Multivariate Analysis Applied to Microwave-Driven Cyanide Polymerization: A Statistical View of a Complex System
Abstract
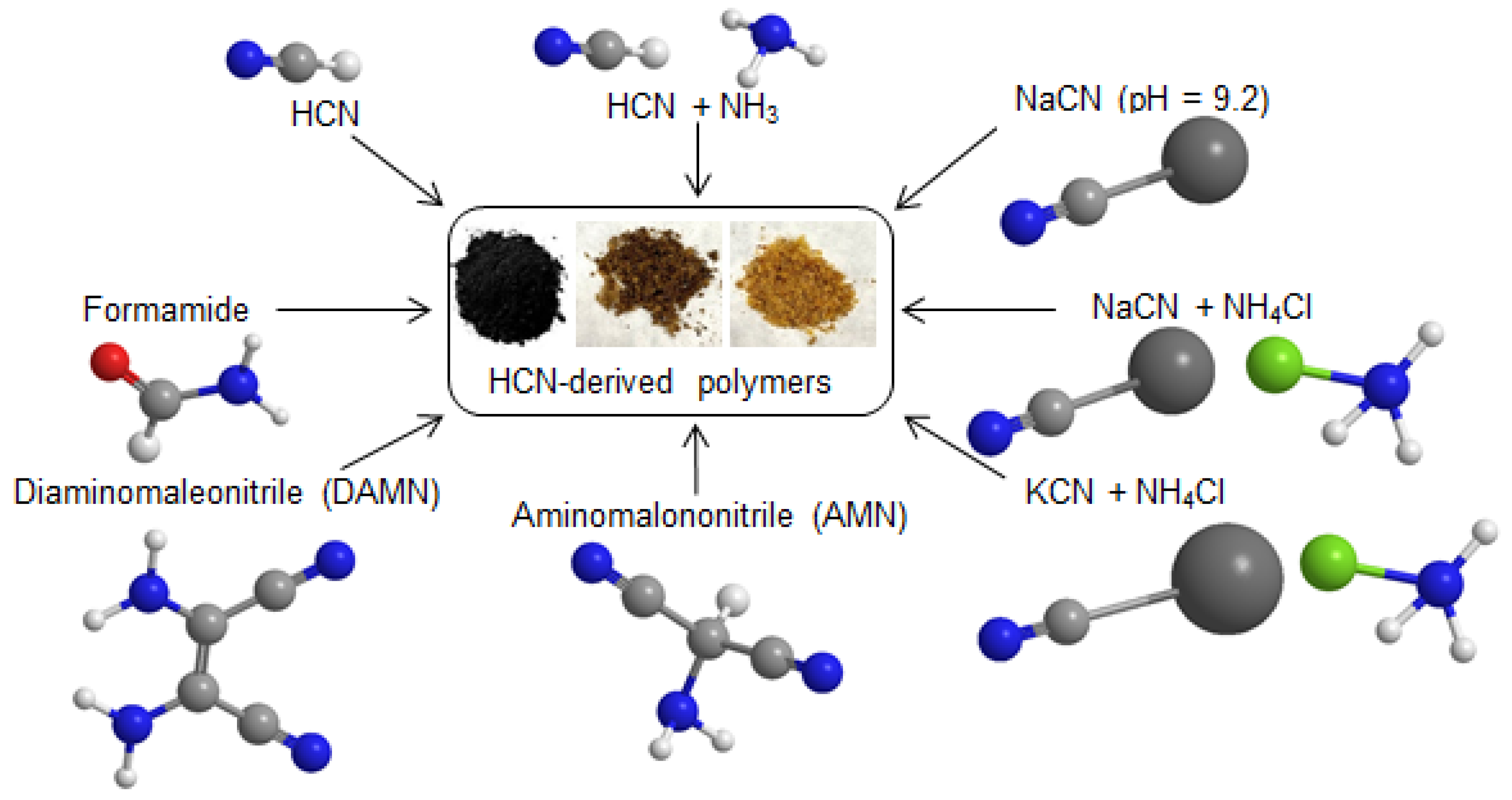
1. Introduction
2. Materials and Methods
2.1. Synthesis of the NH4CN Polymers
2.2. Instrumental Analysis
2.3. Statistical Analysis
3. Results
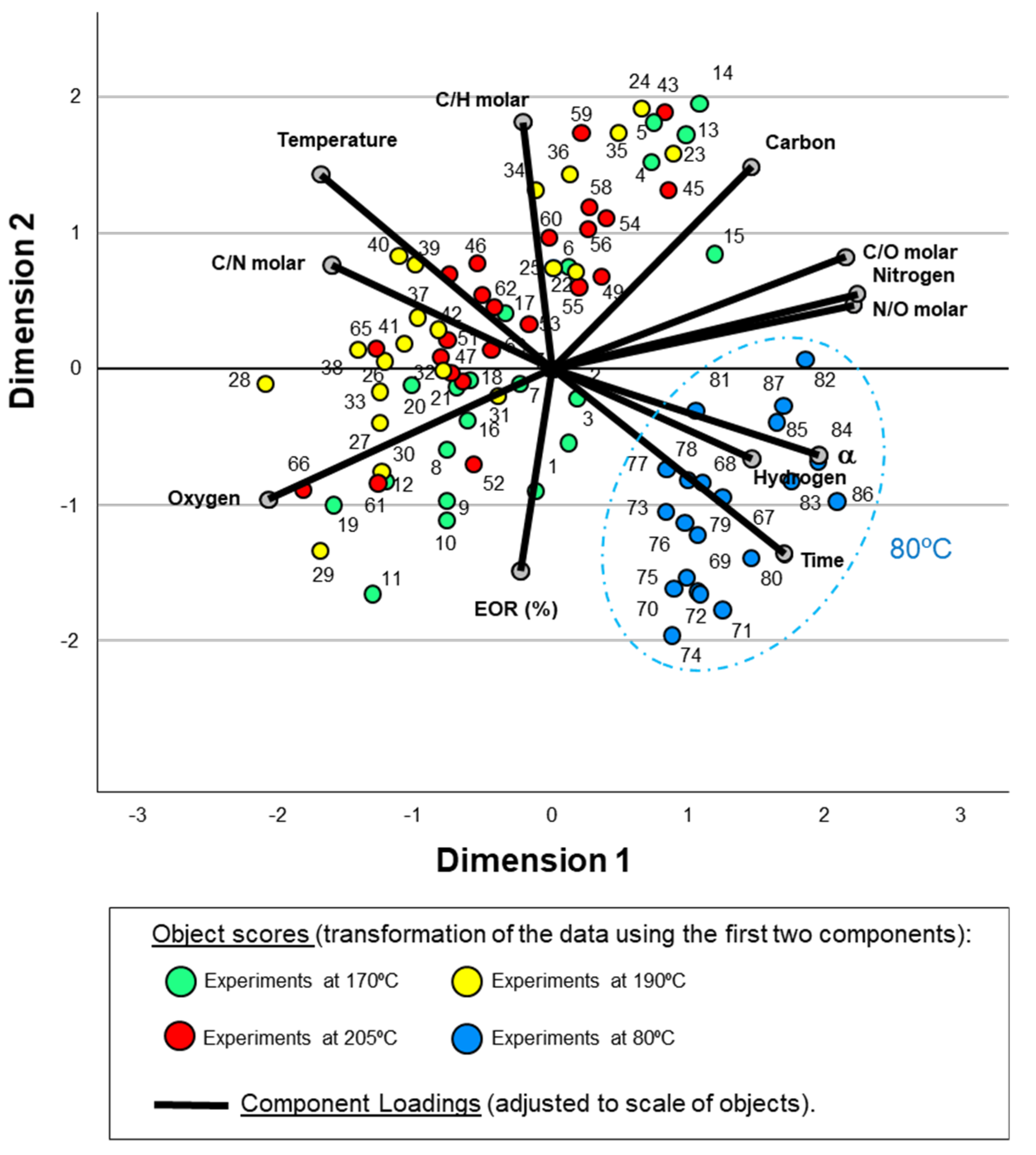
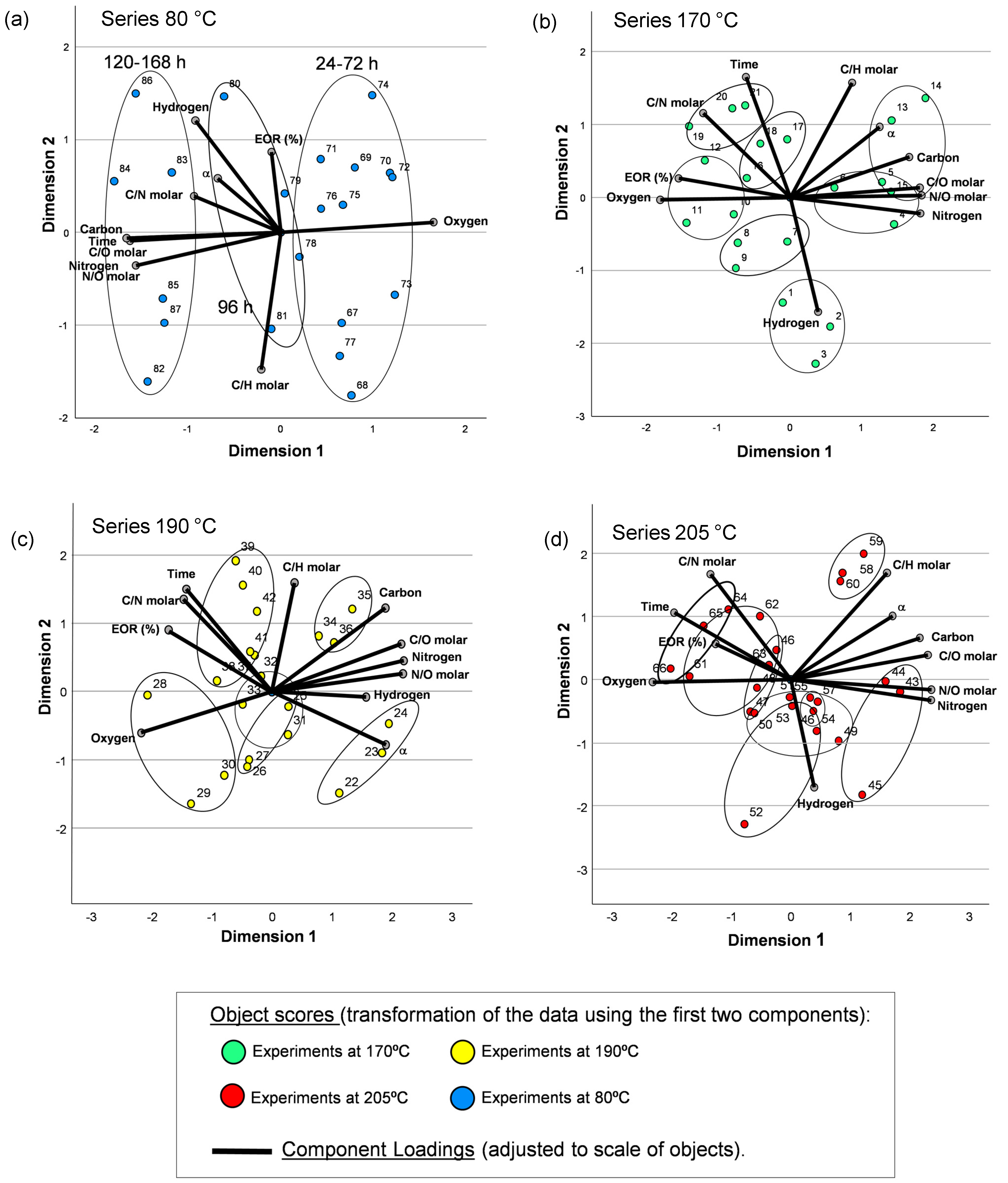
3.1. CATPCA and PCA to Study Cyanide Polymerization Assisted by MWR Tracking by Means of Gel Fractions
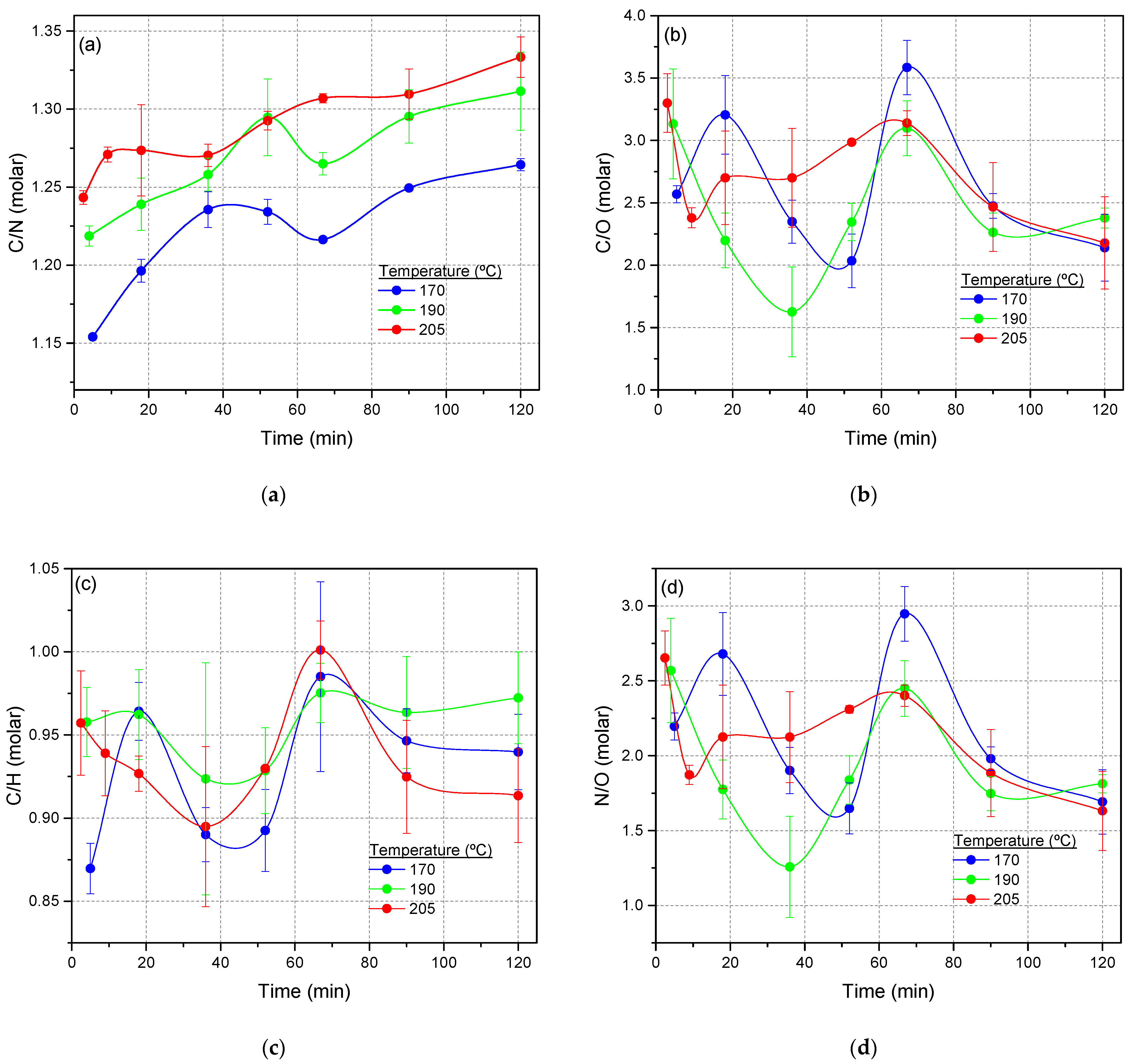
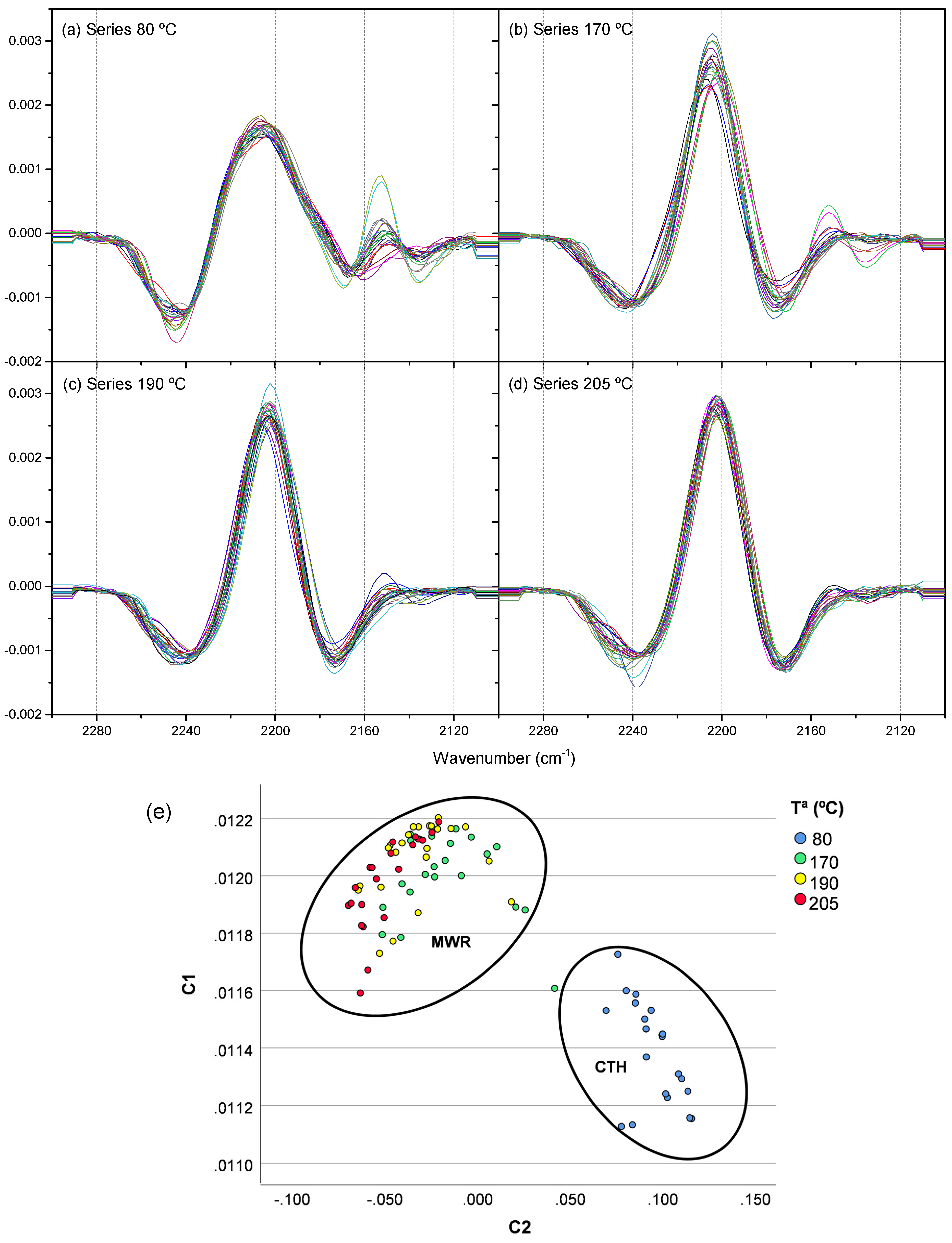
3.1.1. Conversion Limit, Chemical Composition and Data from FTIR Spectroscopy
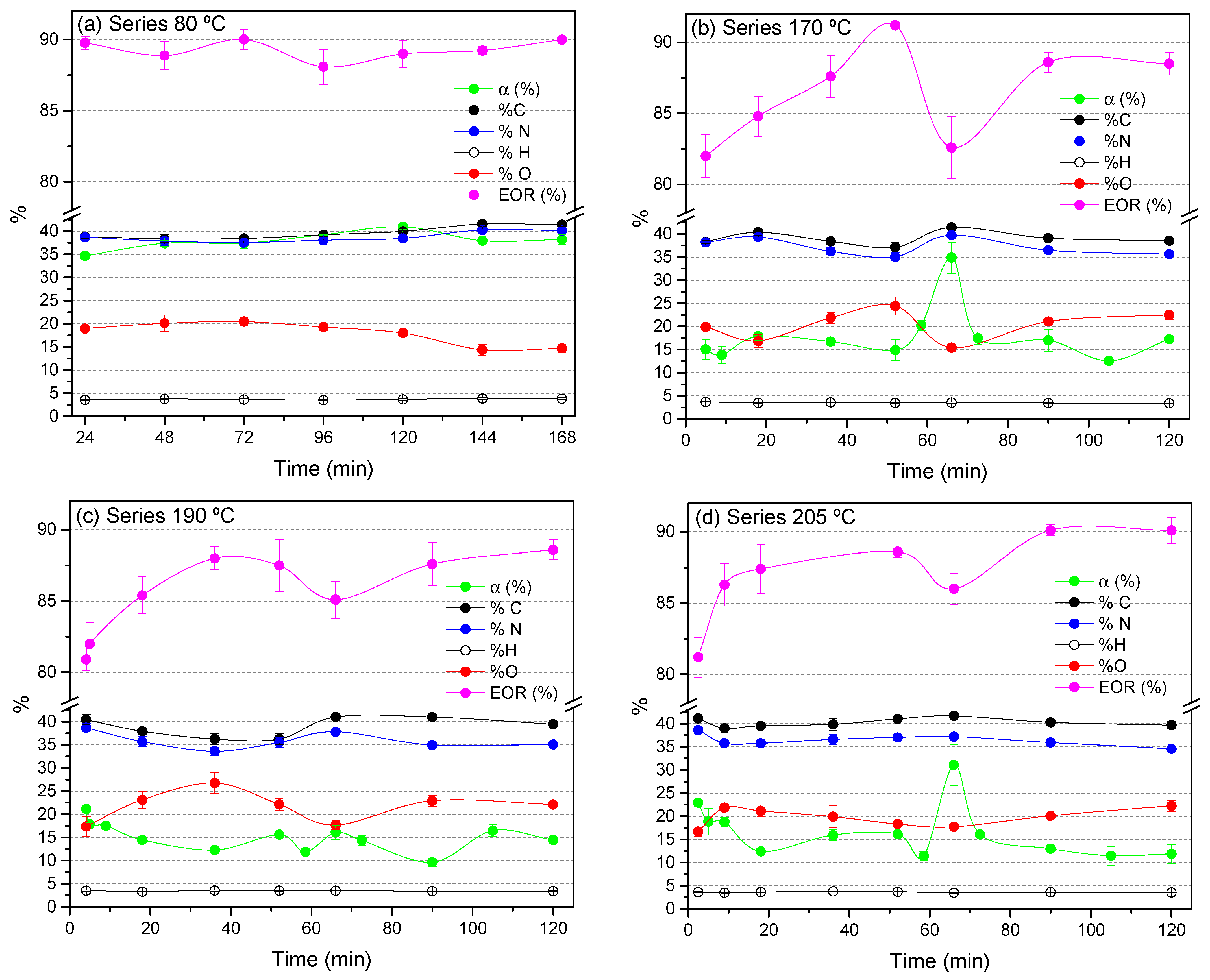
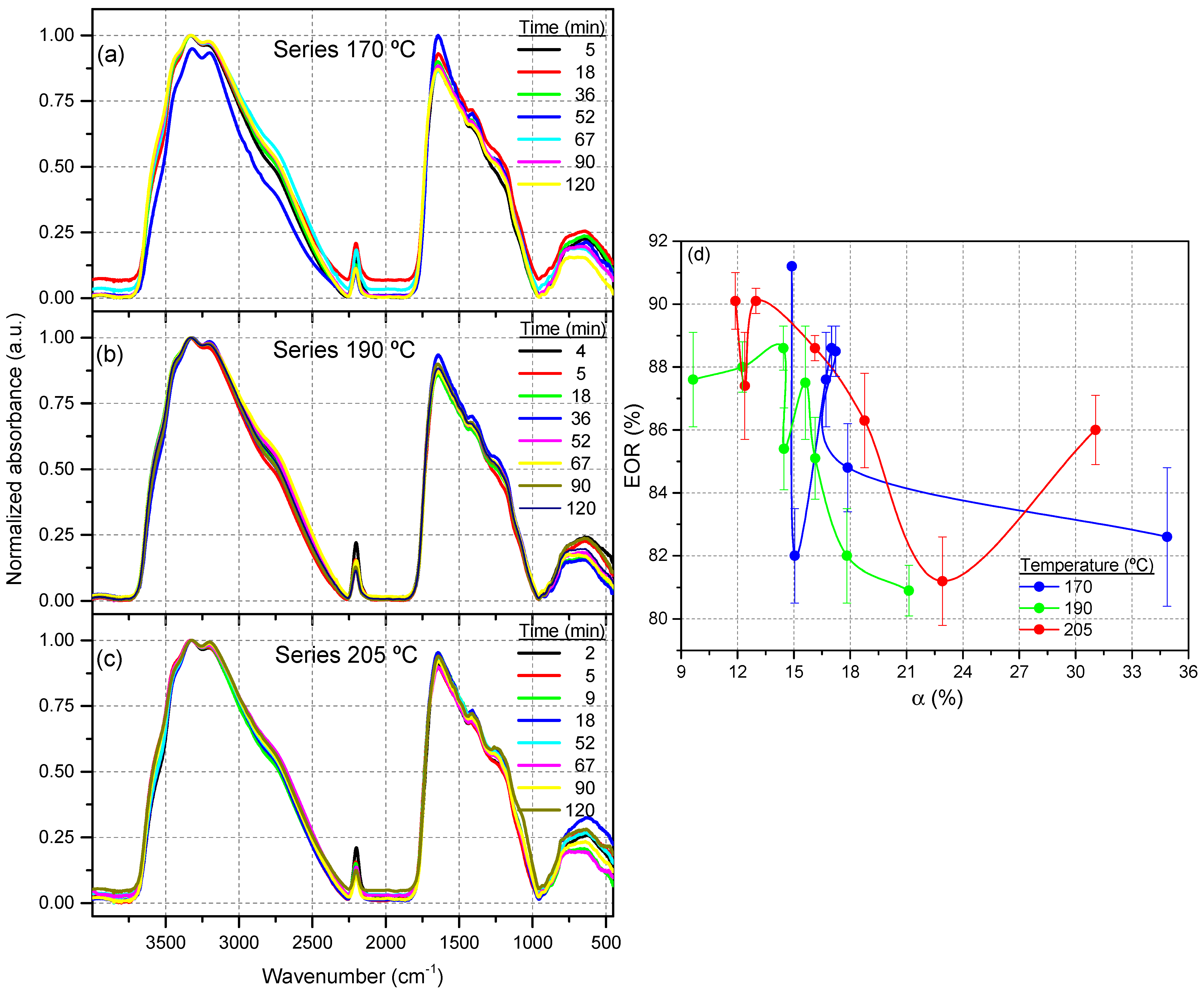
3.1.2. PCA of the FTIR Spectra for the Cyanide Polymers
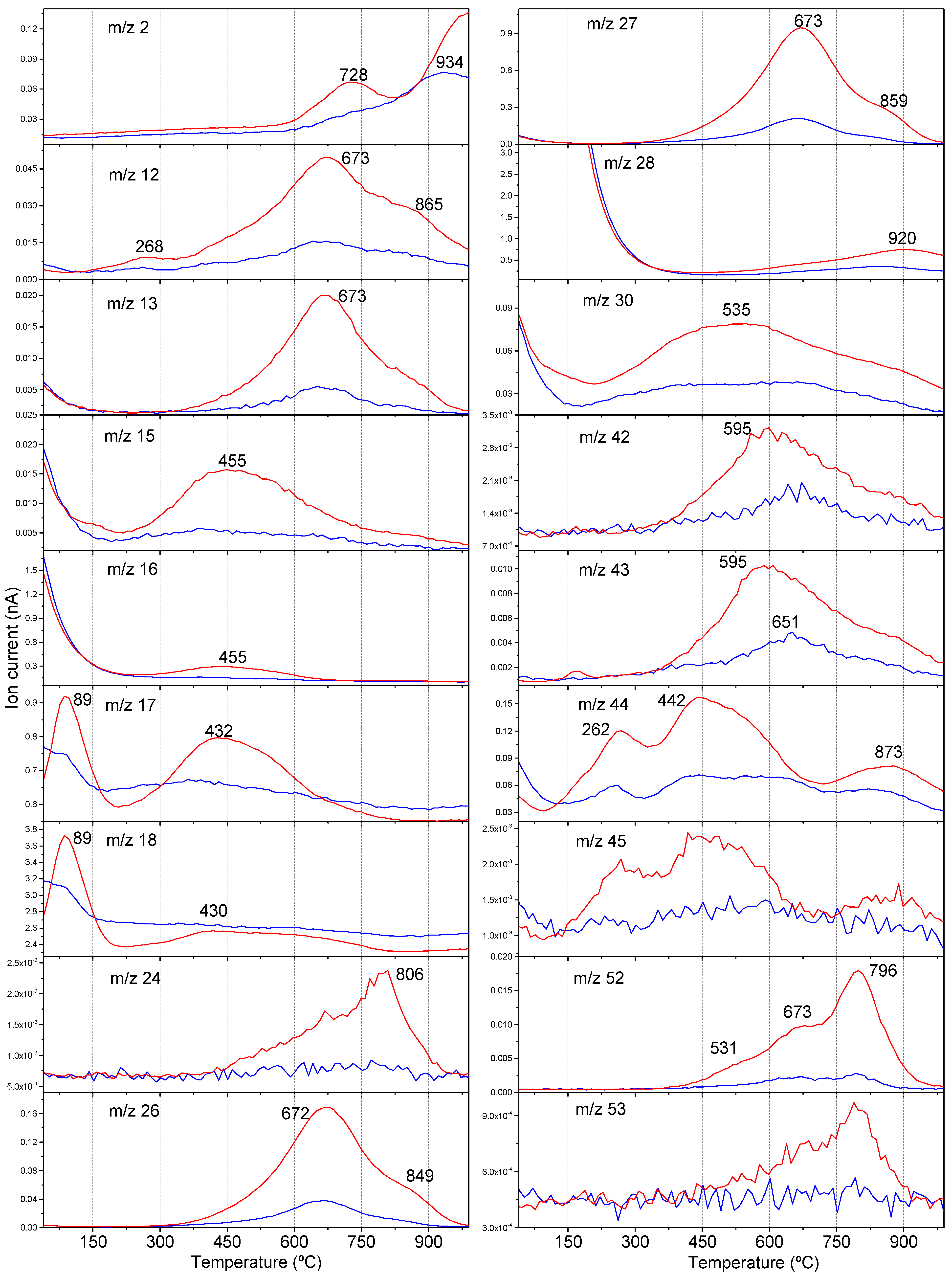
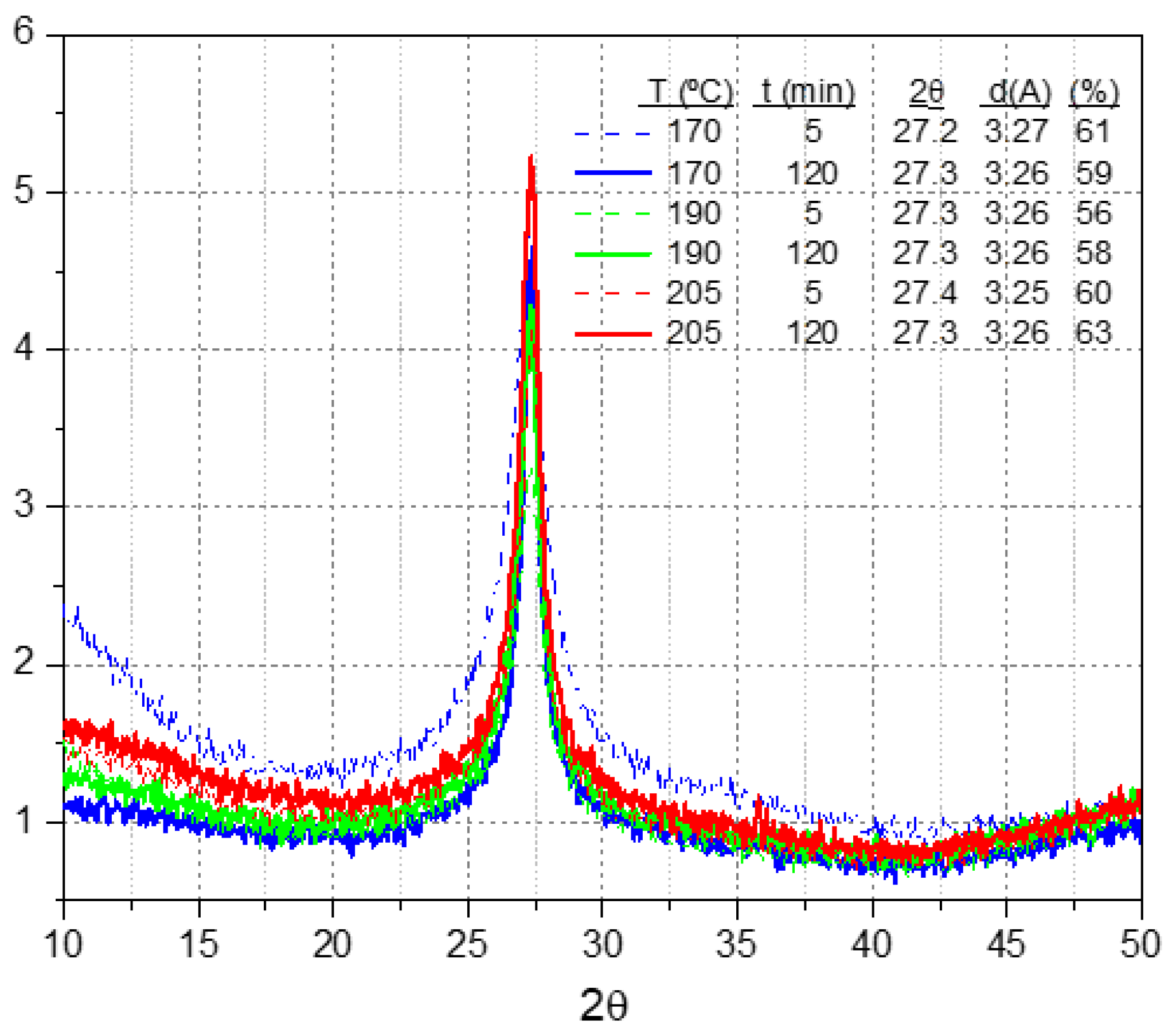
3.1.3. PCA for the Thermal Analysis Data from the Gel Fractions
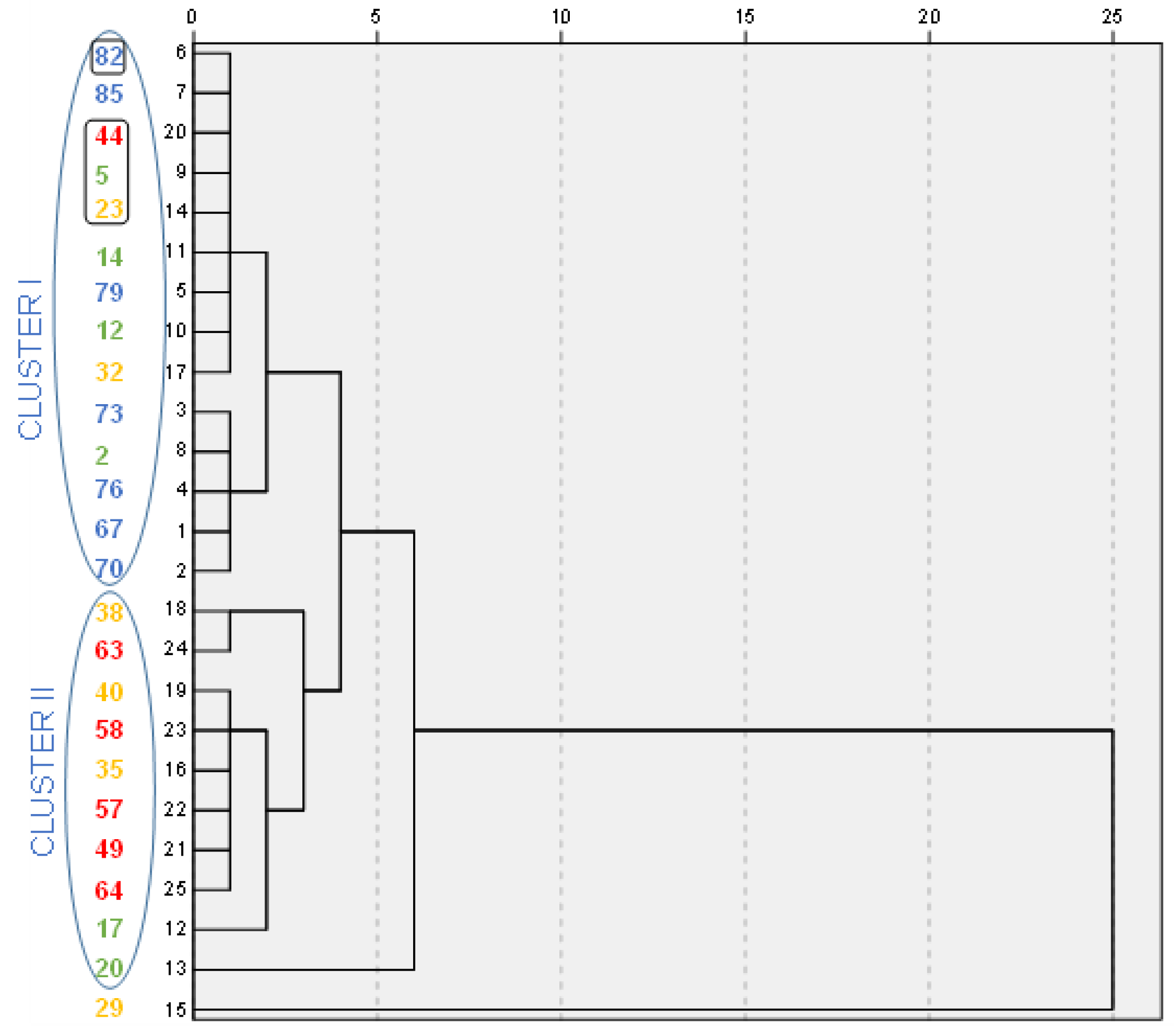
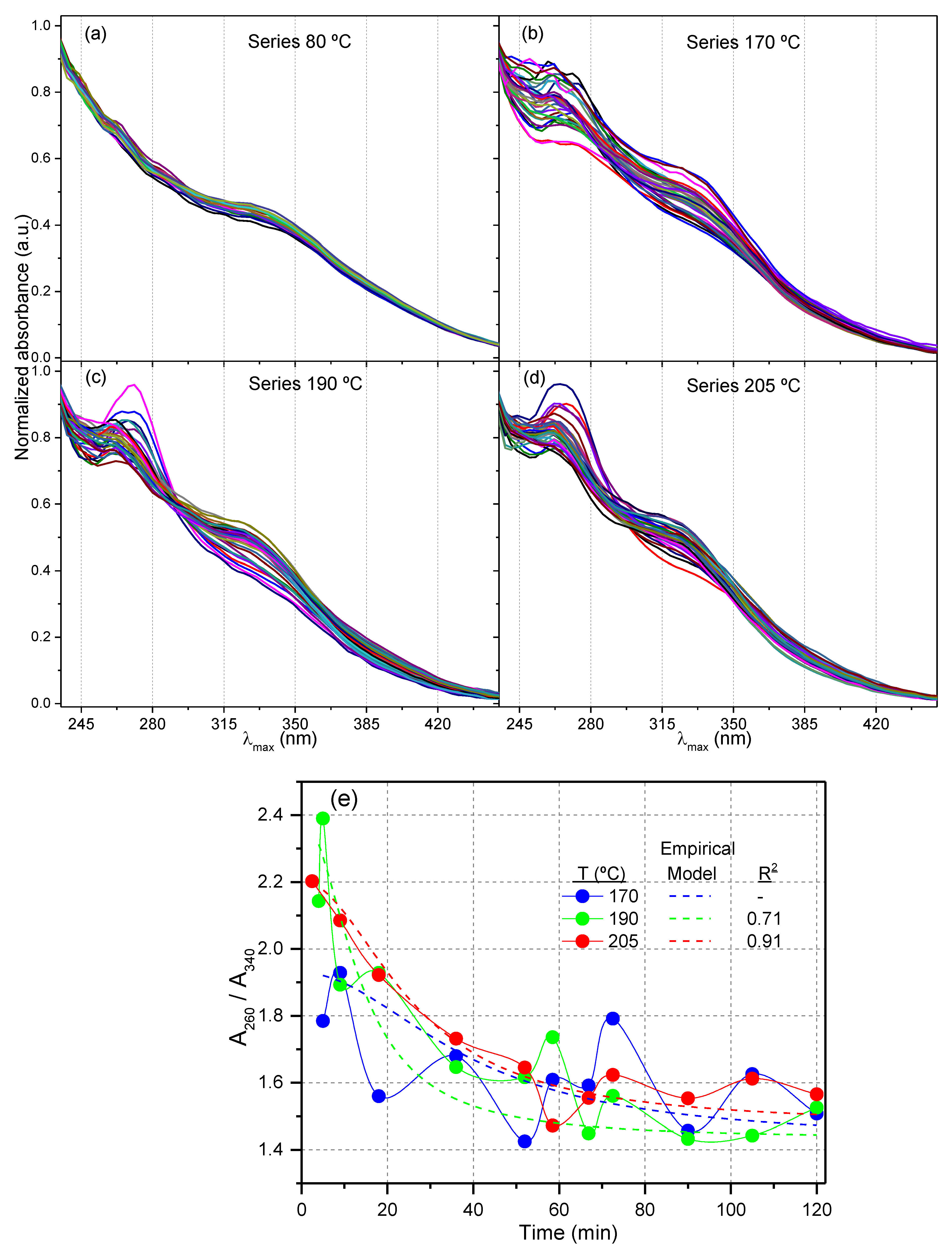
3.2. PCA for UV-Vis Spectra of the Sol Fractions
4. Concluding Remarks and Outlooks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oró, J. Synthesis of Adenine from Ammonium Cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Matthews, C.N.; Moser, R.E. Prebiological Protein Synthesis. Proc. Natl. Acad. Sci. USA 1966, 5, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hagan, W.J. HCN and Chemical Evolution: The Possible Role of Cyano Compounds in Prebiotic Synthesis. Tetrahedron 1984, 40, 1093–1120. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of Abiotic Nucleotide Synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Zorzano, M.-P.; Osuna-Esteban, S. Simple Organics and Biomonomers identified in HCN Polymers. Life 2013, 3, 421–448. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Pérez-Fernández, C.; Mateo-Martí, E. A Comprehensive Review of HCN-derived Polymers. Processes 2021, 9, 597. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; Mompeán, C.; Ruiz-Bermejo, M. A Prebiotic Synthesis of Pterins. Chem. Eur. J. 2015, 21, 13531–13534. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; González-Toril, E.; Mompeán, C.; Ruiz-Bermejo, M. The role of the aqueos aerosols in the “Glyoxylate Scenario”: An experimental approach. Chem. Eur. J. 2016, 26, 12785–12799. