Phase Morphology, Mechanical, and Thermal Properties of Calcium Carbonate-Reinforced Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Composites
2.3. Characterization of the Composites
3. Results and Discussion
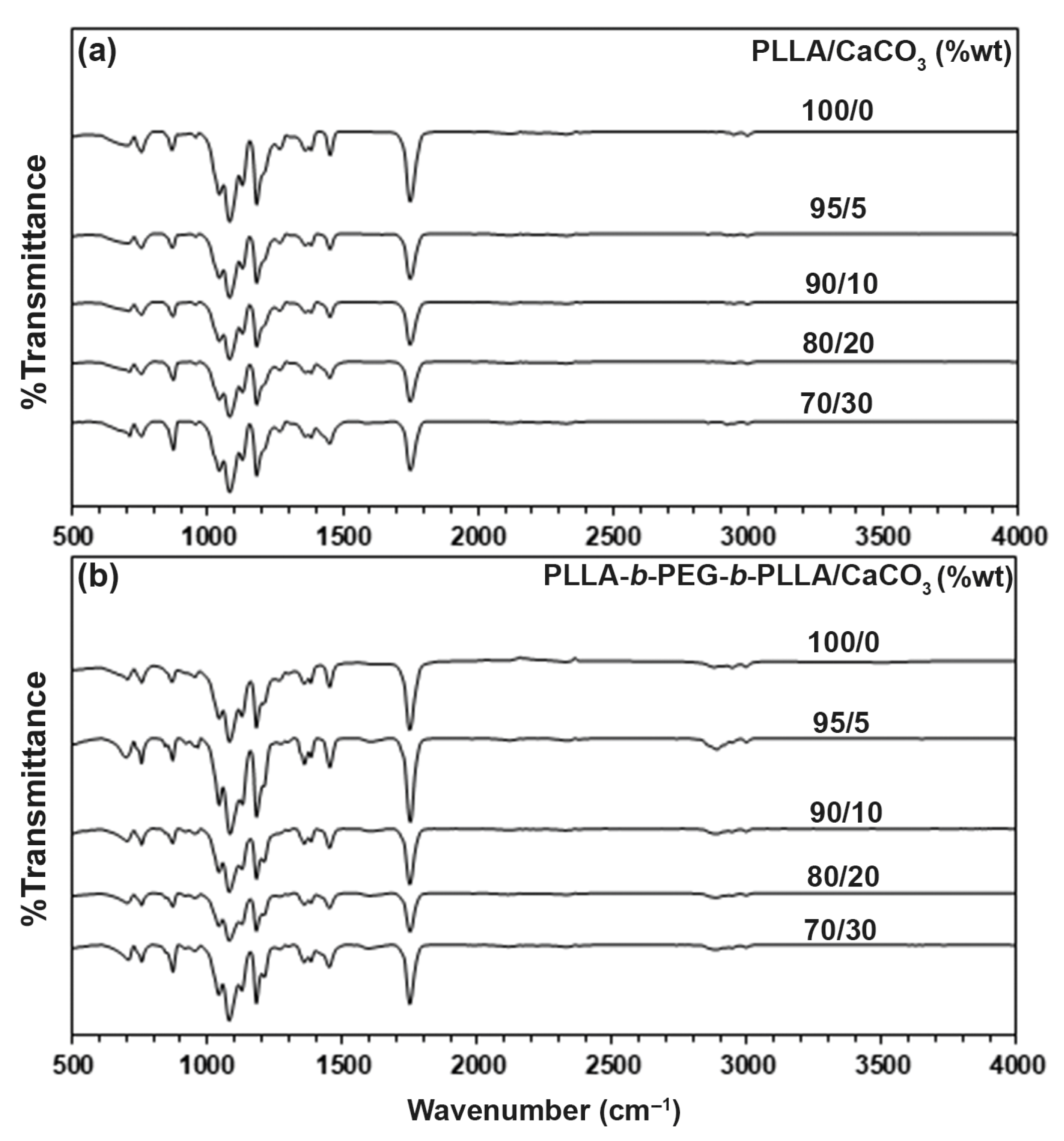
3.1. FTIR
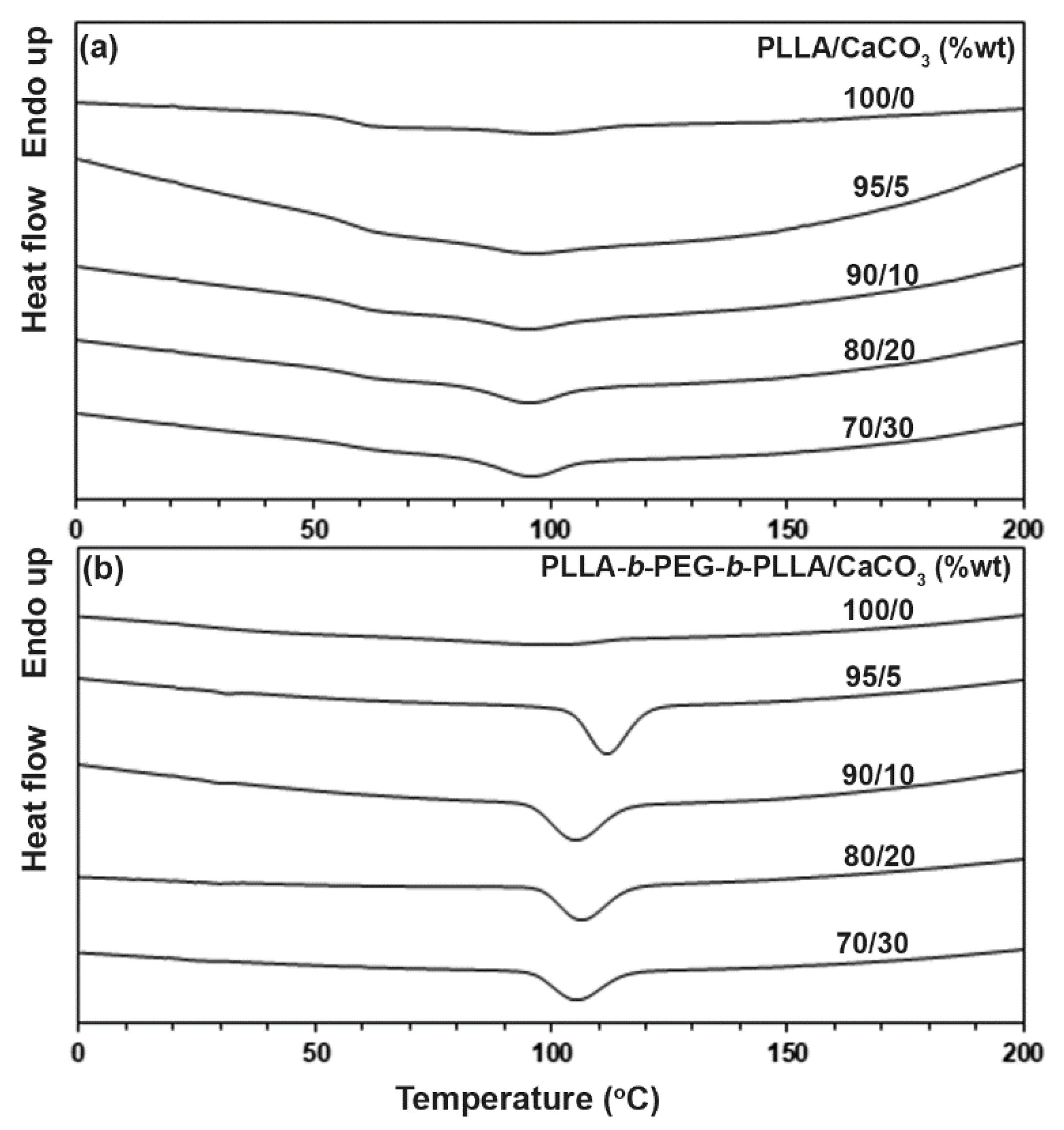
3.2. Thermal Transition Properties
3.3. Thermal Decompositions
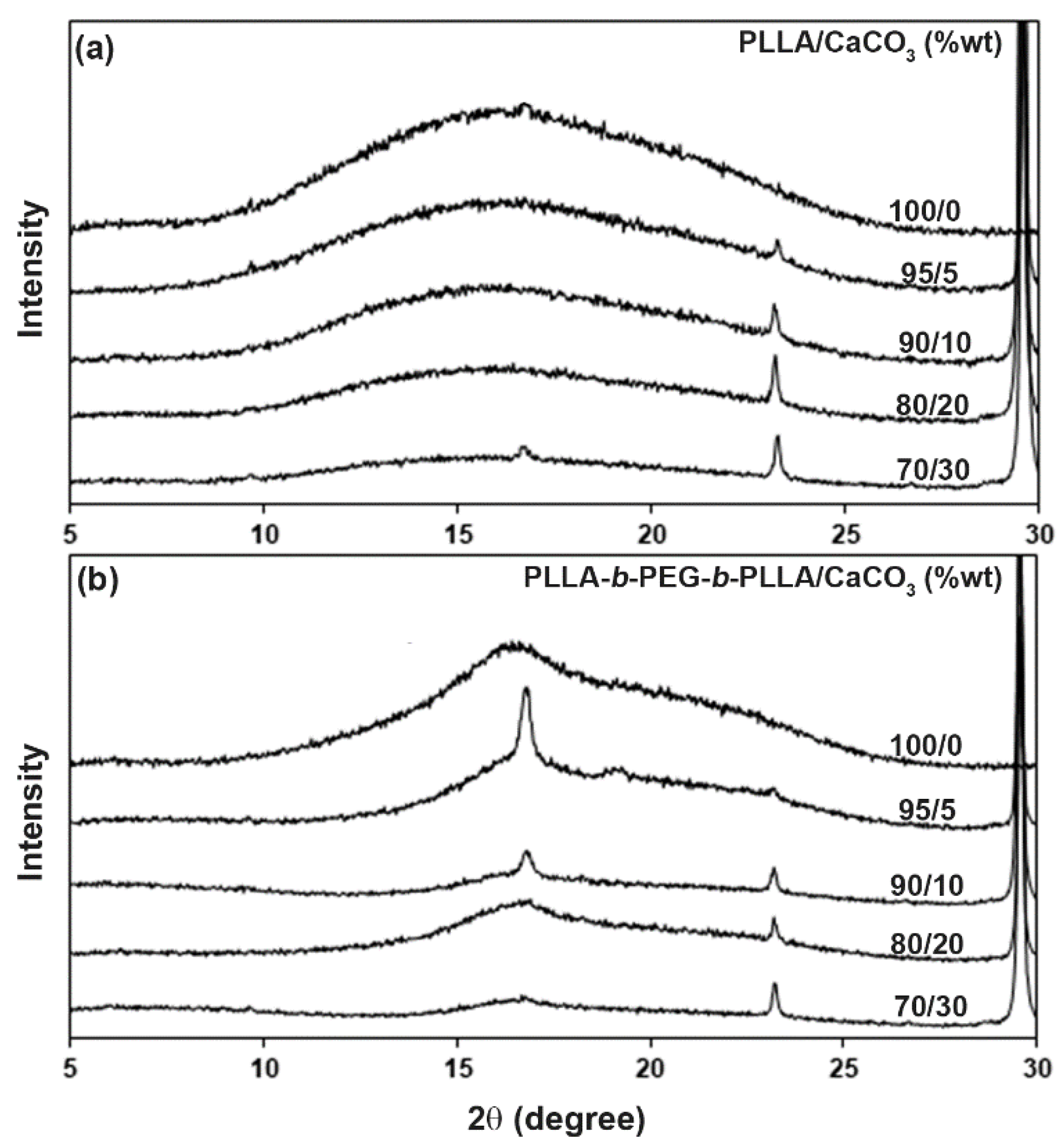
3.4. Crystalline Structures
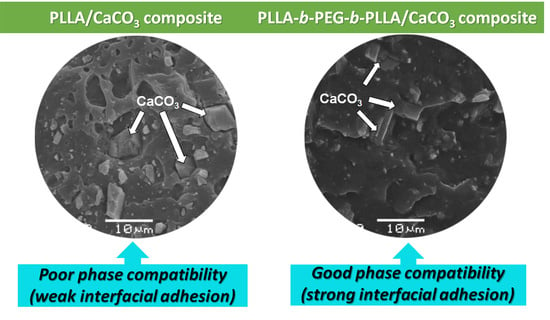
3.5. Phase Morphology
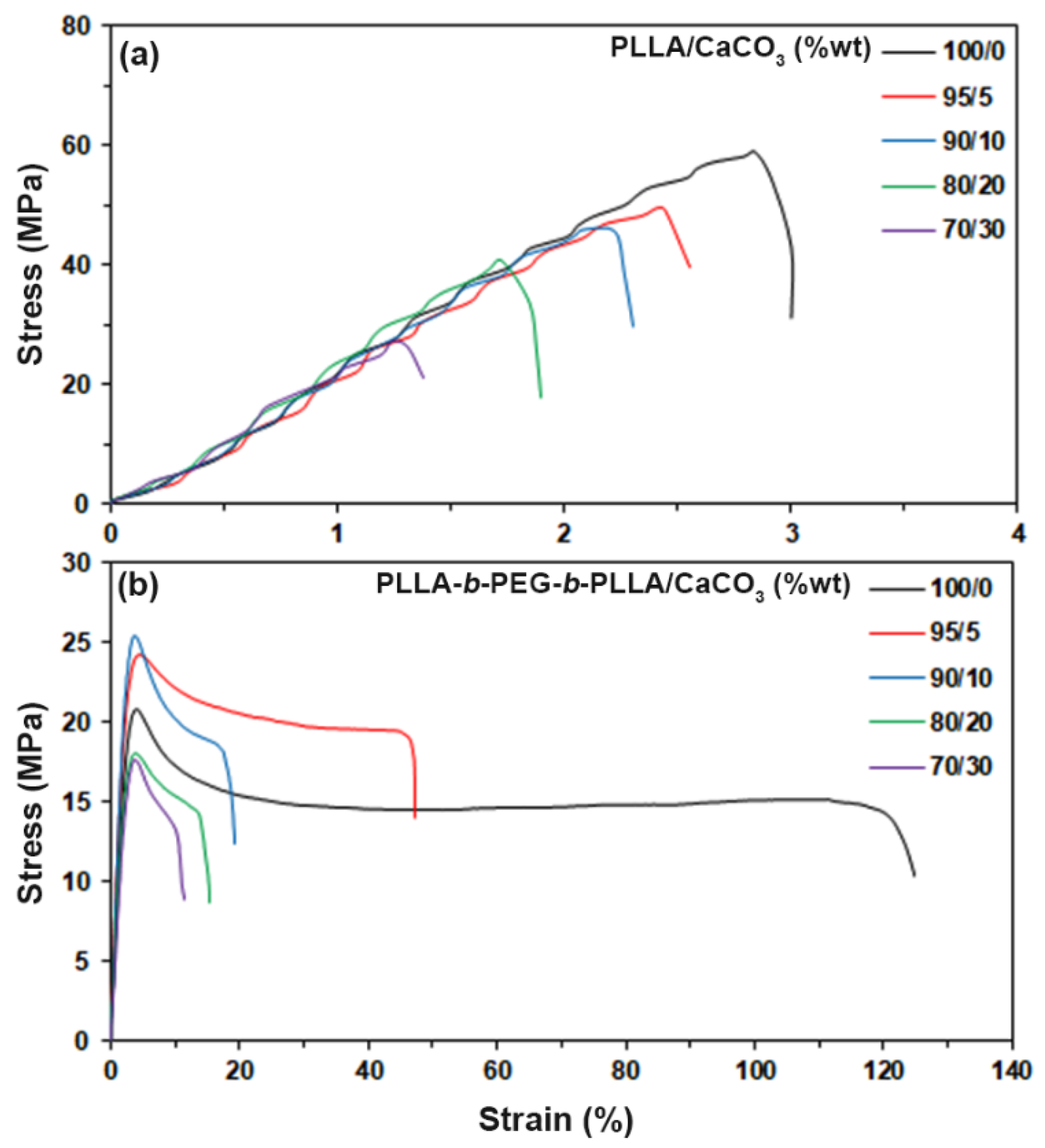
3.6. Tensile Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Tripathi, N.; Misra, M.; Amar, K.; Mohanty, A.K. Durable Polylactic Acid (PLA)-Based Sustainable Engineered Blends and Biocomposites: Recent Developments, Challenges, and Opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Rotiman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Saedi Ardahaei, A.; Dai, Y.; Neisiany, R.E.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The Life Cycle Assessment for Polylactic Acid (PLA) to Make It a Low-Carbon Material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Rihayat, T.; Hadi, A.E.; Aidy, N.; Safitri, A.; Siregar, J.P.; Cionita, T.; Irawan, A.P.; Hamdan, M.H.M.; Fitriyana, D.F. Biodegradation of polylactic acid-based bio-composites reinforced with chitosan and essential oils as anti-microbial material for food packaging. Polymers 2021, 13, 4019. [Google Scholar] [CrossRef]
