Improving Glass Transition Temperature and Toughness of Epoxy Adhesives by a Complex Room-Temperature Curing System by Changing the Stoichiometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Formulation and Cure Schedules
2.3. Kinetic Analysis of the Curing Process Using Differential Scanning Calorimetry (DSC)
2.4. Curing Time Analysis
2.5. Viscoelastic Properties of the Cured Material
2.6. Tensile Testing
2.7. Shear Testing
3. Results and Discussion
3.1. Kinetic Analysis of Curing Process by DSC
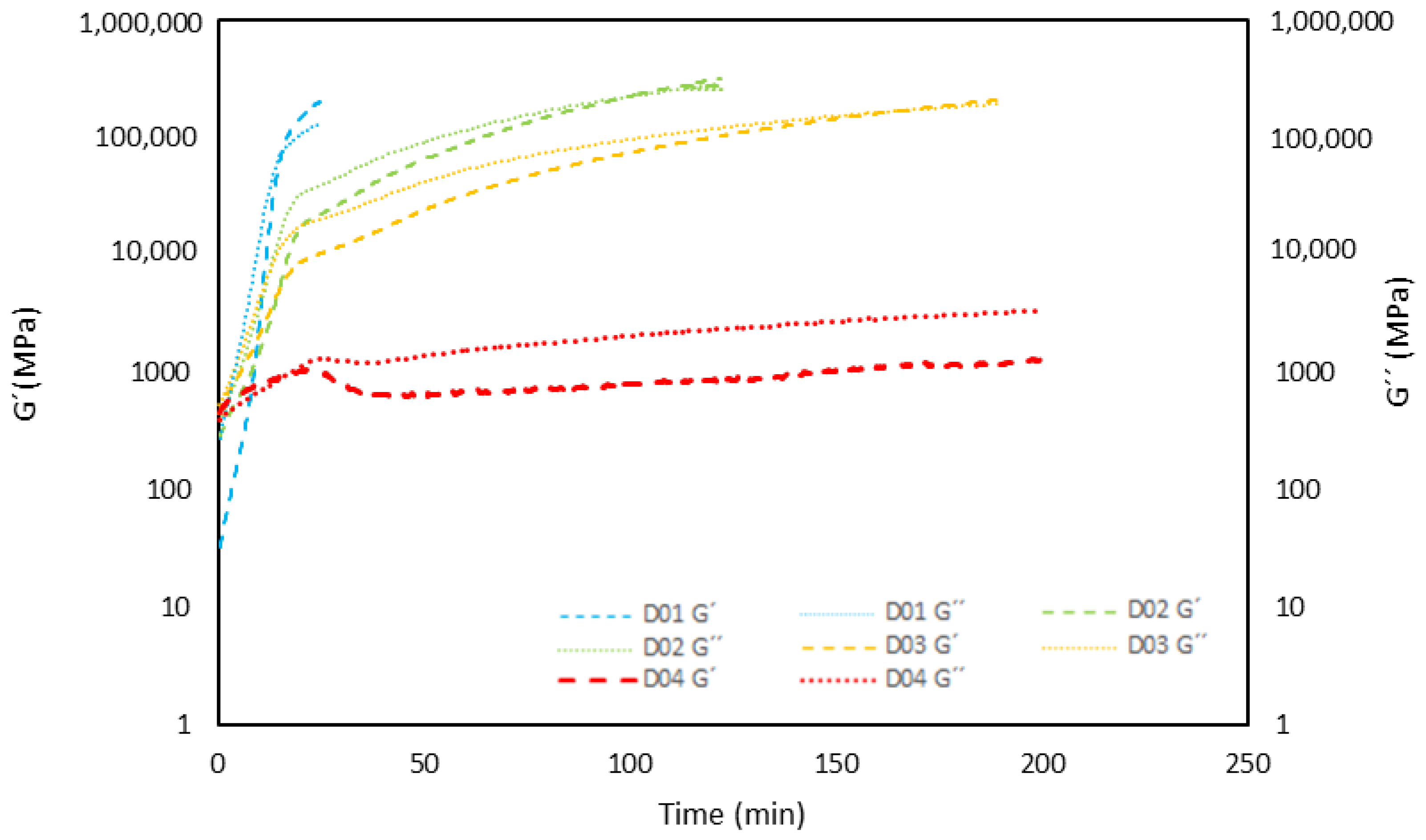
3.2. Rheological Analysis of Curing Process
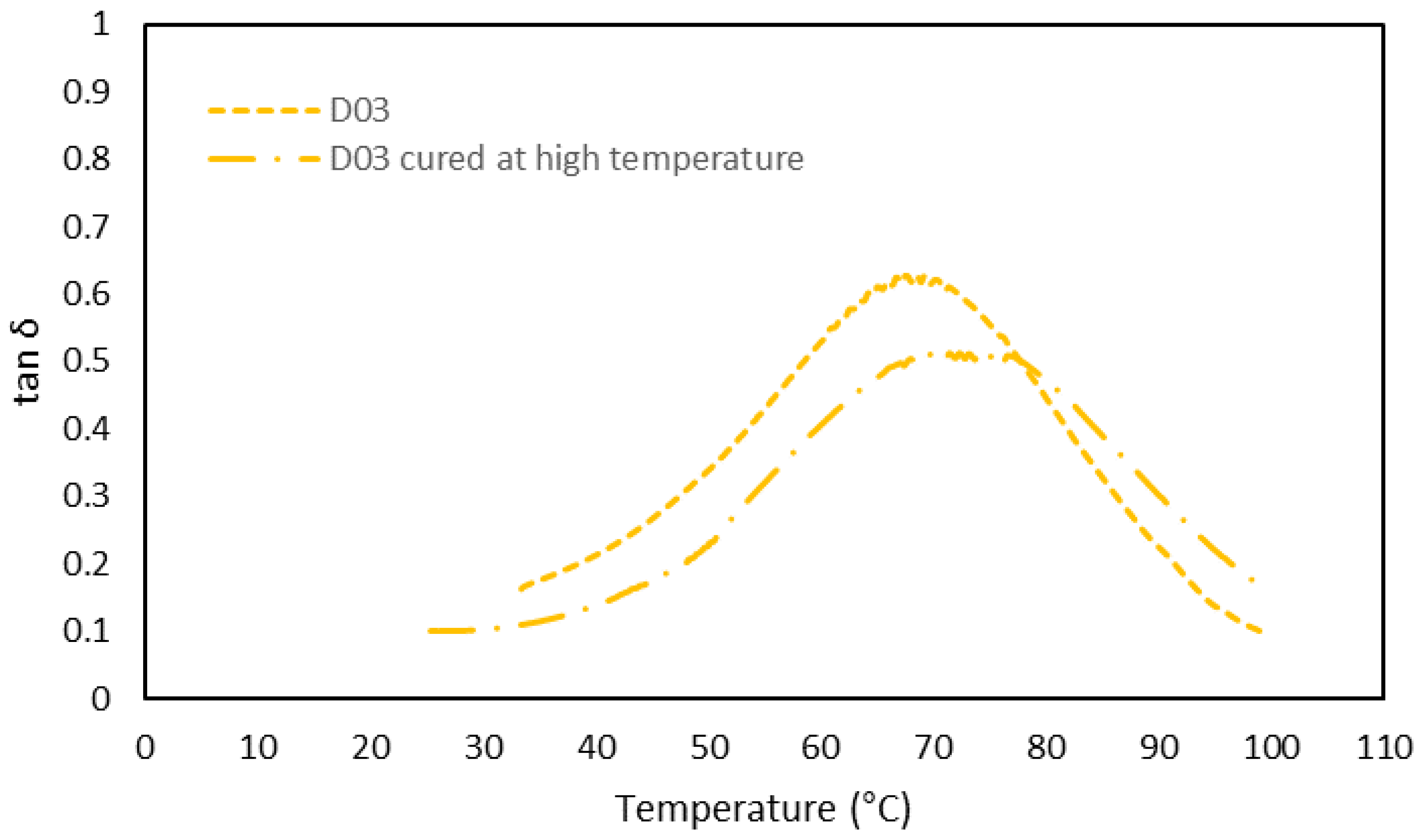
3.3. Thermomechanical Characterization by DMA
3.4. Mechanical Characterization
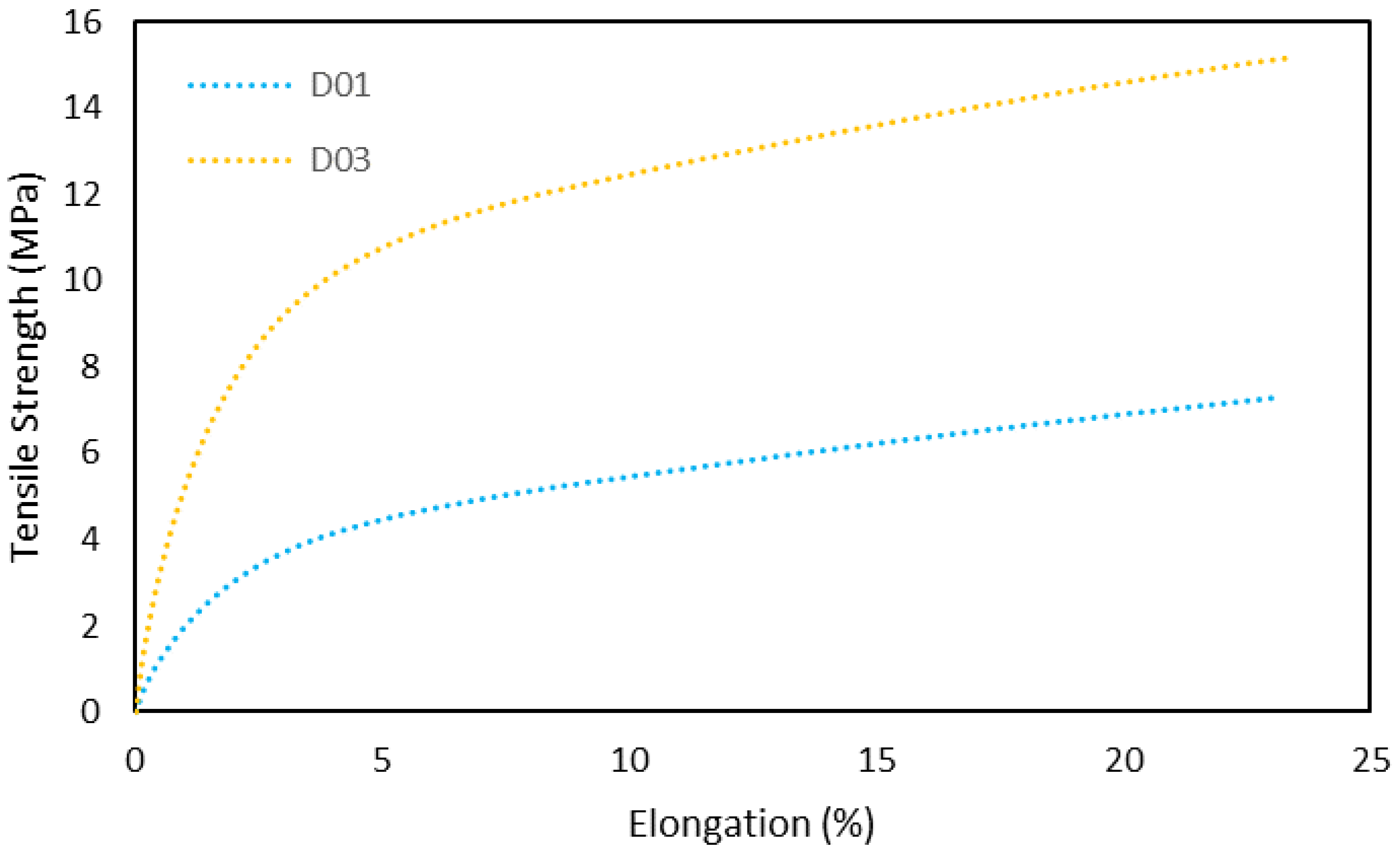
3.4.1. Tensile Strength
3.4.2. Lap Shear Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, F.; Xie, W.; Ma, C.; Wang, D.; El-Bahy, Z.H.; Helal, M.H.; Liu, H.; Du, A.; Guo, Z.; Gu, H. Superb electromagnetic shielding polymer nanocomposites filled with 3-dimensional p-phenylenediamine/aniline copolymer nanofibers@copper foam hybrid nanofillers. Compos. Part B Eng. 2022, 245, 110236. [Google Scholar] [CrossRef]
- Karami, M.H.; Kalaee, M.; Khajavi, R.; Moradi, O.; Zaarei, D. Thermal degradation kinetics of epoxy resin modified with elastomeric nanoparticles. Adv. Compos. Hybrid. Mater. 2022, 5, 390–401. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, H.; Ma, C.; Xu, X.; Wang, Y.; Wang, Z.; Wei, R.; Liu, H.; Liu, C.; Shao, Q.; et al. Trace electrosprayed nanopolystyrene facilitated dispersion of multiwalled carbon nanotubes: Simultaneously strengthening and toughening epoxy. Carbon 2019, 142, 131–140. [Google Scholar] [CrossRef]
- Jing, X.; Wei, J.; Liu, Y.; Song, B.; Liu, Y. Deployment Analysis of Aramid Fiber Reinforced Shape- Memory Epoxy Resin Composites. Eng. Sci. 2020, 11, 44–53. [Google Scholar] [CrossRef]