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Gálvez-Martínez, S.; Mateo-Martí, E.; Colín-García, M. A serpentinite lizardite-HCN interaction leading the increasing of molecular complexity in an alkaline hydrothermal scenario: Implications for the origin of life studies. Life 2021, 11, 661. [Google Scholar] [CrossRef]
- Hortal, L.; Pérez-Fernández, C.; de la Fuente, J.L.; Valles, P.; Mateo-Martí, E.; Ruiz-Bermejo, M. A Dual Perspective on the Microwave-Assisted Synthesis of HCN Polymers towards the Chemical Evolution and Design of Functional Materials. Sci. Rep. 2020, 10, 22350. [Google Scholar] [CrossRef]
- Pérez-Fernández, C.; Vega, J.; Rayo-Pizarroso, P.; Mateo-Marti, E.; Ruiz-Bermejo, M. Prebiotic synthesis of noncanonical nucleobases under plausible alkaline hydrothermal conditions. Sci. Rep. 2022, 12, 15140. [Google Scholar] [CrossRef] [PubMed]
- Sandström, H.; Rahm, M. The Beginning of HCN Polymerization: Iminoacetonitrile Formation and Its Implications in Astrochemical Environments. ACS Earth Space Chem. 2021, 5, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Bhattacharyya, D.; Anoop, A. Prebiotic Chemistry of HCN Tetramerization by Automated Reaction Search. Chem. Eur. J. 2018, 24, 4885–4894. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.; Andersen, T.; Flamm, C.; Hanczyc, M.M.; Merkle, D.; Stadler, P.F. Navigating the Chemical Space of HCN Polymerization and Hydrolysis: Guiding Graph Grammars by Mass Spectrometry Data. Entropy 2013, 15, 4066–4083. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common Origins of RNA, Protein and Lipid Precursors in a Cyanosulfidic Protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Das, T.; Ghule, S.; Vanka, K. Insights into the Origin of Life: Did It Begin from HCN and H2O? ACS Cent. Sci. 2019, 5, 1532–1540. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Molaverdikhani, K.; Pudritz, R.E.; Henning, T.; Cerrillo, K.E. Toward RNA Life on Early Earth: From Atmospheric HCN to Biomolecule Production in Warm Little Ponds. Astrophys. J. 2022, 932, 9. [Google Scholar] [CrossRef]
