting pins in thoracotomy. Horacic Cardiovasc. Surg. 1999, 47, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Griffet, J.; Chevallier, A.; El Hayek, T.; Odin, G.; Pebeyre, B.; Accorsi, E. Diaphyseal fractures treated by polylactide and hydroxyapatite pins. Experimental study in rat. J. Mater. Sci. —Mater. Med. 1999, 10, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.W.; Yao, C.H.; Chen, Y.S.; Hsieh, T.C.; Lin, J.H.; Hsing, W.H. Manufacturing and Properties of PLA Absorbable Surgical Suture. Text. Res. J. 2008, 78, 958–965. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wu, G.H.; Zhang, X.F.; Yu, J.J.; Liu, M.F.; Zhang, Y.; Wang, P.; Yin, X.L.; Zhang, J.; Li, F.; et al. Preparation and properties of poly (lactic acid) (PLA) suture loaded with PLA microspheres enclosed drugs (PM-Ds). J. Text. Inst. 2019, 110, 1596–1605. [Google Scholar] [CrossRef]
- DeJong, L.E.S.; DeBerardino, T.M.; Brooks, D.E.; Judson, K. In vivo comparison of a metal versus a biodegradable suture anchor. Arthrosc.—J. Arthrosc. Relat. Surg. 2004, 20, 511–516. [Google Scholar] [CrossRef]
- Liu, S.Q.; Yu, J.J.; Li, H.M.; Wang, K.W.; Wu, G.H.; Wang, B.W.; Liu, M.F.; Zhang, Y.; Wang, P.; Zhang, J.; et al. Controllable Drug Release Behavior of Polylactic Acid (PLA) Surgical Suture Coating with Ciprofloxacin (CPFX)-Polycaprolactone (PCL)/Polyglycolide (PGA). Polymers 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C-Mater. 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Shukla, V.R.; Varadarajan, K.M.; Kumar, S. Microarchitected 3D printed polylactic acid (PLA) nanocomposite scaffolds for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 103, 103576. [Google Scholar] [CrossRef]
- Fu, S.J.; Zhang, P.H. Chitosan-gelatin enhanced antibacterial and biological properties of PLA and PGA braided threads for juvenile pseudomyopia treatment. Text. Res. J. 2021, 91, 2053–2062. [Google Scholar] [CrossRef]
- Shin, J.J.; Park, T.J.; Kim, B.Y.; Kim, C.M.; Suh, D.H.; Lee, S.J.; Moon, H.R.; Ryu, H.J. Comparative effects of various absorbable threads in a rat model. J. Cosmet. Laser Ther. 2019, 21, 158–162. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Giamporcaro, A.; De Caro, S.; Tranquillo, E.; Catauro, M. Threads Made with Blended Biopolymers: Mechanical, Physical and Biological Features. Polymers 2019, 11, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Song, Z.; Shi, B.; Ding, J.; Zhuang, X.; Zhang, X.; Fu, C.; Chen, X. A comparative study of preventing postoperative tendon adhesion using electrospun polyester membranes with different degradation kinetics. Sci. China Chem. 2015, 58, 1159–1168. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Qi, N. Controllable structure, properties, and degradation of the electrospun PLGA/PLA-blended nanofibrous scaffolds. J. Appl. Polym. Sci. 2012, 125, E468–E476. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, C.; Yue, Y.; Guo, W.; Wu, Y.; Wu, Q. Mechanical properties and in vitro degradation of electrospun bio-nanocomposite mats from PLA and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 301–308. [Google Scholar] [CrossRef]
- Wagner, F.C.; Polossek, L.; Yilmaz, T.; Jaeger, M.; Maier, D.; Feucht, M.J.; Sudkamp, N.P.; Reising, K. Biodegradable magnesium vs. polylactide pins for radial head fracture stabilization: A biomechanical study. J. Shoulder Elb. Surg. 2021, 30, 365–372. [Google Scholar] [CrossRef]