- Gu, H.; Ma, C.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Putnam-Neeb, A.A.; Kaiser, J.M.; Hubbard, A.M.; Street, D.P.; Dickerson, M.B.; Nepal, D.; Baldwin, L.A. Self-healing and polymer welding of soft and stiff epoxy thermosets via silanolates. Adv. Compos. Hybrid Mater. 2022, 5, 3068–3080. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Kumar, B.; Agumba, D.O.; Pham, D.H.; Latif, M.; Dinesh Kim, H.C.; Alrobei, H.; Kim, J. Recent Research Progress on Lignin-Derived Resins for Natural Fiber Composite Applications. Polymers 2021, 13, 1162. [Google Scholar] [CrossRef]
- Kumar, B.; Agumba, D.O.; Pham, D.H.; Kim, H.C.; Kim, J. Recent progress in bio-based eugenol resins: From synthetic strategies to structural properties and coating applications. J. Appl. Polym. Sci. 2022, 139, 51532. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Sánchez Huerta, R.S.; Ledezma Pérez, A.S.; García Valdez, A.E. Synthesis of a Curing Agent Derived from Limonene and the Study of Its Performance to Polymerize a Biobased Epoxy Resin Using the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers 2022, 14, 2192. [Google Scholar] [CrossRef]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw Hill Professional: New York, NY, USA, 2005. [Google Scholar]
- Sindt, O.; Perez, J.; Gerard, J.F. Molecular architecture-mechanical behaviour relationships in epoxy networks. Polymer 1996, 37, 2989–2997. [Google Scholar] [CrossRef]
- Torres, J.M.; Wang, C.; Coughlin, E.B.; Bishop, J.P.; Register, R.A.; Riggleman, R.A.; Christopher, M.S.; Vogth, B.D. Influence of chain stiffness on thermal and mechanical properties of polymer thin films. Macromolecules 2011, 44, 9040–9045. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, B. Glass transition temperature and molecular parameters of polymer. J. Polymer 1991, 32, 471–478. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G. Polymer free volume and its connection to the glass transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Hale, A.; Crhistopher, W.M. Glass transition temperature as a function of conversion in thermosetting polymers. Macromolecules 1991, 24, 2610–2621. [Google Scholar] [CrossRef]
- Bicerano, J.; Sammler, R.L.; Carriere, C.J.; Seitz, J.T. Correlation between Glass Transition Temperature and Chain Structure for Randomly Crosslinked High Polymers. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2247–2259. [Google Scholar] [CrossRef]
- Pascault, J.P.; Williamns, R.J. Glass transition temperature versus conversion relationships for thermosetting polymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 85–95. [Google Scholar] [CrossRef]
- Vanlandingham, M.R.; Eduljee, R.F.; Gillespie, J.R.J.W. Relationships between stoichiometry, microstructure, and properties for amine-cured epoxies. J. Polym. Sci. 1999, 71, 699–712. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, C.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Qian, D.; Zhou, J.; Zheng, J.; Cao, J.; Wan, J.; Fan, H. Synthesis, Curing Behaviors and Properties of a Bio-Based Trifunctional Epoxy Silicone Modified Epoxy Thermosets. Polymers 2022, 14, 4391. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. High performance fully bio-based epoxy thermoset from syringaldehyde-derived epoxy monomer cured by furan-derived amine. Green Chem. 2021, 23, 501–510. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Sánchez Huerta, R.S.; Ledezma Pérez, A.S.; García Valdez, A.E. Síntesis de un agente de curado derivado del limoneno y estudio de su desempeño para polimerizar una resina epoxi de base biológica mediante la técnica de fotopolimerización epoxi/tiol-eno. Polímeros 2022, 14, 2022. [Google Scholar]