- Peng, K.; Mubarak, S.; Diao, X.; Cai, Z.; Zhang, C.; Wang, J.; Wu, L. Progress in the Preparation, Properties, and Applications of PLA and Its Composite Microporous Materials by Supercritical CO2: A Review from 2020 to 2022. Polymers 2022, 14, 4320. [Google Scholar] [CrossRef]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Yun, X.; Li, X.; Jin, Y.; Sun, W.; Dong, T. Fast crystallization and toughening of poly(L-lactic acid) by incorporating with poly(ethylene glycol) as a middle block chain. Polym. Sci.—Ser. A 2018, 60, 141–155. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymorama, N. Improvement in melt flow property and flexibility of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) by chain extension reaction for potential use as flexible bioplastics. Mater. Des. 2018, 154, 73–80. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic acid (PLA) biocomposite: Processing, additive manufacturing and advanced applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Rayung, M.; Abu, F.; Ahmad, S.; Fadil, F.; Karim, A.A.; Norizan, M.N.; Sarifuddin, N.; Mat Desa, M.S.Z.; Mohd Basri, M.S.; et al. A Review on Antimicrobial Packaging from Biodegradable Polymer Composites. Polymers 2022, 14, 174. [Google Scholar] [CrossRef]
- Barletta, M.; Puopolo, M. Thermoforming of compostable PLA/PBS blends reinforced with highly hygroscopic calcium carbonate. J. Manuf. Processes 2020, 56 Pt A, 1185–1192. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, B.H.; Choi, J.H.; Yoon, J.-S. Mechanical properties and thermal stability of poly(L-lactide)/calcium carbonate composites. J. Appl. Polym. Sci. 2008, 109, 3087–3092. [Google Scholar] [CrossRef]
- Piekarska, K.; Piorkowska, E.; Bojda, J. The influence of matrix crystallinity, filler grain size and modification on properties of PLA/calcium carbonate composites. Polym. Test. 2017, 62, 203–209. [Google Scholar] [CrossRef]
- Kumar, V.; Dev, A.; Gupta, A.P. Studies of poly(lactic acid) based calcium carbonate nanocomposites. Compos. B Eng. 2014, 56, 184–188. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, G.; Siligardi, C.; Ori, G.; Lazzeri, A. Comparison of precipitated calcium carbonate/polylactic acid and halloysite/polylactic acid nanocomposites. J. Nanomater. 2015, 16, 67. [Google Scholar] [CrossRef]