- Zhao, J.R.; Wang, M.M.; Xu, J.; Yang, Y. Progress in absorbable polymeric thread used as acupoint embedding material. Polym. Test. 2021, 101, 107298. [Google Scholar] [CrossRef]
- Pascual-Gonzalez, C.; de la Vega, J.; Thompson, C.; Fernandez-Blazquez, J.P.; Herraez-Molinero, D.; Biurrun, N.; Lizarralde, I.; Del Rio, J.S.; Gonzalez, C.; LLorca, J.L. Processing and mechanical properties of novel biodegradable poly-lactic acid/Zn 3D printed scaffolds for application in tissue regeneration. J. Mech. Behav. Biomed. Mater. 2022, 132, 105290. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.H.; Lee, J.H.; Jeong, B.Y.; Ahn, S.K.; Choi, Y.M.; Choi, D.J.; Chang, J.H. Antimicrobial and biodegradable PLGA medical sutures with natural grapefruit seed extracts. Mater. Lett. 2013, 95, 40–43. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, H.; Kontopoulou, M. Hydrolytic degradation of branched PLA produced by reactive extrusion. Polym. Degrad. Stab. 2018, 158, 228–237. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(D,L-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef] [PubMed]
- Bendix, D. Chemical synthesis of polylactide and its copolymers for medical applications. Polym. Degrad. Stab. 1998, 59, 129–135. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, W.; Zhou, S.; Trajtman, A.; Alfa, M. Electrospun PEG–PLA nanofibrous membrane for sustained release of hydrophilic antibiotics. J. Appl. Polym. Sci. 2010, 118, 588–595. [Google Scholar] [CrossRef]
- Leones, A.; Peponi, L.; Lieblich, M.; Benavente, R.; Fiori, S. In Vitro Degradation of Plasticized PLA Electrospun Fiber Mats: Morphological, Thermal and Crystalline Evolution. Polymers 2020, 12, 2975. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhang, X.; He, M.Y.; Li, J.C. Degradation of PGA, prepared by reactive extrusion polymerization, in water, humid, and dry air, and in a vacuum. J. Mater. Res. 2020, 35, 1846–1856. [Google Scholar] [CrossRef]
- Chu, C.C. Hydrolytic Degradation of Polyglycolic Acid—Tensile-Strength and Crystallinity Study. J. Appl. Polym. Sci. 1981, 26, 1727–1734. [Google Scholar] [CrossRef]
- Ang, H.Y.; Huang, Y.Y.; Lim, S.T.; Wong, P.; Joner, M.; Foin, N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J. Thorac. Dis. 2017, 9, S923–S934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Cooper, J.A.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Min, B.-M.; Lee, S.J.; Lee, T.S.; Park, W.H. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly(lactide-co-glycolide). J. Appl. Polym. Sci. 2005, 95, 193–200. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, M.S.; Jeon, O.; Choi, C.Y.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Sherwood, J.K.; Riley, S.L.; Palazzolo, R.; Brown, S.C.; Monkhouse, D.C.; Coates, M.; Griffith, L.G.; Landeen, L.K.; Ratcliffe, A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002, 23, 4739–4751. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.Y.; Foin, N. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef]