- D’Ischia, M.; Manini, P.; Moracci, M.; Saladino, R.; Ball, V.; Thissen, H.; Evans, R.A.; Puzzarini, C.; Barone, V. Astrochemistry and Astrobiology: Materials Science in Wonderland? Int. J. Mol. Sci. 2019, 20, 4079. [Google Scholar] [CrossRef]
- Thissen, H.; Evans, R.A.; Ball, V. Films and Materials Derived from Aminomalonnonitrile. Processes 2021, 9, 82. [Google Scholar] [CrossRef]
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-Chemistry Inspired Polymer Coatings for Biomedical and Material Science Applications. NPG Asia Mater. 2015, 7, e225. [Google Scholar] [CrossRef]
- Menzies, D.J.; Ang, A.; Thissen, H.; Evans, R.A. Adhesive Prebiotic Chemistry Inspired Coatings for Bone Contacting Applications. ACS Biomater. Sci. Eng. 2017, 3, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Liao, T.-Y.; Thissen, H.; Tsai, W.-B. One-Step Aminomalononitrile-Based Coatings Containing Zwitterionic Copolymers for the Reduction of Biofouling and the Foreign Body Response. ACS Biomater. Sci. Eng. 2019, 5, 6454–6462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Chiang, Y.-L.; Chang, Y.-H.; Thissen, H.; Tsai, S.-W. An aqueous-based process to bioactivate poly(ε-caprolactone)/mesoporous bioglass composite surfaces by prebiotic chemistry-inspired polymer coatings for biomedical applications. Colloids Surf. B Biointerfaces 2021, 205, 111913. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.-Y.; Easton, C.D.; Thissen, H.; Tsai, W.-B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. ACS Biomater. Sci. Eng. 2020, 6, 3349–3360. [Google Scholar] [CrossRef]
- Ball, V. Antioxidant Activity of Films Inspired by Prebiotic Chemistry. Mater. Lett. 2021, 285, 129050. [Google Scholar] [CrossRef]
- Pérez-Fernández, C.; Ruiz-Bermejo, M.; Gálvez-Martínez, S.; Mateo-Martí, E. A XPS study of HCN-derived films on pyrite surfaces: A prebiotic chemistry standpoint towards the development of protective coatings. RSC Adv. 2021, 11, 20109–20117. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; García-Armada, P.; Mateo-Martí, E.; de la Fuente, J.L. HCN-derived polymers from thermally induced polymerization of diaminomaleonitrile: A non-enzymatic peroxide sensor based on prebiotic chemistry. Eur. Polym. J. 2022, 162, 110897. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, Y.; Su, Y.; Ge, C.; Jin, B.; Li, Z.; Wu, S. Preparation and Characterization of Poly-Hydrogen Cyanide Nanofibers with High Visible Light Photocatalytic Activity. Catal. Commun. 2014, 46, 197–200. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Carretero-González, J.; García-Fernández, L.; Aguilar, M.R. A comparative study of HCN polymers synthesized from NH4CN or DAMN polymerization in aqueous media: New perspectives for prebiotic chemistry and material science. Chem. Eur. J. 2019, 25, 11437–11455. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; García-Armada, P.; Valles, P.; de la Fuente, J.L. Semiconducting soft submicron particles from the microwave-driven polymerization of diaminomaleonitrile. Polymers 2022, 14, 3460. [Google Scholar] [CrossRef]
- Jung, J.; Menzies, D.J.; Thissen, H.; Easton, C.D.; Evans, R.A.; Henry, R.; Deletic, A.; McCarthy, D.T. New prebiotic chemistry inspired filter media for stormwater/greywater disinfection. J. Hazard. Mater. 2019, 378, 120749. [Google Scholar] [CrossRef] [PubMed]
- Rahm, M.; Lunine, J.I.; Usher, D.A.; Shalloway, D. Polymorphism and Electronic Structure of Polyimine and Its Potential Significance for Prebiotic Chemistry on Titan. Proc. Natl. Acad. Sci. USA 2016, 113, 8121–8126. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, B.; Zhang, L.; Yin, L.; Wang, X. Synthesis of layered carbonitrides from biotic molecules for photoredox transformations. Angew. Chem. Int. Ed. 2017, 56, 6627. [Google Scholar] [CrossRef] [PubMed]
- Toh, R.J.; Evans, R.; Thissen, H.; Voelcker, N.H.; d’Ischia, M.; Ball, V. Deposition of Aminomalononitrile-Based Films: Kinetics, Chemistry, and Morphology. Langmuir 2019, 35, 9896–9903. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Ruiz-Bermejo, M.; de la Fuente, J.L. Modelling the Kinetics and Structural Property Evolution of a Versatile Reaction: Aqueous HCN Polymerization. Phys. Chem. Chem. Phys. 2018, 20, 17353–17366. [Google Scholar] [CrossRef] [PubMed]
- Mas, I.; de la Fuente, J.L.; Ruiz-Bermejo, M. Temperature Effect on Aqueous NH4CN Polymerization: Relationship between Kinetic Behaviour and Structural Properties. Eur. Polym. J. 2020, 132, 109719. [Google Scholar] [CrossRef]
