Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup of the AP Plasma System
2.3. Characterisation of AP-PP-lim Nano-Thin Films
2.4. Microbiological Activity
3. Results and Discussion
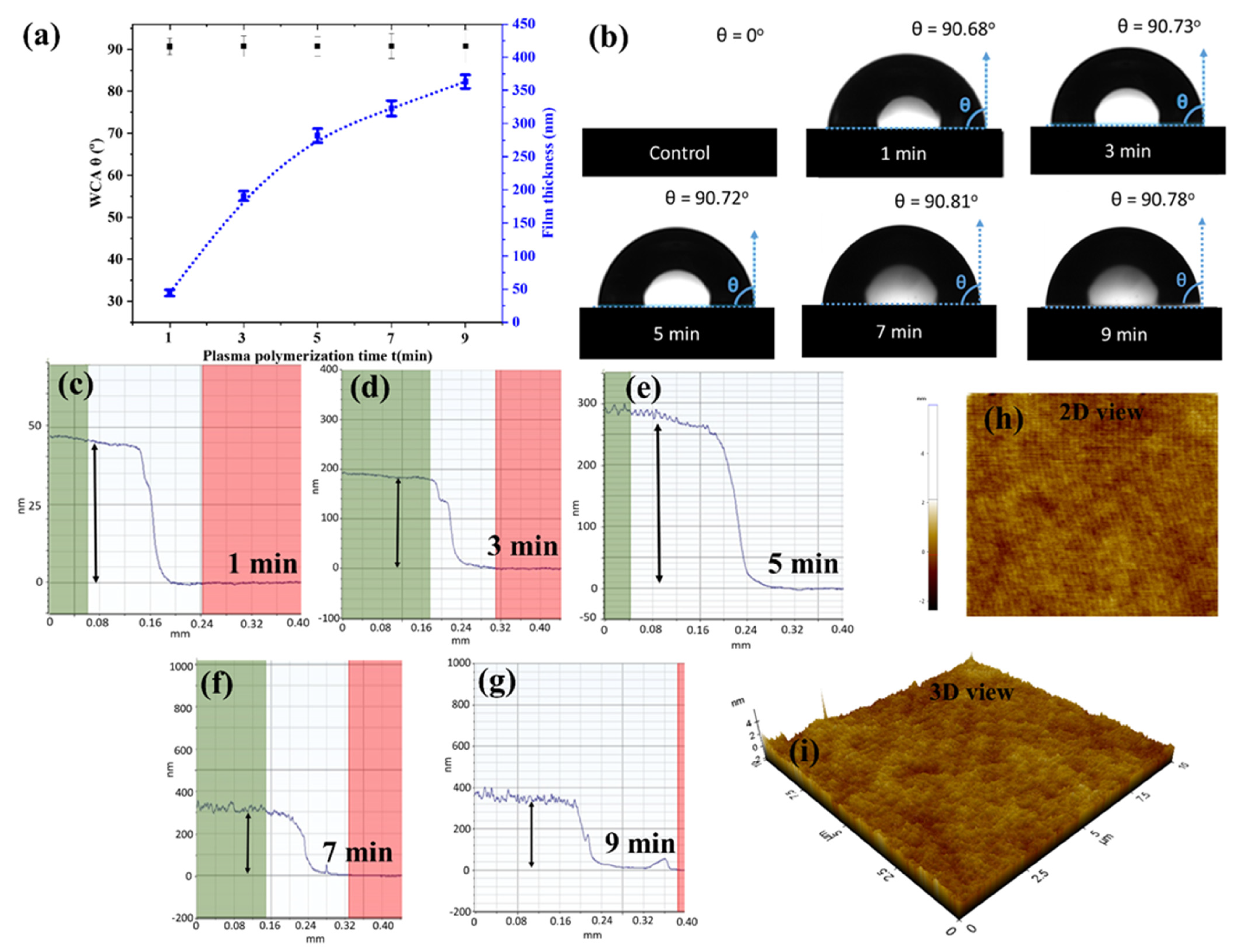
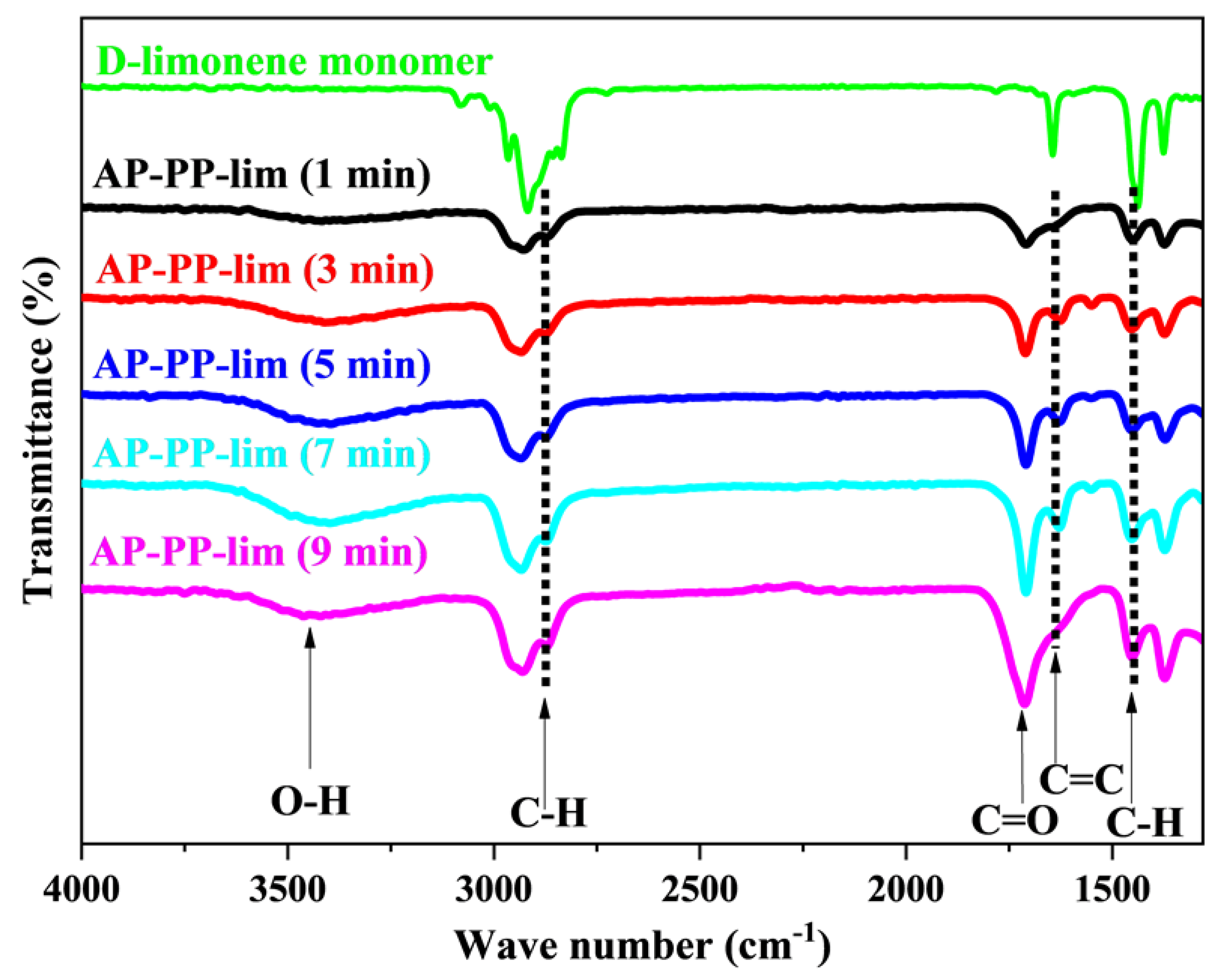
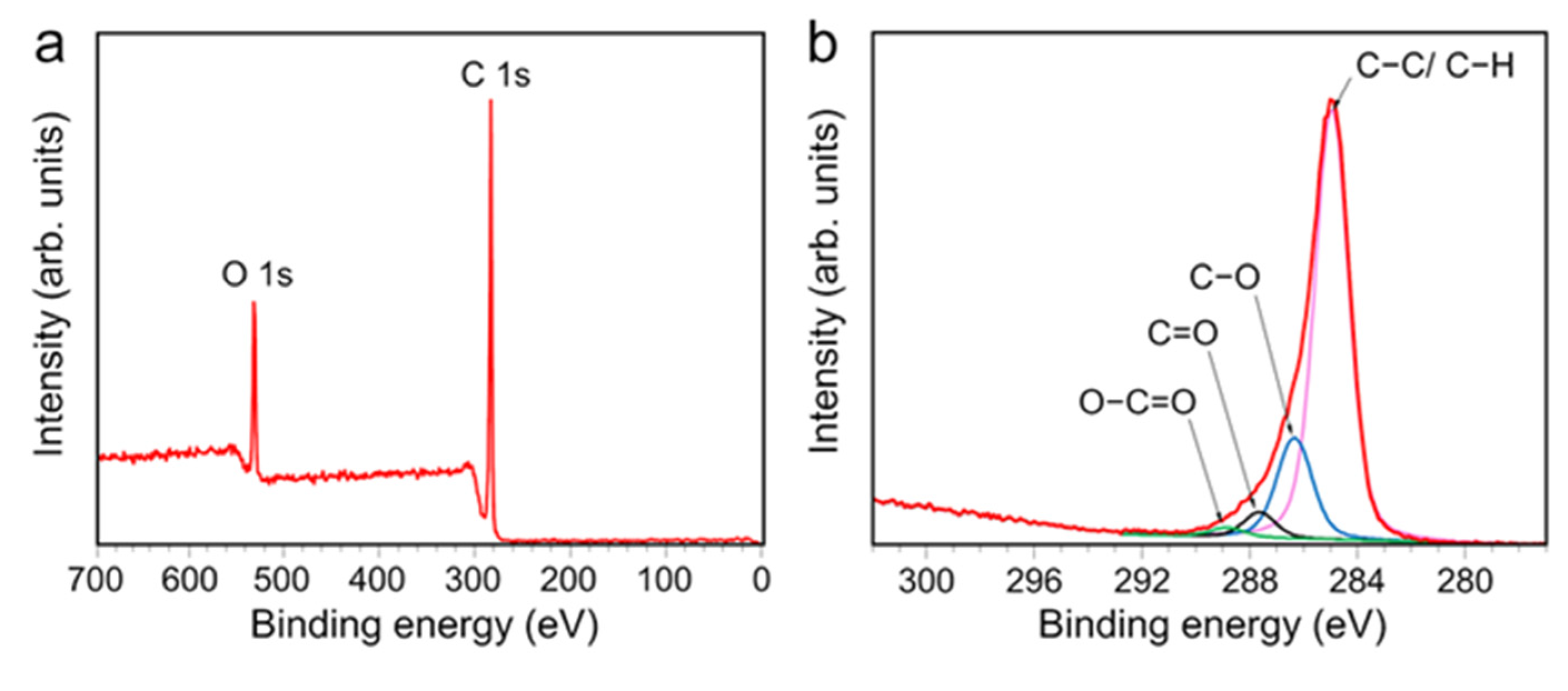
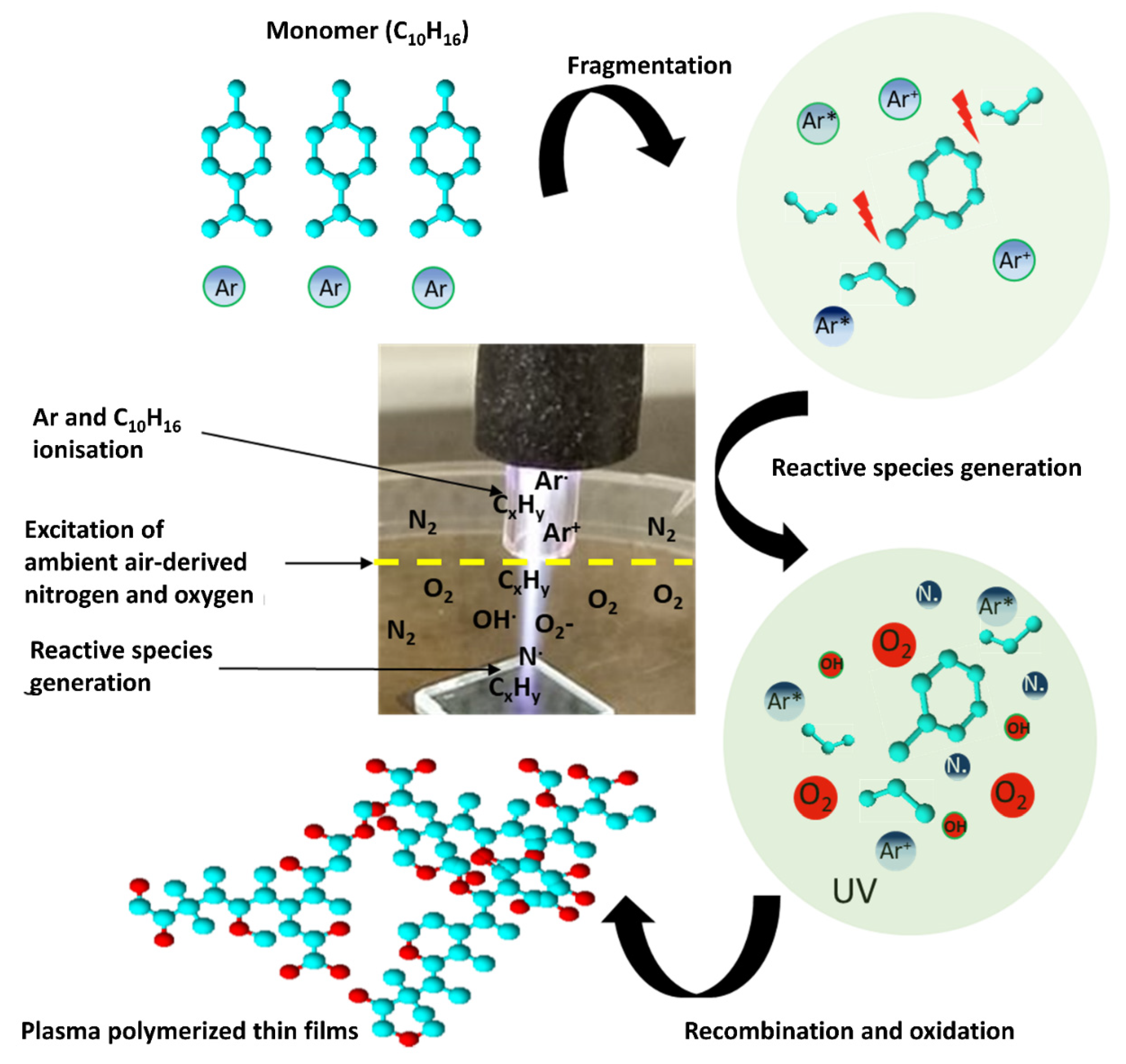
3.1. Characteristics of AP-PP-lim Nano-Thin Films
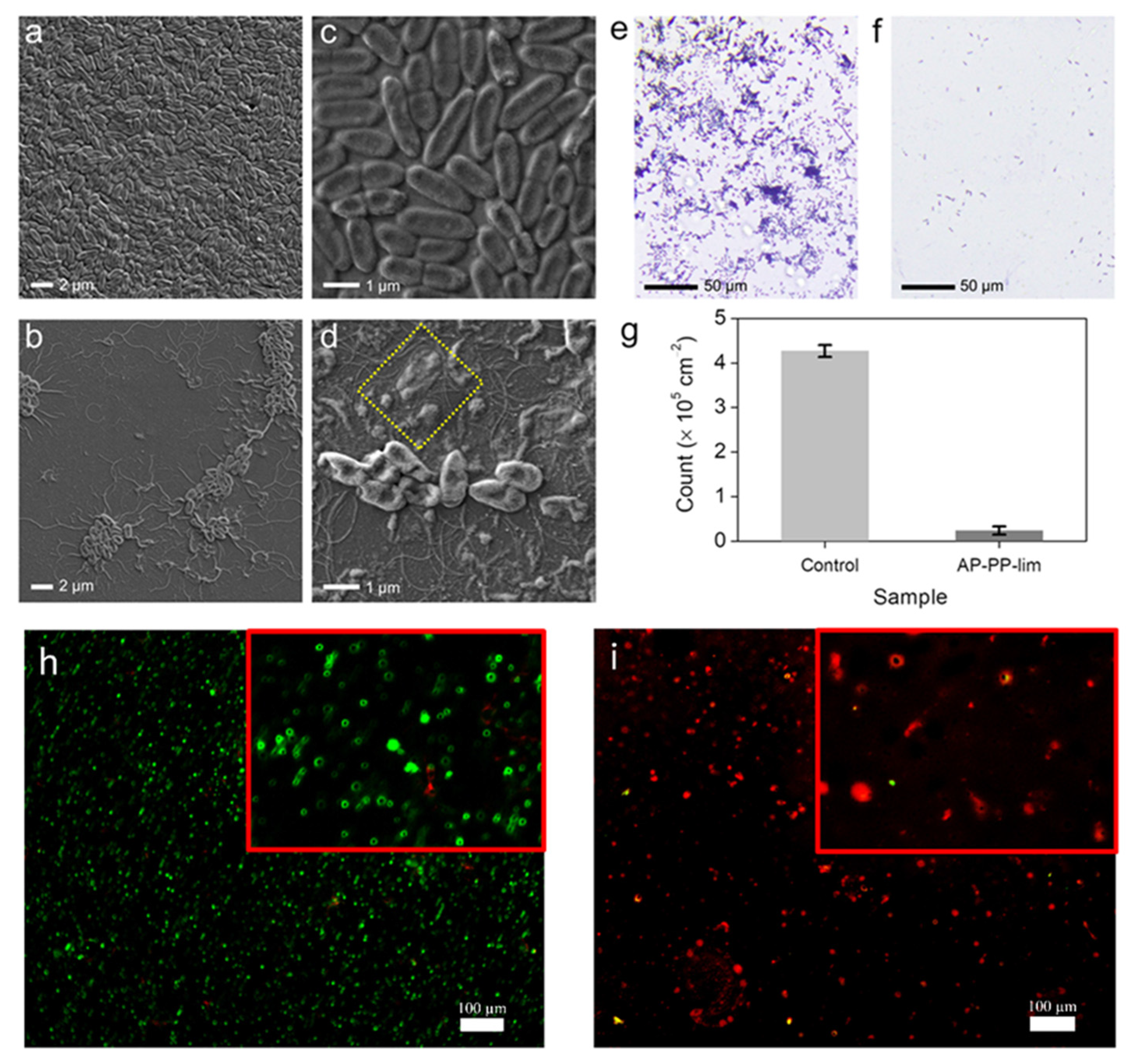
3.2. Antimicrobial Performance of AP-PP-lim Nano-Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, C.H.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant Abiotic Stress Proteomics: The Major Factors Determining Alterations in Cellular Proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Casanovas, H.J.; la Rosa, M.D.; Bello-Lemus, Y.; Rasperini, G.; Acosta-Hoyos, A.J. Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay. Healthcare 2022, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Masood, A.; Ahmed, N.; Mohd Razip Wee, M.F.; Haniff, M.A.S.M.; Mahmoudi, E.; Patra, A.; Siow, K.S. Pulsed Plasma Polymerisation of Carvone: Characterisations and Antibacterial Properties. Surf. Innov. 2022, 40, 1–13. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic Approach of Multifaceted Antibacterial Mechanism of Limonene Traced in Escherichia Coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing Implant-Related Infections: Active Release Strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M. Immobilized Antibiotics to Prevent Orthopaedic Implant Infections. Adv. Drug Del. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials Approaches to Treating Implant-Associated Osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef]
- Cong, Y.; Quan, C.; Liu, M.; Liu, J.; Huang, G.; Tong, G.; Yin, Y.; Zhang, C.; Jiang, Q. Alendronate-Decorated Biodegradable Polymeric Micelles for Potential Bone-Targeted Delivery of Vancomycin. J. Biomater. Sci. Polym. Ed. 2015, 26, 629–643. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun Polyurethane/Keratin/Agnp Biocomposite Mats for Biocompatible and Antibacterial Wound Dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef]