- Onuma, Y.; Ormiston, J.; Serruys, P.W. Bioresorbable Scaffold Technologies. Circ. J. 2011, 75, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y. Preparation, structure, and in vitro degradation behavior of the electrospun poly(lactide-co-glycolide) ultrafine fibrous vascular scaffold. Fibers Polym. 2012, 13, 754–761. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. J. Control. Release 2020, 320, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.P.; Du, F.S.; Jin, W.H.; Yang, J.Y.; Xu, Y. In vitro degradation of poly(caprolactone), poly(lactide) and their block copolymers: Influence of composition, temperature and morphology. React. Funct. Polym. 1997, 32, 161–168. [Google Scholar] [CrossRef]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic polyesters. I. The degradation of poly(ϵ-caprolactone) in vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Zarayneh, S.; Sepahi, A.A.; Jonoobi, M.; Rasouli, H. Comparative antibacterial effects of cellulose nanofiber, chitosan nanofiber, chitosan/cellulose combination and chitosan alone against bacterial contamination of Iranian banknotes. Int. J. Biol. Macromol. 2018, 118, 1045–1054. [Google Scholar] [CrossRef]
- Boryniec, S.; Strobin, G.; Struszczyk, H.; Niekraszewicz, A.; Kucharska, M. GPC Studies of Chitosan Degradation. International J. Polym. Anal. Charact. 1997, 3, 359–368. [Google Scholar] [CrossRef]
- Jouybari, M.H.; Hosseini, S.; Mahboobnia, K.; Boloursaz, L.A.; Moradi, M.; Irani, M. Simultaneous controlled release of 5-FU, DOX and PTX from chitosan/PLA/5-FU/g-C3N4-DOX/g-C3N4-PTX triaxial nanofibers for breast cancer treatment in vitro. Colloids Surf. B-Biointerfaces 2019, 179, 495–504. [Google Scholar] [CrossRef]
- Aggarwal, U.; Goyal, A.K.; Rath, G. Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 75, 125–132. [Google Scholar] [CrossRef]
- Anaraki, N.A.; Rad, L.R.; Irani, M.; Haririan, I. Fabrication of PLA/PEG/MWCNT Electrospun Nanofibrous Scaffolds for Anticancer Drug Delivery. J. Appl. Polym. Sci. 2015, 132, 41286. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Xu, C.; Xi, Z.; Ren, Y.; Song, Q.; Lu, S. Novel pH-sensitive drug-loaded electrospun nanofibers based on regenerated keratin for local tumor chemotherapy. Text. Res. J. 2020, 90, 2336–2349. [Google Scholar] [CrossRef]
- Wulf, K.; Arbeiter, D.; Matschegewski, C.; Teske, M.; Huling, J.; Schmitz, K.-P.; Grabow, N.; Kohse, S. Smart releasing electrospun nanofibers-poly: L.lactide fibers as dual drug delivery system for biomedical application. Biomed. Mater. 2020, 16, 015022. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Q.-S.; Ding, Y.-P.; Zhu, Z.-C. Preparation of Cisplatin Composite Micro/Nanofibers and Antitumor Activity In Vitro Against Human Tumor spc-a-1 Cells. Nano 2011, 6, 325–332. [Google Scholar] [CrossRef]
- Keridou, I.; Franco, L.; Turon, P.; del Valle, L.J.; Puiggali, J. Scaffolds with Tunable Properties Constituted by Electrospun Nanofibers of Polyglycolide and Poly(epsilon-caprolactone). Macromol. Mater. Eng. 2018, 303, 1800100. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Y.; Yuan, C.; Sun, P.; Xu, Q.; Lin, D.; Han, Z.; Xu, X.; Zhou, Q.; Deng, J. Fabrication of astaxanthin-loaded electrospun nanofiber-based mucoadhesive patches with water-insoluble backing for the treatment of oral premalignant lesions. Mater. Des. 2022, 223, 111131. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsieh, C.-H.; Huang, Y.-T.; Chu, S.-Y.; Chen, C.-M.; Lee, W.-J.; Liu, S.-J. Enhanced Paclitaxel Efficacy to Suppress Triple-Negative Breast Cancer Progression Using Metronomic Chemotherapy with a Controlled Release System of Electrospun Poly-d-l-Lactide-Co-Glycolide (PLGA) Nanofibers. Cancers 2021, 13, 3350. [Google Scholar] [CrossRef]