- Pérez-Fernández, C.; Valles, P.; González-Toril, E.; Mateo-Martí, E.; de la Fuente, J.L.; Ruiz-Bermejo, M. Tuning the morphology in the nanoscale of NH4CN polymers synthesized by microwave radiation: A comparative study. Polymers 2022, 14, 57. [Google Scholar] [CrossRef]
- Holm, N.G. (Ed.) Marine Hydrothermal Systems and the Origin of Life; Springer: Dordrecht, The Netherlands, 1992; pp. 15–31. ISBN 978-94-010-5216-0. [Google Scholar]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal Vents and the Origin of Life. Nat. Rev. Microbiol. 2008, 11, 805–814. [Google Scholar] [CrossRef]
- Colín-García, M.; Villafañe-Barajas, S.; Camprubí, A.; Ortega-Gutiérrez, F.; Colás, V.; Negrón-Mendoza, A. Prebiotic chemistry in hydrothermal vent systems. In Handbook of Astrobiology, 1st ed.; Kolb, V.M., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 297–329. ISBN 978-13-151-5996-6. [Google Scholar]
- Rimmer, P.B.; Shorttle, O. Origin of Life’s Building Blocks in Carbon-and Nitrogen-Rich Surface Hydrothermal Vents. Life 2019, 9, 12. [Google Scholar] [CrossRef]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubí, E.; Lane, N. The Origin of Life in Alkaline Hydrothermal Vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef]
- Burcar, B.T.; Barge, L.M.; Trail, D.; Watson, E.B.; Russell, M.J.; McGown, L.B. RNA Oligomerization in Laboratory Analogues of Alkaline Hydrothermal Vent Systems. Astrobiology 2015, 15, 509–522. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Figueira, F.L.; Van Steenberge, P.H.M.; D’hooge, D.R.; Zhou, Y.-N.; Luo, Z.-H. Bridging principal component analysis and method of moments based parameter estimation for grafting of polybutadiene with styrene. Chem. Eng. J. 2021, 425, 130463. [Google Scholar] [CrossRef]
- Chiangraeng, N.; Armstrong, M.; Manokruang, K.; Lee, V.S.; Jiranusornkul, S.; Nimmanpipug, P. Characteristic Structural Knowledge for Morphological Identification and Classification in Meso-Scale Simulations Using Principal Component Analysis. Polymers 2021, 13, 2581. [Google Scholar] [CrossRef] [PubMed]