- Fang, B.; Jiang, Y.; Nüsslein, K.; Rotello, V.M.; Santore, M.M. Antimicrobial Surfaces Containing Cationic Nanoparticles: How Immobilized, Clustered, and Protruding Cationic Charge Presentation Affects Killing Activity and Kinetics. Colloids Surf. B. Biointerfaces 2015, 125, 255–263. [Google Scholar] [CrossRef]
- Fuchs, A.D.; Tiller, J.C. Contact-Active Antimicrobial Coatings Derived from Aqueous Suspensions. Angew. Chem. Int. Ed. 2006, 45, 6759–6762. [Google Scholar] [CrossRef]
- Milović, N.M.; Wang, J.; Lewis, K.; Klibanov, A.M. Immobilized N-Alkylated Polyethylenimine Avidly Kills Bacteria by Rupturing Cell Membranes with No Resistance Developed. Biotechnol. Bioeng. 2005, 90, 715–722. [Google Scholar] [CrossRef]
- Förch, R.; Chifen, A.N.; Bousquet, A.; Khor, H.L.; Jungblut, M.; Chu, L.Q.; Zhang, Z.; Osey-Mensah, I.; Sinner, E.K.; Knoll, W. Recent and Expected Roles of Plasma-Polymerized Films for Biomedical Applications. Chem. Vap. Deposition 2007, 13, 280–294. [Google Scholar] [CrossRef]
- Franklin, A.D. Nanomaterials in Transistors: From High-Performance to Thin-Film Applications. Science 2015, 349, aab2750. [Google Scholar] [CrossRef]
- Montemor, M.d.F. Functional and Smart Coatings for Corrosion Protection: A Review of Recent Advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Zare, M.; Zare, M.; Butler, J.A.; Ramakrishna, S. Nanoscience-Led Antimicrobial Surface Engineering to Prevent Infections. ACS Appl. Nano Mater. 2021, 4, 4269–4283. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive Antifouling and Active Self-Disinfecting Antiviral Surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Ohl, A.; Lüthen, F.; Bergemann, C.; Nebe, B.; Rychly, J.; Walschus, U.; Schlosser, M.; Liefeith, K. Capability of Differently Charged Plasma Polymer Coatings for Control of Tissue Interactions with Titanium Surfaces. J. Adhes. Sci. Technol. 2010, 24, 1191–1205. [Google Scholar] [CrossRef]