- Tseng, Y.-Y.; Wang, Y.-C.; Su, C.-H.; Yang, T.-C.; Chang, T.-M.; Kau, Y.-C.; Liu, S.-J. Concurrent delivery of carmustine, irinotecan, and cisplatin to the cerebral cavity using biodegradable nanofibers: In vitro and in vivo studies. Colloids Surf. B-Biointerfaces 2015, 134, 254–261. [Google Scholar] [CrossRef]
- Lim, M.M.; Sultana, N. Drug Loading, Drug Release and In vitro Degradation of Poly(Caprolactone) Electrospun Fibers. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 356–359. [Google Scholar]
- Mozaffari, S.; Seyedabadi, S.; Alemzadeh, E. Anticancer efficiency of doxorubicin and berberine-loaded PCL nanofibers in preventing local breast cancer recurrence. J. Drug Deliv. Sci. Technol. 2021, 67, 102984. [Google Scholar] [CrossRef]
- Kaplan, J.A.; Liu, R.; Freedman, J.D.; Padera, R.; Schwartz, J.; Colson, Y.L.; Grinstaff, M.W. Prevention of lung cancer recurrence using cisplatin-loaded superhydrophobic nanofiber meshes. Biomaterials 2016, 76, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Gaiser, S.; Luisier, N.; Hegemann, D.; Ferguson, S.J.; Fortunato, G.; Rossi, R.M. pH-Responsive Chitosan/Alginate Polyelectrolyte Complexes on Electrospun PLGA Nanofibers for Controlled Drug Release. Nanomaterials 2021, 11, 1850. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Shen, M.; Tomás, H.; Zhou, B.; Shi, X. Antitumor Efficacy of Doxorubicin-Loaded Electrospun Attapulgite–Poly(lactic-co-glycolic acid) Composite Nanofibers. J. Funct. Biomater. 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Yuan, Y.; Choi, K.; Choi, S.O.; Kim, J. Doxorubicin Release Controlled by Induced Phase Separation and Use of a Co-Solvent. Materials 2018, 11, 681. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Liu, T.; Liu, S.; Jing, X. Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv. 2014, 23, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, S.; Zheng, R.; Zhao, X.; Chen, X.; Fan, C.; Cui, W. Smart electrospun fibrous scaffolds inhibit tumor cells and promote normal cell proliferation. Rsc. Adv. 2014, 4, 51696–51702. [Google Scholar] [CrossRef]
- Giannetti, R.; Abraham, G.A.; Rivero, G. The role of emulsion parameters in tramadol sustained-release from electrospun mats. Mater. Sci. Eng. C 2019, 99, 1493–1501. [Google Scholar] [CrossRef]
- Iqbal, S.; Rashid, M.H.; Arbab, A.S.; Khan, M. Encapsulation of Anticancer Drugs (5-Fluorouracil and Paclitaxel) into Polycaprolactone (PCL) Nanofibers and In Vitro Testing for Sustained and Targeted Therapy. J. Biomed. Nanotechnol. 2017, 13, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. Rsc. Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Jin, Z.; Chen, H.; Liang, L.; Li, Y.; Wang, G.; Zhang, J.; Xu, T. Electrospun poly (L-lactic acid)/gelatine membranes loaded with doxorubicin for effective suppression of glioblastoma cell growth in vitro and in vivo. Regen. Biomater. 2021, 8, rbab043. [Google Scholar] [CrossRef]
- Laha, A.; Sharma, C.S.; Majumdar, S. Electrospun gelatin nanofibers as drug carrier: Effect of crosslinking on sustained release. Mater. Today Proc. 2016, 3, 3484–3491. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Mortazavi, S.A.; Kobarfard, F.; Jaafari, M.R.; Hashemi, A.; Farhadnejad, H.; Feyzi-barnaji, B. Electrospun Doxorubicin-loaded PEO/PCL core/sheath nanofibers for chemopreventive action against breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 64, 102576. [Google Scholar] [CrossRef]
- Xiao, Y.; Fan, Y.; Tu, W.; Ning, Y.; Zhu, M.; Liu, Y.; Shi, X. Multifunctional PLGA microfibrous rings enable MR imaging-guided tumor chemotherapy and metastasis inhibition through prevention of circulating tumor cell shedding. Nano Today 2021, 38, 101123. [Google Scholar] [CrossRef]