- Héberger, K.; Milczewska, K.; Voelkel, A. Principal component analysis of polymer-solvent and filler-solvent interactions by inverse gas chromatography. Colloids Surf. A Physicochem. Eng. Asp. 2005, 260, 29–37. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F.; Ruckebusch, C.; Vitale, R. A Chemometric Strategies for Spectroscopy-Based Food Authentication. Appl. Sci. 2020, 10, 6544. [Google Scholar] [CrossRef]
- Mohammadi, M.; Khanmohammadi Khorrami, M.; Vatani, A.; Ghasemzadeh, H.; Vatanparast, H.; Bahramian, A.; Fallah, A. Rapid determination and classification of crude oils by ATR-FTIR spectroscopy and chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118157. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, M.; Martini, E.; Castrucci, M.; Campanella, L.; Coppa, A. Chemometric Comparison of Data Files Using Several Thermal Analytical Techniques for Dating Fossil Bones from Two Old Burial Sites. Curr. Anal. Chem. 2021, 17, 536–544. [Google Scholar] [CrossRef]
- Francisco-Fernández, M.; Tarrío-Saavedra, J.; Naya, S.; López-Beceiro, J.; Artiaga, R. Statistical classification of early and late wood through the growth rings using thermogravimetric analysis. J. Therm. Anal. Calorim. 2017, 127, 499–506. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; Moreno, M.; de la Fuente, J.L.; Briones, C.; Ruiz-Bermejo, M. Experimental Conditions Affecting the Kinetics of Aqueous HCN Polymerization as Revealed by UV-Vis Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and Canodraw for Windows User’s Guide: Software for Canonical Community Ordination; Microcomputer Power: New York, NY, USA, 2002. [Google Scholar]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Rogero, C.; Menor-Salván, C.; Osuna-Esteban, S.; Martín-Gago, J.A. New insights into the characterization of insoluble black HCN polymers. Chem. Biodiver. 2012, 9, 25–40. [Google Scholar] [CrossRef]
- Liebman, S.A.; Pesce-Rodriguez, R.A.; Matthews, C.N. Organic Analysis of Hydrogen Cyanide Polymers: Prebiotic and Extraterrestrial Chemistry. Adv. Space Res. 1995, 15, 71–80. [Google Scholar] [CrossRef]
- Umemoto, K.; Takahasi, M.; Yokota, K. Studies on the Structure of HCN Oligomers. Orig. Life Evol. Biosph. 1987, 17, 283–293. [Google Scholar] [CrossRef]