- Cao, N.; Miao, Y.; Zhang, D.; Boukherroub, R.; Lin, X.; Ju, H.; Li, H. Preparation of Mussel-Inspired Perfluorinated Polydopamine Film on Brass Substrates: Superhydrophobic and Anti-Corrosion Application. Prog. Org. Coat. 2018, 125, 109–118. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Y.; Sun, C.; Yu, S.; Liu, M.; Gao, C. Thin-Film Composite Membranes Formed by Interfacial Polymerization with Natural Material Sericin and Trimesoyl Chloride for Nanofiltration. J. Membr. Sci. 2014, 471, 381–391. [Google Scholar] [CrossRef]
- Gupta, P.; Nayak, K.K. Characteristics of Protein-Based Biopolymer and Its Application. Polym. Eng. Sci. 2015, 55, 485–498. [Google Scholar] [CrossRef]
- Woloszyn, J.D.; Hesse, P.; Hungenberg, K.D.; McAuley, K.B. Parameter Selection and Estimation Techniques in a Styrene Polymerization Model. Macromol. React. Eng. 2013, 7, 293–310. [Google Scholar] [CrossRef]
- Yasuda, H.; Bumgarner, M.; Marsh, H.; Morosoff, N. Plasma Polymerization of Some Organic Compounds and Properties of the Polymers. J. Polym. Sci., Polym. Chem. Ed. 1976, 14, 195–224. [Google Scholar] [CrossRef]
- Kumar, A.; Al-Jumaili, A.; Prasad, K.; Bazaka, K.; Mulvey, P.; Warner, J.; Jacob, M.V. Pulse Plasma Deposition of Terpinen-4-Ol: An Insight into Polymerization Mechanism and Enhanced Antibacterial Response of Developed Thin Films. Plasma Chem. Plasma Process. 2020, 40, 339–355. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Bazaka, K.; Jacob, M.V. Retention of Antibacterial Activity in Geranium Plasma Polymer Thin Films. Nanomaterials 2017, 7, 270. [Google Scholar] [CrossRef]