- Hu, J.; Wei, J.; Liu, W.; Chen, Y. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a drug delivery system by emulsion electrospinning. J. Biomat. Sci.-Polym. E 2013, 24, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Kaviannasab, E.; Semnani, D.; Khorasani, S.N.; Varshosaz, J.; Khalili, S.; Ghahreman, F. Core-shell nanofibers of poly (epsilon-caprolactone) and Polyvinylpyrrolidone for drug delivery system. Mater. Res. Express 2019, 6, 115015. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, N.; Li, J.; Wang, H.; Wang, C.; Zhang, Z.; Gu, J.g.; Wang, M.; Han, C.C.; Xu, S.; et al. Dual-Functional Esophageal Stent Coating Composed of Paclitaxel-Loaded Electrospun Membrane and Protective Film. J. Biomed. Nanotechnol. 2019, 15, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Graham-Gurysh, E.G.; Moore, K.M.; Schorzman, A.N.; Lee, T.; Zamboni, W.C.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Tumor Responsive and Tunable Polymeric Platform for Optimized Delivery of Paclitaxel to Treat Glioblastoma. ACS Appl. Mater. Interfaces 2020, 12, 19345–19356. [Google Scholar] [CrossRef] [PubMed]
- Khashi, M.; Hassanajili, S.; Golestaneh, S.I. Electrospun Poly-lactic Acid/Chitosan Nanofibers Loaded with Paclitaxel for Coating of a Prototype Polymeric Stent. Fibers Polym. 2018, 19, 1444–1453. [Google Scholar] [CrossRef]
- Xie, J.; Tan, R.S.; Wang, C.-H. Biodegradable microparticles and fiber fabrics for sustained delivery of cisplatin to treat C6 glioma in vitro. J. Biomed. Mater. Res. A 2008, 85A, 897–908. [Google Scholar] [CrossRef]
- Luo, H.L.; Zhang, Y.; Yang, Z.W.; Zuo, G.F.; Zhang, Q.C.; Yao, F.L.; Wan, Y.Z. Encapsulating doxorubicin-intercalated lamellar nanohydroxyapatite into PLGA nanofibers for sustained drug release. Curr. Appl. Phys. 2019, 19, 1204–1210. [Google Scholar] [CrossRef]
- Ye, C.Q.; Zhao, J.L.; Zheng, Y.T.; Wu, C.Y.; Chen, Y.; Wu, H.; An, X.; Huang, M.X.; Wang, S.G. Preparation of Poly(lactic-co-glycolic acid)-Based Composite Microfibers for Postoperative Treatment of Tumor in NIR I and NIR II Biowindows. Macromol. Biosci. 2018, 18, e1800206. [Google Scholar] [CrossRef]
- Jaworska, J.; Smolarczyk, R.; Musiał-Kulik, M.; Cichoń, T.; Karpeta-Jarząbek, P.; Włodarczyk, J.; Stojko, M.; Janeczek, H.; Kordyka, A.; Kaczmarczyk, B.; et al. Electrospun paclitaxel delivery system based on PGCL/PLGA in local therapy combined with brachytherapy. Int. J. Pharm. 2021, 602, 120596. [Google Scholar] [CrossRef]
- Guo, Y.; Ghobeira, R.; Sun, Z.; Shali, P.; Morent, R.; De Geyter, N. Atmospheric pressure plasma jet treatment of PLA/PAni solutions: Enhanced morphology, improved yield of electrospun nanofibers and concomitant doping behaviour. Polymer 2022, 262, 125502. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, W.; Tremblay, P.-L.; Zhang, T. Electrostimulation of fibroblast proliferation by an electrospun poly (lactide-co-glycolide)/polydopamine/chitosan membrane in a humid environment. Colloids Surf. B Biointerfaces 2022, 220, 112902. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos Carriles, Y.; Suetel, M.; Henze, S.; Álvarez Brito, R.; Mueller, W.-D. Electrospun meshes of poly (n-butyl cyanoacrylate) and their potential applications for drug delivery and tissue engineering. Int. J. Pharm. 2021, 606, 120735. [Google Scholar] [CrossRef]