- Ye, S.; Cramer, N.B.; Bowman, C.N. Relationship between glass transition temperature and polymerization temperature for cross-linked photopolymers. Macromolecules 2011, 44, 490–494. [Google Scholar] [CrossRef]
- Carbas, R.J.; Marques, E.A.S.; Silva, L.F.M.; Lopes, A.M. Effect of Cure Temperature on the Glass Transition Temperature and Mechanical Properties of Epoxy Adhesives. J. Adhes. 2014, 90, 104–119. [Google Scholar] [CrossRef]
- Peng, X.; Gillham, J.K. Time-temperature-transformation (TTT) cure diagrams: Relationship between Tg and the temperature and time of cure for epoxy systems. J. Polym. Sci. 1985, 30, 4685–4696. [Google Scholar] [CrossRef]
- Chan, L.C.; Naé, H.N.; Gillham, J.K. Time-temperature-transformation (TTT) diagrams of high Tg epoxy systems: Competition between cure and thermal degradation. J. Polym. Sci. 1984, 29, 3307–3327. [Google Scholar] [CrossRef]
- Michel, M.; Ferrier, E. Effect of curing temperature conditions on glass transition temperature values of epoxy polymer used for wet lay-up applications. Constr. Build. Mater. 2020, 231, 117206. [Google Scholar] [CrossRef]
- Oleinik, E.F. Epoxy-aromatic amine networks in the glassy state structure and properties. Adv. Polym. Sci. 1986, 80, 49–99. [Google Scholar]
- Pizzi, A. (Ed.) Handbook of Adhesive Technology; Marcel Dekker, Inc.: New City, NY, USA, 2003. [Google Scholar]
- Fernández-Francos, X.; Konuray, A.-O.; Belmonte, A.; De la Flor, S.; Serra, À.; Ramis, X. Sequential curing of off-stoichiometric thiol-epoxy thermosets with a custom-tailored structure. Polym. Chem. 2016, 7, 2280–2290. [Google Scholar] [CrossRef] [Green Version]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014.