- Chan, Y.W.; Siow, K.S.; Ng, P.Y.; Gires, U.; Majlis, B.Y. Plasma Polymerized Carvone as an Antibacterial and Biocompatible Coating. Mater. Sci. Eng. C 2016, 68, 861–871. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Zhai, C.; Hung, J.; Shineh, G.; Stewart, C.A.C.; Fadzil, A.A.; Ionescu, M.; Gan, Y.; Wise, S.G.; Akhavan, B. Mechanically Robust Nitrogen-Rich Plasma Polymers: Biofunctional Interfaces for Surface Engineering of Biomedical Implants. Mater. Today Adv. 2021, 12, 100188. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Arefi-Khonsari, F. Low and Atmospheric Plasma Polymerisation of Nanocoatings for Bio-Applications. Surf. Innov. 2015, 3, 63–83. [Google Scholar] [CrossRef]
- Deng, X.; Leys, C.; Vujosevic, D.; Vuksanovic, V.; Cvelbar, U.; De Geyter, N.; Morent, R.; Nikiforov, A. Engineering of Composite Organosilicon Thin Films with Embedded Silver Nanoparticles Via Atmospheric Pressure Plasma Process for Antibacterial Activity. Plasma Process. Polym. 2014, 11, 921–930. [Google Scholar] [CrossRef]
- Khan, M.; Rehman, N.; Khan, S.; Ullah, N.; Masood, A.; Ullah, A. Spectroscopic Study of Co2 and Co2–N2 Mixture Plasma Using Dielectric Barrier Discharge. AIP Adv. 2019, 9, 085015. [Google Scholar] [CrossRef]
- Lou, B.-S.; Lai, C.-H.; Chu, T.-P.; Hsieh, J.-H.; Chen, C.-M.; Su, Y.-M.; Hou, C.-W.; Chou, P.-Y.; Lee, J.-W. Parameters Affecting the Antimicrobial Properties of Cold Atmospheric Plasma Jet. J. Clin. Med. Res. 2019, 8, 1930. [Google Scholar] [CrossRef]
- Ma, C.; Nikiforov, A.; De Geyter, N.; Morent, R.; Ostrikov, K.K. Plasma for Biomedical Decontamination: From Plasma-Engineered to Plasma-Active Antimicrobial Surfaces. Curr. Opin. Chem. Eng. 2022, 36, 100764. [Google Scholar] [CrossRef]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical Applications of Cold Atmospheric Plasma: State of the Science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of in-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef]