- Hosseini, A.; Ramezani, S.; Tabibiazar, M.; Mohammadi, M.; Golchinfar, Z.; Mahmoudzadeh, M.; Jahanban-Esfahlan, A. Immobilization of α-amylase in ethylcellulose electrospun fibers using emulsion-electrospinning method. Carbohydr. Polym. 2022, 278, 118919. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J. An antibacterial coaxial electrospun polylactic acid/silk fibroin loaded with nano-ZnO and Voltaren. Mater. Lett. 2022, 325, 132804. [Google Scholar] [CrossRef]
- Liu, J.; Chang, M.-J.; Du, H.-L. Facile preparation of cross-linked porous poly(vinyl alcohol) nanofibers by electrospinning. Mater. Lett. 2016, 183, 318–321. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Shahsavari, Z.; Shahhosseini, S.; ten Hagen, T.L.M.; Haeri, A. Piperine-loaded electrospun nanofibers, an implantable anticancer controlled delivery system for postsurgical breast cancer treatment. Int. J. Pharm. 2022, 624, 121990. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Qi, Y.; Zhou, D.; Xie, Z.; Jing, X.; Chen, X.; Huang, Y. Time-programmed DCA and oxaliplatin release by multilayered nanofiber mats in prevention of local cancer recurrence following surgery. J. Control. Release 2016, 235, 125–133. [Google Scholar] [CrossRef]
- Ramachandran, R.; Junnuthula, V.R.; Gowd, G.S.; Ashokan, A.; Thomas, J.; Peethambaran, R.; Thomas, A.; Unni, A.K.; Panikar, D.; Nair, S.V.; et al. Theranostic 3-Dimensional nano brain-implant for prolonged and localized treatment of recurrent glioma. Sci. Rep. 2017, 7, 43271. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Ma, J.; Ni, G.; Yang, G.; Zhou, S. Programmable Codelivery of Doxorubicin and Apatinib Using an Implantable Hierarchical-Structured Fiber Device for Overcoming Cancer Multidrug Resistance. Small 2019, 15, e1804397. [Google Scholar] [CrossRef] [PubMed]
- Bei, H.-P.; Xu, T.; Zhou, J.; Dong, Z.; Wang, Y.; Wong, K.-Y.; Wang, H.; Zhao, X. Evaporation-based, co-axial lock-and-key fibrous reservoir for long-term prevention of hypertrophic scars. Appl. Mater. Today 2022, 27, 101463. [Google Scholar] [CrossRef]
- Padmakumar, S.; Paul-Prasanth, B.; Pavithran, K.; Vijaykumar, D.K.; Rajanbabu, A.; Sivanarayanan, T.B.; Kadakia, E.; Amiji, M.M.; Nair, S.V.; Menon, D. Long-term drug delivery using implantable electrospun woven polymeric nanotextiles. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 274–284. [Google Scholar] [CrossRef] [PubMed]


| Polymer | Degradation Duration in Crystalline Forms | Degradation Duration in Electrospun Forms | Hydrophobicity | Intended Use |
|---|---|---|---|---|
PLA 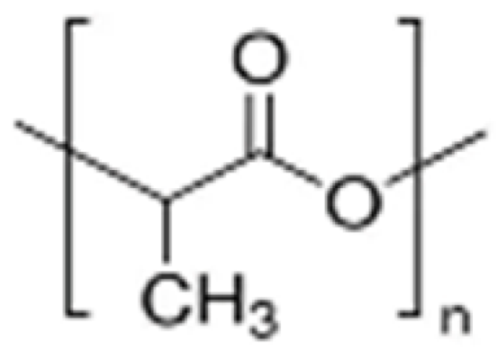 | More than one year [35,36,37]. | Approximately three months, less than half a year [38,39]. | Hydrophobic. | Long-term structural materials [9,28] and delivery systems [61,62,63,64]. |
PGA 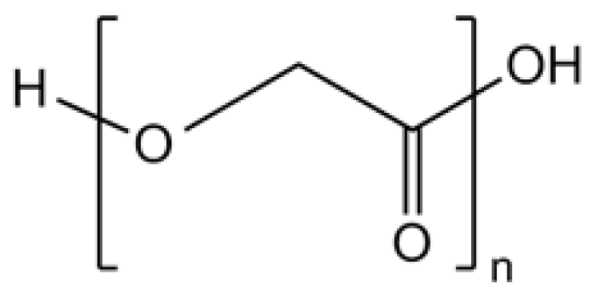 | A few months [41,42]. | Approximately or less than one month [43,44]. | Hydrophilic. | Tissue engineering material [35,43]. and tuning down the degradation period of long-term delivery systems [65,66]. |
PLGA  | More than one year [49,50,51]. | Approximately two months [52]. | Hydrophilicity proportional to the GA ratio. | Mid-term flexible structural materials [29,31] and delivery system [67,68]. |
PCL  | Up to two years [55]. | A 30% decline in fiber diameter after around three months [69]. | Hydrophobic. | Long-term structural materials [25,69] and delivery system [70,71]. |