- Cataldo, F.; Lilla, E.; Ursini, O.; Angelini, G. TGA-FT-IR Study of Pyrolysis of Poly(Hydrogen Cyanide) Synthesized from Thermal Decomposition of Formamide. Implications in Cometary Emissions. J. Anal. Appl. Pyrolysis 2010, 87, 24–44. [Google Scholar] [CrossRef]
- Khare, B.N.; Sagan, C.; Thompson, W.R.; Arakawa, E.T.; Meisse, C.; Tuminello, P.S. Optical Properties of Poly-HCN and Their Astronomical Applications. Can. J. Chem. 1994, 72, 678–694. [Google Scholar] [CrossRef]
- Meulman, J.J.; Heiser, W.J. SPSS Categories 20.0; IBM: Chicago, IL, USA, 2011. [Google Scholar]
- De la Fuente, J.L.; Ruiz-Bermejo, M.; Nna-Mvondo, D.; Minard, R.D. Further progress into the thermal characterization of HCN polymers. Polym. Degrad. Stab. 2014, 110, 241–251. [Google Scholar] [CrossRef]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, X.; Yu, K.; Wan, C.; Wang, Y.; Huang, S.; Sun, Q.; Huang, J. Identification of myocardial fibrosis by ATR-FTIR spectroscopy combined with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120238. [Google Scholar] [CrossRef]
- Nitrosetein, T.; Wongwattanakul, M.; Chonanant, C.; Leelayuwat, C.; Charoensri, N.; Jearanaikoon, P.; Lulitanond, A.; Wood, B.R.; Tippayawat, P.; Heraud, P. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy combined with chemometric modelling for the classification of clinically relevant Enterococci. J. App. Microbiol. 2020, 130, 982–993. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Marín-Yaseli, M.R. The influence of reactions conditions in aqueous HCN polymerization on the polymer thermal degradation properties. J. Anal. App. Pyrolysis 2017, 124, 103–112. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Colín-García, M. Characterization of HCN-derived thermal polymers: Implications for chemical evolution. Processes 2020, 8, 968. [Google Scholar] [CrossRef]
- Sharma, S.; Arya, A.; Cruz, R.; Cleaves, H.J. Automated Exploration of Prebiotic Chemical Reaction Space: Progress and Perspectives. Life 2021, 11, 1140. [Google Scholar] [CrossRef]
- Guttenberg, N.; Virgo, N.; Chandru, K.; Scharf, C.; Mamajanov, I. Bulk measurements of messy chemistries are needed for a theory of the origins of life. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160347. [Google Scholar] [CrossRef]