- Gerchman, D.; Bones, B.; Pereira, M.; Takimi, A. Thin Film Deposition by Plasma Polymerization Using D-Limonene as a Renewable Precursor. Prog. Org. Coat. 2019, 129, 133–139. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H. Qcm-D and Xps Study of Protein Adsorption on Plasma Polymers with Sulfonate and Phosphonate Surface Groups. Colloids Surf. B. Biointerfaces 2019, 173, 447–453. [Google Scholar] [CrossRef]
- Ahmed, N.; Masood, A.; Siow, K.S.; Wee, M.; Haron, F.F.; Patra, A.; Nayan, N.; Soon, C.F.J.P.C.; Processing, P. Effects of Oxygen (O2) Plasma Treatment in Promoting the Germination and Growth of Chili. Plasma Chem. Plasma Process. 2022, 42, 91–108. [Google Scholar] [CrossRef]
- Madkour, A.E.; Tew, G.N. Towards Self-Sterilizing Medical Devices: Controlling Infection. Polym. Int. 2008, 57, 6–10. [Google Scholar] [CrossRef]
- Moyes, R.B.; Reynolds, J.; Breakwell, D.P. Differential Staining of Bacteria: Gram Stain. Curr. Protoc. Microbiol. 2009, 15, A.3C.1–A.3C.8. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Ju, M.; Milbrandt, N.B.; Tsai, Y.H.; Navarreto-Lugo, M.; Visperas, A.; Klika, A.; Barsoum, W.; Higuera-Rueda, C.A.; Samia, A.C.S. Photoactivated Gold Nanorod Hydrogel Composite Containing D-Amino Acids for the Complete Eradication of Bacterial Biofilms on Metal Alloy Implant Materials. ACS Appl. Nano Mater. 2020, 3, 5862–5873. [Google Scholar] [CrossRef]
- Srinivasan, S.; McKinley, G.H.; Cohen, R.E. Assessing the Accuracy of Contact Angle Measurements for Sessile Drops on Liquid-Repellent Surfaces. Langmuir 2011, 27, 13582–13589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sang, D.K.; Du, Z.; Zhang, C.; Tian, M.; Mi, J. Interfacial Structures, Surface Tensions, and Contact Angles of Diiodomethane on Fluorinated Polymers. J. Phys. Chem. C 2014, 118, 10143–10152. [Google Scholar] [CrossRef]
- Belibel, R.; Avramoglou, T.; Garcia, A.; Barbaud, C.; Mora, L. Effect of Chemical Heterogeneity of Biodegradable Polymers on Surface Energy: A Static Contact Angle Analysis of Polyester Model Films. Mater. Sci. Eng. C 2016, 59, 998–1006. [Google Scholar] [CrossRef]