- UNE-EN 2263-1; Aerospace series. Non-metallic materials. Structural adhesives. Test method. Part 1: Single lap shear. AENOR: Madrid, Spain, 2018.
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
- Bijender, K.; Swarup, R.; Dickens, O.A.; Duc, H.P.; Jaehwan, K. Effect of bio-based derived epoxy resin on interfacial adhesion of cellulose film and applicability towards natural jute fiber-reinforced composites. Int. J. Biol. Macromol. 2022, 222, 1304–1313. [Google Scholar]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. New catalysts for diglycidyl ether of bisphenol A curing based on thiol-epoxy click reaction. Eur. Polym. J. 2014, 59, 377–386. [Google Scholar] [CrossRef]
- Ooi, S.K.; Cook, W.D.; Simon, G.P.; Such, C. DSC studies of the curing mechanism and kinetics of DGEBA using imidazole curing agents. Polymer 2000, 41, 3639–3649. [Google Scholar] [CrossRef]
- Fernandez-Francos, X.; Cook, W.D.; Serra, A.; Ramis, X.; Liang, G.G.; Salla, J.M. Crosslinking of mixtures of DGEBA with 1,6-dioxaspiro [4,4]nonan-2,7-dione initiated by tertiary amines. Part IV. Effect of hydroxyl groups on initiation and curing kinetics. Polymer 2010, 51, 26–34. [Google Scholar] [CrossRef]

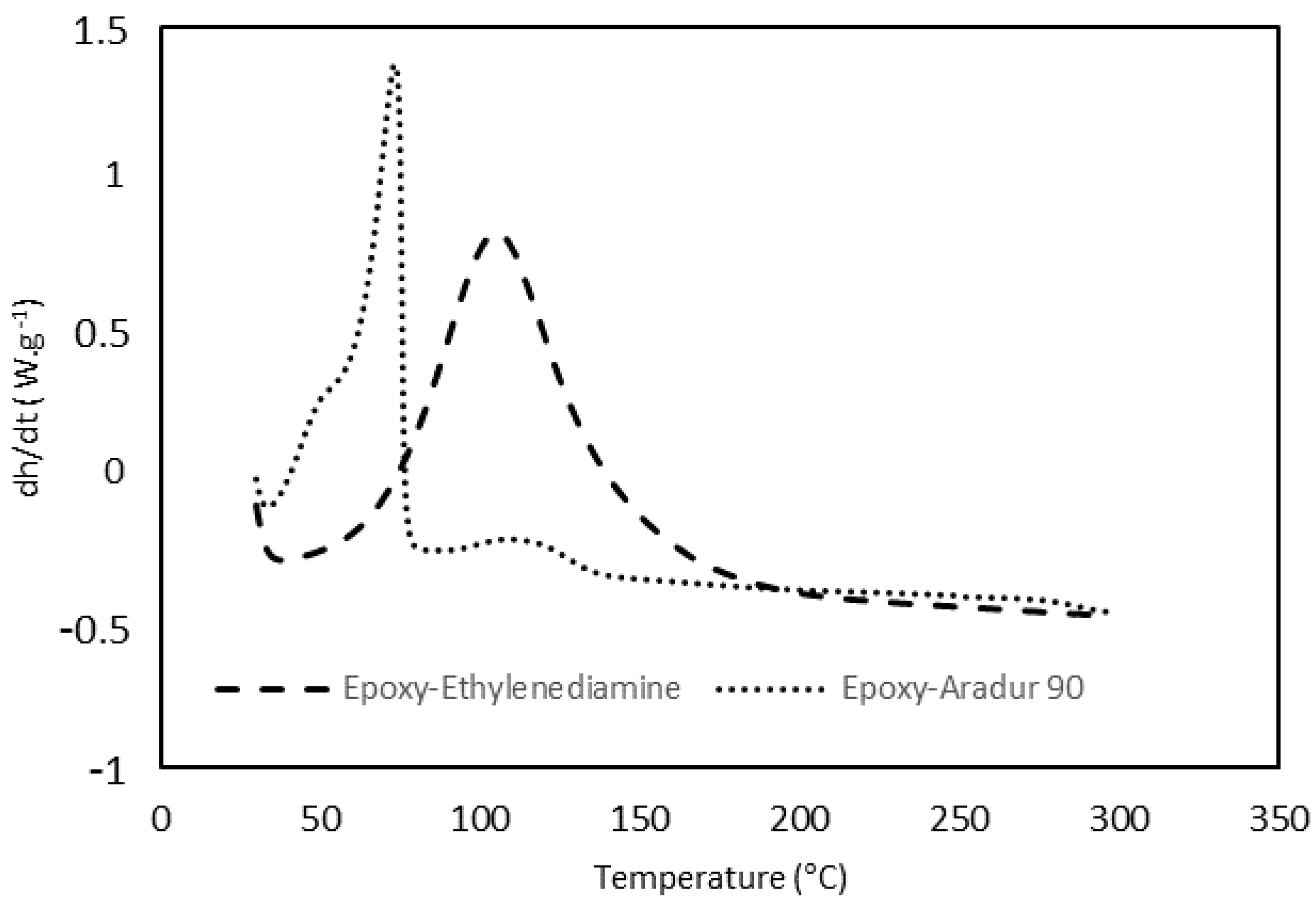
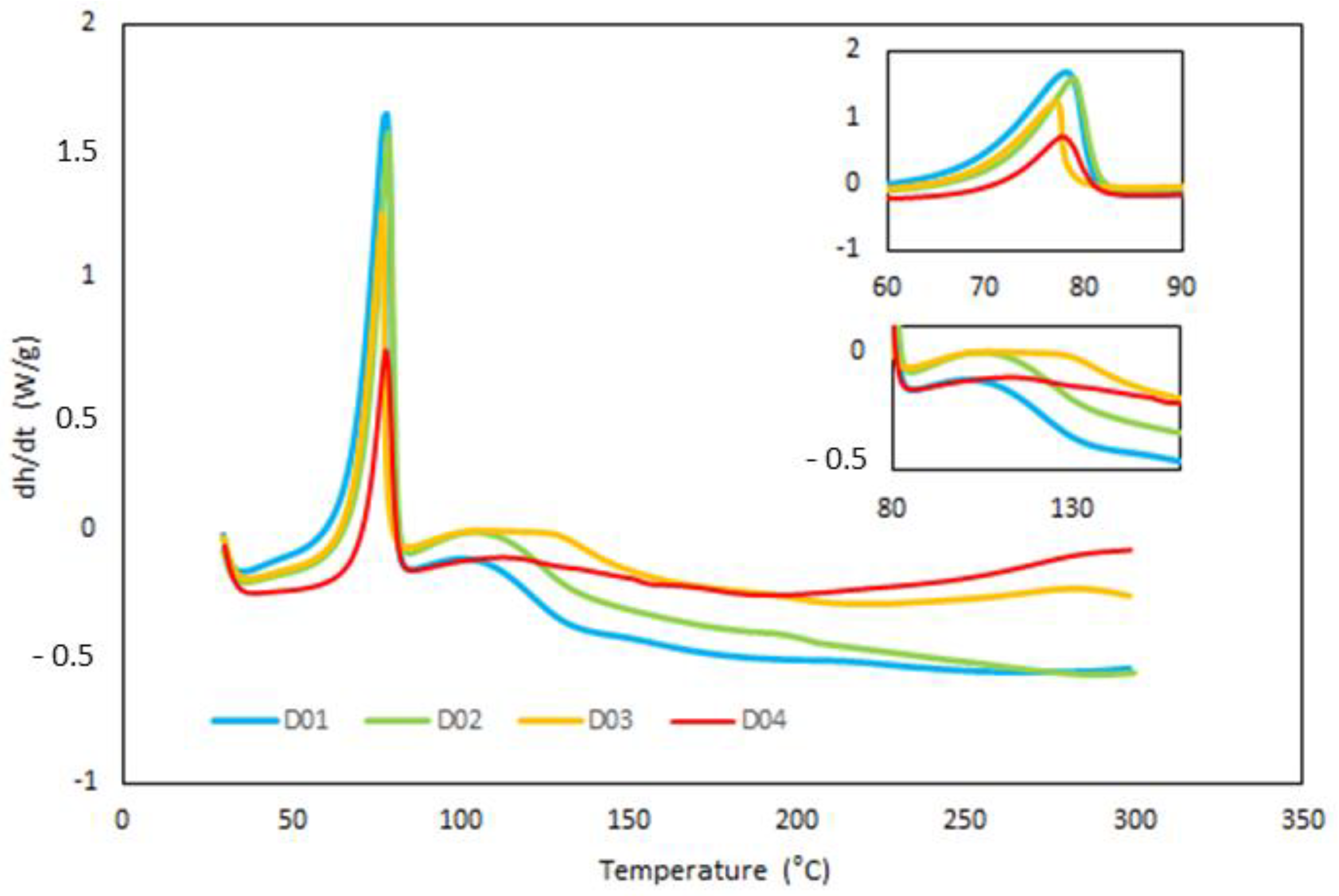

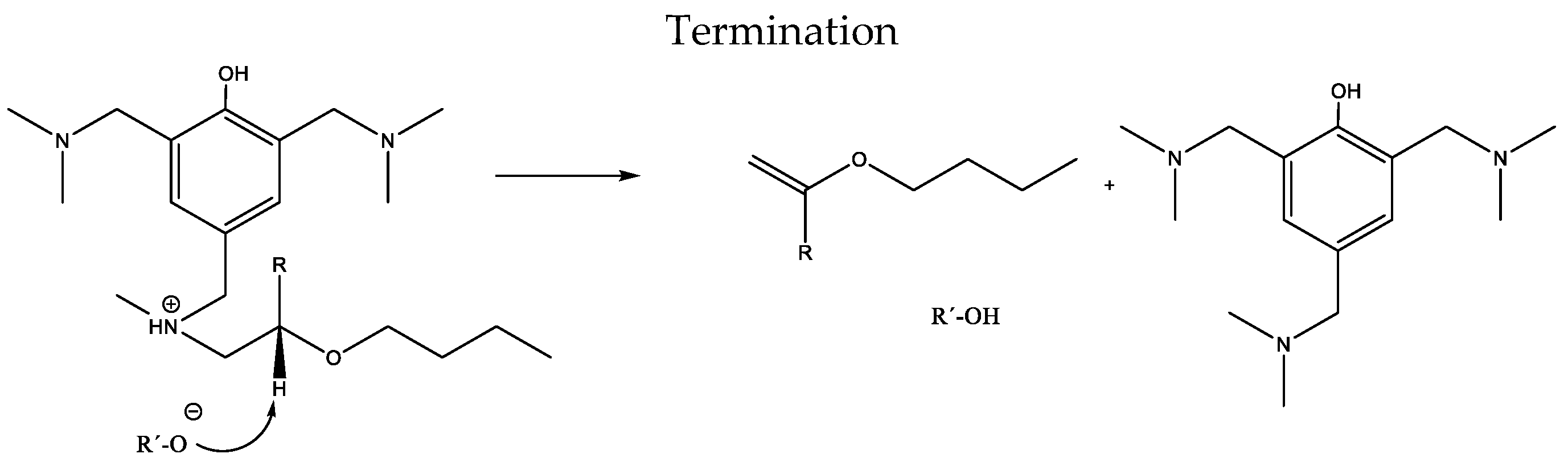
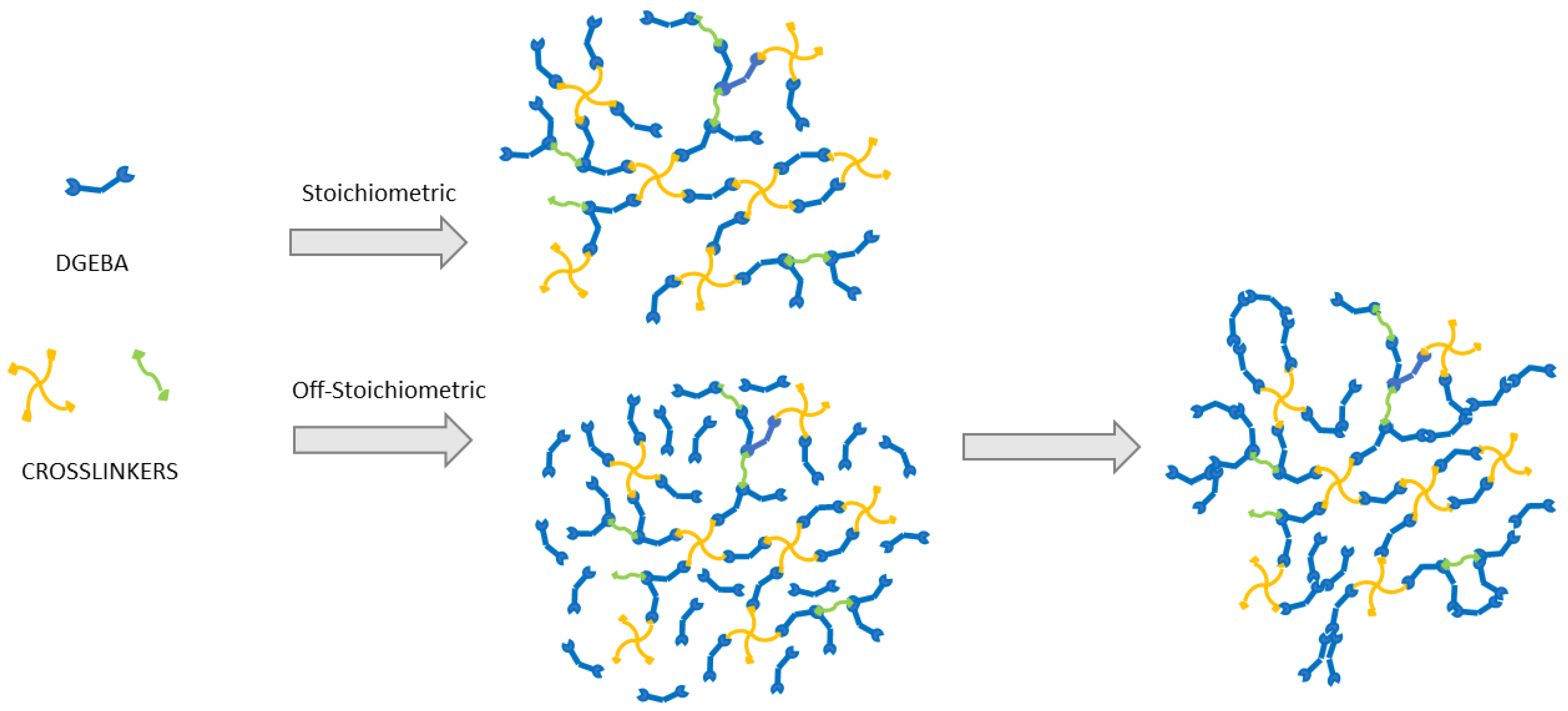


| Sample | Weight Ratio Epoxy/Crosslinker | Cure Time |
|---|---|---|
| D01 (Reference) | 1:1 | r.t. (1 week) |
| D02 | 1.5:1 | r.t. (1 week) |
| D03 | 2:1 | r.t. (1 week) |
| D04 | 4:1 | r.t. (1 week) |