- Asadi, Z.; Javadi, A.; Mohammadzadeh, F.; Alavi, K. Investigation on the role of nanoclay and nanocalcium carbonate on morphology, rheology, crystallinity and mechanical properties of binary and ternary nanocomposites based on PLA. Int. J. Polym. Anal. Charact. 2021, 26, 1–16. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Baimark, Y. Thermal, morphological and mechanical properties of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide)/thermoplastic starch blends. Carbohydr. Polym. 2022, 283, 119155. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
- Volpe, V.; Filitto, M.; Klofacova, V.; Santis, F.; Roberto Pantani, R. Effect of mold opening on the properties of PLA samples obtained by foam injection molding. Polym. Eng. Sci. 2018, 58, 475–484. [Google Scholar] [CrossRef]
- Baimark, Y.; Srihanam, P.; Srisuwan, Y.; Phromsopha, T. Enhancement in Crystallizability of Poly(L-Lactide) Using Stereocomplex-Polylactide Powder as a Nucleating Agent. Polymers 2022, 14, 4092. [Google Scholar] [CrossRef]
- Cuevas-Carballo, Z.B.; Duarte-Aranda, S.; Canch’ e-Escamilla, G. Properties and biodegradation of thermoplastic starch obtained from grafted starches with poly(lactic acid). J. Polym. Environ. 2019, 27, 2607–2617. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Jiang, S.; Chen, X.; An, L. Crystal structure and morphology influenced by shear effect of poly(l-lactide) and its melting behavior revealed by WAXD, DSC and in-situ POM. Polymer 2011, 52, 3478–3487. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rigid filler toughening in PLA-calcium carbonate composites: Effect of particle surface treatment and matrix plasticization. Eur. Polym. J. 2019, 113, 78–88. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Alba, J.; Tarrés, Q.; Espinach, F.X.; Mutjé, P.; Delgado-Aguilar, M. Characterization of CaCO3 filled poly(lactic) acid and bio polyethylene materials for building applications. Polymers 2021, 13, 3323. [Google Scholar] [CrossRef]
- Mohd Zain, A.H.; Kahar, A.W.M.; Hanafi, I. Solid-state photocross-linking of cassava starch: Improvement properties of thermoplastic starch. Polym. Bull. 2018, 75, 3341–3356. [Google Scholar] [CrossRef]
- Mohd Zain, A.H.; Kahar, A.W.M.; Hanafi, I. Effect of calcium carbonate incorporation on the properties of low linear density polyethylene/thermoplastic starch blends. J. Eng. Sci. 2019, 15, 97–108. [Google Scholar] [CrossRef]
- Bindhu, B.; Renisha, R.; Roberts, L.; Varghese, T.O. Boron nitride reinforced polylactic acid composites film for packaging: Preparation and properties. Polym. Test. 2018, 66, 172–177. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Luo, C.; Chen, Y.; Zhou, Y.; Yao, B.; Dong, L.; Du, X.; Ji, J. Effect of talc and diatomite on compatible, morphological, and mechanical behavior of PLA/PBAT blends. e-Polymers 2021, 21, 234–243. [Google Scholar] [CrossRef]

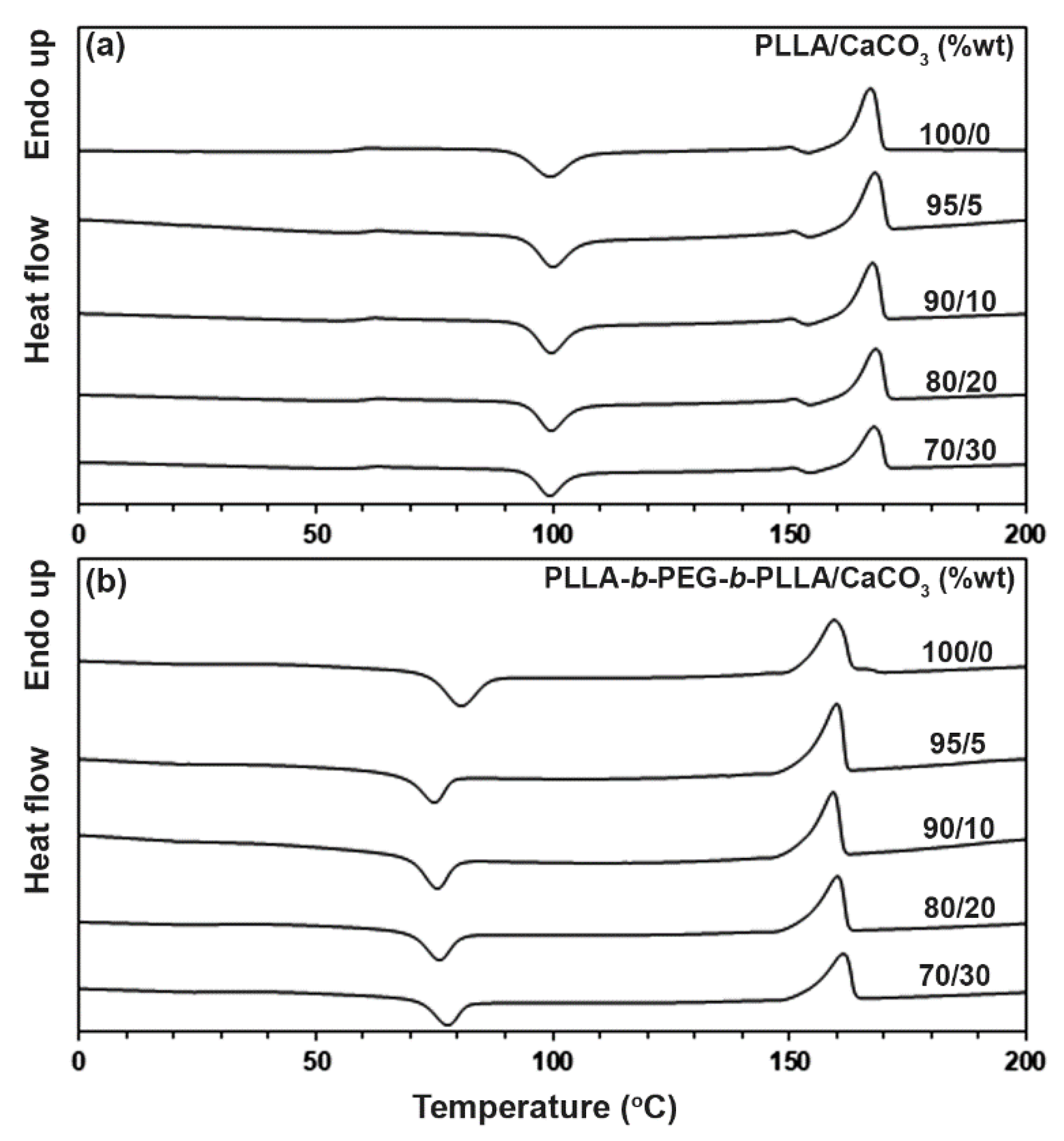



| Sample | Tg (°C) a | Tcc (°C) b | Tm (°C) c | DSC − Xc (%) d |
|---|---|---|---|---|
| PLLA/CaCO3 (%wt) | ||||
| 100/0 | 58 | 100 | 167 | 9.2 |
| 95/5 | 59 | 100 | 168 | 10.4 |
| 90/10 | 59 | 100 | 168 | 9.8 |
| 80/20 | 60 | 100 | 168 | 11.4 |
| 70/30 | 59 | 100 | 168 | 10.2 |
| PLLA-b-PEG-b-PLLA/CaCO3 (%wt) | ||||
| 100/0 | 30 | 81 | 159 | 13.9 |
| 95/5 | 30 | 75 | 160 | 18.4 |
| 90/10 | 30 | 76 | 159 | 18.2 |
| 80/20 | 31 | 76 | 160 | 17.9 |
| 70/30 | 32 | 78 | 161 | 17.1 |
| Sample | Tc (°C) a | ΔHc (J/g) b |
|---|---|---|
| PLLA/CaCO3 (%wt) | ||
| 100/0 | 99 | 5.5 |
| 95/5 | 96 | 5.8 |
| 90/10 | 95 | 5.5 |
| 80/20 | 96 | 6.3 |
| 70/30 | 96 | 6.1 |
| PLLA-b-PEG-b-PLLA/CaCO3 (%wt) | ||
| 100/0 | 99 | 11.7 |
| 95/5 | 112 | 31.7 |
| 90/10 | 106 | 28.1 |
| 80/20 | 105 | 27.5 |
| 70/30 | 105 | 22.9 |
| Sample | 5%–Td (°C) a | Residue Weight at 600 °C (%) b | PLLA–Td,max (°C) c | PEG–Td,max (°C) d |