- Fahmy, A.; Mix, R.; Schönhals, A.; Friedrich, J. Surface and Bulk Structure of Thin Spin Coated and Plasma-Polymerized Polystyrene Films. Plasma Chem. Plasma Process. 2012, 32, 767–780. [Google Scholar] [CrossRef]
- Jacob, M.V.; Easton, C.D.; Anderson, L.J.; Bazaka, K. Rf Plasma Polymerised Thin Films from Natural Resources. Int. J. Mod. Phys. Conf. Ser. 2014, 32, 1460319. [Google Scholar] [CrossRef]
- Zhao, C.; Li, L.; Wang, Q.; Yu, Q.; Zheng, J. Effect of Film Thickness on the Antifouling Performance of Poly (Hydroxy-Functional Methacrylates) Grafted Surfaces. Langmuir 2011, 27, 4906–4913. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface Characteristics Influencing Bacterial Adhesion to Polymeric Substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Derdar, H.; Belbachir, M.; Harrane, A. A Green Synthesis of Polylimonene Using Maghnite-H+, an Exchanged Montmorillonite Clay, as Eco-Catalyst. Bull. Chem. React. Eng. Catal. 2019, 14, 69–78. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Polymers Containing Sulfur and Their Co-Polymers with 1, 7-Octadiene: Chemical and Structural Analysis. Plasma Process. Polym. 2017, 14, 1600044. [Google Scholar] [CrossRef]
- Palaniappan, S.; Narayana, B. Temperature Effect on Conducting Polyaniline Salts: Thermal and Spectral Studies. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2431–2436. [Google Scholar] [CrossRef]
- Burkey, D.D.; Gleason, K.K. Structure and Thermal Properties of Thin Film Poly (A-Methylstyrene) Deposited Via Plasma-Enhanced Chemical Vapor Deposition. Chem. Vap. Depos. 2003, 9, 65–71. [Google Scholar] [CrossRef]
- Easton, C.; Jacob, M. Ageing and Thermal Degradation of Plasma Polymerised Thin Films Derived from Lavandula Angustifolia Essential Oil. Polym. Degrad. Stab. 2009, 94, 597–603. [Google Scholar] [CrossRef]