Chitosan  | Approximately one month [58]. | Limited amount of whole process degradation data. | Hydrophilic. | Short-term drug delivery system and tuning down the degradation period of other long-term delivery systems [59,60], specialty in antibacterial ability [57], and pH-responsive delivery [72]. |
| Chemo Drug | Polymer Information | Techniques | Release Profile and Maximum Released % |
|---|---|---|---|
| DOX | PCL 80 kD | Blending | ~9 days logistic to ~30% [70] |
| Shell PCL 80 kD + Core PEO 300 kD | Coaxial | ~10 days logistic to ~60% [82] | |
| PLA 380 kD + Gelatin (Type A from porcine skin, 210–250 g Bloom) | Immersion Coating | ~6 days logistic to ~30% [80] | |
| PLA 186 kD + PEG 4 kD | Blending (High Shear) with an enhancer | 30 days exponential to ~85% [61] | |
| PLGA 50:50 81 kD + branched PEI 25 kD + PVA 85–124 kD | Surface Mod Coating | 50 days logistic to ~50% [83] | |
| Core Chitosan (75–85% deacetylated 200 kD) + Shell PLA 150 kD | Coaxial Tri-Layer + Complex Encapsulation | ~30 days logistic to ~80% [59] | |
| 5-FU | PLA pellets 200 kD | Complex Encapsulation | ~4 days logistic to ~90% [62] |
| PLGA 50:50 10 kD + gelatin (type A from porcine skin, 300 bloom) | Blending | ~10 days logistic to ~90% [84] | |
| Shell PCL 80 kD + Core PVA 89–98 kD | Coaxial | ~25 days logistic to ~80% [78] | |
| Core PVP + Shell PCL 70% + PVP 30% PCL 80 kD, PVP 360 kD | Coaxial | ~7 days logistic to ~80% [85] | |
| Core Chitosan (75–85% deacetylated 200 kD) + Shell PLA 150 kD | Coaxial Tri-Layer | ~30 days linear to ~80% [59] | |
| PTX | PCL 200 kD + PLGA 75:25 80 kD | Bi-layer | ~9 days logistic to ~35% [86] |
| Core Chitosan (75–85% deacetylated 200 kD) + Shell PLA 150 kD | Coaxial Tri-Layer + Complex Encapsulation | ~21 days logistic to ~80% [59] | |
| Dextran 450–650 kD + PLA 260 kD | Blending | ~35 days linear to ~90% [87] | |
| PLA + Chitosan | Blending | 14 days logistic to ~30% [88] | |
| PLA | Blending | ~40 days logistic to ~80% [63] | |
| PLGA 50:50 33 kD | Blending | ~42 days logistic to ~80% [67] | |
| CIS | PCL 45 kD + Chitosan 310 kD | Blending | 30 days linear to ~65% [60] |
| PCL 70–90 kD | Blending + Release Enhancer | ~70 days linear to ~60% [71] | |
| PLA 85–160 kD + PLGA 50:50 50–70 kD | Blending | ~33 days logistic to ~70% [89] | |
| PLGA 50:50 33 kD | Blending | ~30 days exponential to ~100% [68] | |
| PLA 100 kD | Blending | ~11 days logistic to ~45% [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Chen, H.; Xu, J.; Wang, J.; Wang, H.; Huang, S.; Xu, S. A Review of the Release Profiles and Efficacies of Chemotherapy Drug-Loaded Electrospun Membranes. Polymers 2023, 15, 251. https://doi.org/10.3390/polym15020251
Lin Z, Chen H, Xu J, Wang J, Wang H, Huang S, Xu S. A Review of the Release Profiles and Efficacies of Chemotherapy Drug-Loaded Electrospun Membranes. Polymers. 2023; 15(2):251. https://doi.org/10.3390/polym15020251
Chicago/Turabian StyleLin, Zhenyu, Hao Chen, Jiawei Xu, Jie Wang, Huijing Wang, Shifen Huang, and Shanshan Xu. 2023. "A Review of the Release Profiles and Efficacies of Chemotherapy Drug-Loaded Electrospun Membranes" Polymers 15, no. 2: 251. https://doi.org/10.3390/polym15020251
APA StyleLin, Z., Chen, H., Xu, J., Wang, J., Wang, H., Huang, S., & Xu, S. (2023). A Review of the Release Profiles and Efficacies of Chemotherapy Drug-Loaded Electrospun Membranes. Polymers, 15(2), 251. https://doi.org/10.3390/polym15020251