|---|---|---|---|---|
| PLLA/CaCO3 (%wt) | ||||
| 100/0 | 345 | 0.9 | 379 | - |
| 95/5 | 335 | 5.8 | 369 | - |
| 90/10 | 324 | 9.8 | 362 | - |
| 80/20 | 307 | 21.2 | 355 | - |
| 70/30 | 305 | 28.8 | 351 | - |
| PLLA-b-PEG-b-PLLA/CaCO3 (%wt) | ||||
| 100/0 | 282 | 0.4 | 309 | 421 |
| 95/5 | 302 | 4.7 | 338 | 417 |
| 90/10 | 290 | 10.0 | 335 | 419 |
| 80/20 | 285 | 19.8 | 324 | 417 |
| 70/30 | 276 | 28.7 | 311 | 409 |
| Sample | XRD − Xc (%) a |
|---|---|
| PLLA/CaCO3 (%wt) | |
| 100/0 | - |
| 95/5 | - |
| 90/10 | - |
| 80/20 | - |
| 70/30 | 1.5 |
| PLLA-b-PEG-b-PLLA/CaCO3 (%wt) | |
| 100/0 | 10.8 |
| 95/5 | 16.4 |
| 90/10 | 17.5 |
| 80/20 | 17.6 |
| 70/30 | 18.4 |
| Sample | Stress at Yield (MPa) | Stress at Break (MPa) | Strain at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| PLLA/CaCO3 (%wt) | ||||
| 100/0 | - | 59.4 ± 4.6 | 3.3 ± 1.2 | 923 ± 26 |
| 95/5 | - | 52.1 ± 3.1 | 2.6 ± 0.8 | 817 ± 31 |
| 90/10 | - | 44.8 ± 3.6 | 2.5 ± 1.1 | 605 ± 23 |
| 80/20 | - | 41.7 ± 5.3 | 1.8 ± 0.6 | 425 ± 38 |
| 70/30 | - | 27.4 ± 2.6 | 1.5 ± 0.7 | 332 ± 16 |
| PLLA-b-PEG-b-PLLA/CaCO3 (%wt) | ||||
| 100/0 | 21.8 ± 3.4 | 18.9 ± 3.2 | 108.3 ± 8.6 | 280 ± 27 |
| 95/5 | 25.2 ± 2.6 | 21.6 ± 2.4 | 42.1 ± 5.4 | 396 ± 14 |
| 90/10 | 24.9 ± 4.1 | 18.5 ± 3.6 | 20.7 ± 3.1 | 345 ± 38 |