| Sample | First Peak | Second Peak | ||
|---|---|---|---|---|
| ΔHexo (J·g−1) | T°max (°C) | ΔHexo (J·g−1) | T°max (°C) | |
| D01 | 127.2 | 78 | 74.3 | 106 |
| D02 | 99.3 | 79 | 79.4 | 107 |
| D03 | 76.7 | 77 | 10.,7 | 124 |
| D04 | 48.8 | 77 | 49.5 | 128 |
| Sample | Max. Tensile Strength (MPa) | Elongation | Young’s Modulus (MPa) |
|---|---|---|---|
| D01 | 4.9 ± 0.63 | 23.5 ± 1.4 | 15.8 ± 1.1 |
| D03 | 14.3 ± 1.5 | 23.2 ± 2.7 | 59 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruíz de Azúa, O.; Agulló, N.; Arbusà, J.; Borrós, S. Improving Glass Transition Temperature and Toughness of Epoxy Adhesives by a Complex Room-Temperature Curing System by Changing the Stoichiometry. Polymers 2023, 15, 252. https://doi.org/10.3390/polym15020252
Ruíz de Azúa O, Agulló N, Arbusà J, Borrós S. Improving Glass Transition Temperature and Toughness of Epoxy Adhesives by a Complex Room-Temperature Curing System by Changing the Stoichiometry. Polymers. 2023; 15(2):252. https://doi.org/10.3390/polym15020252
Chicago/Turabian StyleRuíz de Azúa, Oiane, Núria Agulló, Jordi Arbusà, and Salvador Borrós. 2023. "Improving Glass Transition Temperature and Toughness of Epoxy Adhesives by a Complex Room-Temperature Curing System by Changing the Stoichiometry" Polymers 15, no. 2: 252. https://doi.org/10.3390/polym15020252
APA StyleRuíz de Azúa, O., Agulló, N., Arbusà, J., & Borrós, S. (2023). Improving Glass Transition Temperature and Toughness of Epoxy Adhesives by a Complex Room-Temperature Curing System by Changing the Stoichiometry. Polymers, 15(2), 252. https://doi.org/10.3390/polym15020252