- Clouet, F.; Shi, M. Interactions of Polymer Model Surfaces with Cold Plasmas: Hexatriacontane as a Model Molecule of High-Density Polyethylene and Octadecyl Octadecanoate as a Model of Polyester. I. Degradation Rate Versus Time and Power. J. Appl. Polym. Sci. 1992, 46, 1955–1966. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Post-Deposition Ageing Reactions of Plasma Derived Polyterpenol Thin Films. Polym. Degrad. Stab. 2010, 95, 1123–1128. [Google Scholar] [CrossRef]
- Alancherry, S.; Bazaka, K.; Jacob, M.V. Rf Plasma Polymerization of Orange Oil and Characterization of the Polymer Thin Films. J. Polym. Environ. 2018, 26, 2925–2933. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Shanks, R.A. Fabrication and Characterization of Rf Plasma Polymerized Thin Films from 3, 7-Dimethyl-1, 6-Octadien-3-Ol for Electronic and Biomaterial Applications. Adv. Mater. Res. 2010, 123, 323–326. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of Plasma Polymerization–Reviewed from a Chemical Point of View. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Drábik, M.; Polonskyi, O.; Kylián, O.; Čechvala, J.; Artemenko, A.; Gordeev, I.; Choukourov, A.; Slavínská, D.; Matolínová, I.; Biederman, H. Super-Hydrophobic Coatings Prepared by Rf Magnetron Sputtering of Ptfe. Plasma Process. Polym. 2010, 7, 544–551. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Whittle, J.D.; Michelmore, A.; Jacob, M.V. Structural Characterization of Γ-Terpinene Thin Films Using Mass Spectroscopy and X-Ray Photoelectron Spectroscopy. Plasma Process. Polym. 2015, 12, 1085–1094. [Google Scholar] [CrossRef]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Kim, D.Y.; Lee, H.-K.; Shin, B.J.; Lee, D.H.; Tae, H.-S. Atmospheric Pressure Plasma Polymerization Synthesis and Characterization of Polyaniline Films Doped with and without Iodine. Materials 2017, 10, 1272. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellvi, S.; Pagán, R.; Garcia-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus Aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Efflux Pumps of Gram-Negative Bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [CrossRef]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-Inspired Cell-Instructive Nanopatterned Arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Elimelech, M. Antibacterial Activity of Electrospun Polymer Mats with Incorporated Narrow Diameter Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces. 2011, 3, 462–468. [Google Scholar] [CrossRef]
- Lee, S.B.; Koepsel, R.R.; Morley, S.W.; Matyjaszewski, K.; Sun, Y.; Russell, A.J. Permanent, Nonleaching Antibacterial Surfaces. 1. Synthesis by Atom Transfer Radical Polymerization. Biomacromolecules 2004, 5, 877–882. [Google Scholar] [CrossRef]
- Kumar, V.; Pulpytel, J.; Giudetti, G.; Rauscher, H.; Rossi, F.; Arefi-Khonsari, F. Amphiphilic Copolymer Coatings Via Plasma Polymerisation Process: Switching and Anti-Biofouling Characteristics. Plasma Process. Polym. 2011, 8, 373–385. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, A.; Ahmed, N.; Razip Wee, M.F.M.; Patra, A.; Mahmoudi, E.; Siow, K.S. Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity. Polymers 2023, 15, 307. https://doi.org/10.3390/polym15020307
Masood A, Ahmed N, Razip Wee MFM, Patra A, Mahmoudi E, Siow KS. Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity. Polymers. 2023; 15(2):307. https://doi.org/10.3390/polym15020307
Chicago/Turabian StyleMasood, Asad, Naeem Ahmed, M. F. Mohd Razip Wee, Anuttam Patra, Ebrahim Mahmoudi, and Kim S. Siow. 2023. "Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity" Polymers 15, no. 2: 307. https://doi.org/10.3390/polym15020307
APA StyleMasood, A., Ahmed, N., Razip Wee, M. F. M., Patra, A., Mahmoudi, E., & Siow, K. S. (2023). Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity. Polymers, 15(2), 307. https://doi.org/10.3390/polym15020307