| 80/20 | 18.5 ± 3.8 | 13.5 ± 2.1 | 17.5 ± 2.5 | 218 ± 23 |
| 70/30 | 17.8 ± 2.7 | 13.2 ± 1.7 | 11.6 ± 2.4 | 219 ± 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srihanam, P.; Thongsomboon, W.; Baimark, Y. Phase Morphology, Mechanical, and Thermal Properties of Calcium Carbonate-Reinforced Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastics. Polymers 2023, 15, 301. https://doi.org/10.3390/polym15020301
Srihanam P, Thongsomboon W, Baimark Y. Phase Morphology, Mechanical, and Thermal Properties of Calcium Carbonate-Reinforced Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastics. Polymers. 2023; 15(2):301. https://doi.org/10.3390/polym15020301
Chicago/Turabian StyleSrihanam, Prasong, Wiriya Thongsomboon, and Yodthong Baimark. 2023. "Phase Morphology, Mechanical, and Thermal Properties of Calcium Carbonate-Reinforced Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastics" Polymers 15, no. 2: 301. https://doi.org/10.3390/polym15020301
APA StyleSrihanam, P., Thongsomboon, W., & Baimark, Y. (2023). Phase Morphology, Mechanical, and Thermal Properties of Calcium Carbonate-Reinforced Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastics. Polymers, 15(2), 301. https://doi.org/10.3390/polym15020301