- Dubois, D.; Carrasco, N.; Petrucciani, M.; Vettier, L.; Tigrine, S.; Pernot, P. In situ investigation of neutrals involved in the formation of Titan tholins. Icarus 2019, 317, 182–196. [Google Scholar] [CrossRef]
- Perrin, Z.; Carrasco, N.; Chatain, A.; Jovanovic, L.; Vettier, L.; Ruscassier, N.; Cernogora, G. An Atmospheric Origin for HCN-Derived Polymers on Titan. Processes 2021, 9, 965. [Google Scholar] [CrossRef]
- Rengel, M.; Shulyak, D.; Hartogh, P.; Sagawa, H.; Moreno, R.; Jarchow, C.; Breitschwerdt, D. Ground-based HCN submillimetre measurements in Titan’s atmosphere: An intercomparison with Herschel observations. A&A 2022, 658, A88. [Google Scholar]
- Kim, S.J.; Lee, D.W.; Sim, C.K.; Seon, K.I.; Courtin, R.; Geballe, T.R. Retrieval of haze properties and HCN concentrations from the three-micron spectrum of Titan. J. Quant. Spectrosc. Radiat. Transf. 2018, 210, 197–203. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Molaverdikhani, K.; Pudritz, R.E.; Henning, T.; Hébrard, E. HCN Production in Titan’s Atmosphere: Coupling Quantum Chemistry and Disequilibrium Atmospheric Modeling. Astrophys. J. 2020, 901, 110. [Google Scholar] [CrossRef]
- Matthews, C.N. Dark Matter in the Solar System: Hydrogen Cyanide Polymers. Orig. Life Evol. Biosph. 1991, 21, 421–434. [Google Scholar] [CrossRef]
- Matthews, C.N. Hydrogen Cyanide Polymers: From Laboratory to Space. Planet. Space Sci. 1995, 43, 1365–1370. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Fernández, C.; González-Toril, E.; Mateo-Martí, E.; Ruiz-Bermejo, M. Multivariate Analysis Applied to Microwave-Driven Cyanide Polymerization: A Statistical View of a Complex System. Polymers 2023, 15, 410. https://doi.org/10.3390/polym15020410
Pérez-Fernández C, González-Toril E, Mateo-Martí E, Ruiz-Bermejo M. Multivariate Analysis Applied to Microwave-Driven Cyanide Polymerization: A Statistical View of a Complex System. Polymers. 2023; 15(2):410. https://doi.org/10.3390/polym15020410
Chicago/Turabian StylePérez-Fernández, Cristina, Elena González-Toril, Eva Mateo-Martí, and Marta Ruiz-Bermejo. 2023. "Multivariate Analysis Applied to Microwave-Driven Cyanide Polymerization: A Statistical View of a Complex System" Polymers 15, no. 2: 410. https://doi.org/10.3390/polym15020410
APA StylePérez-Fernández, C., González-Toril, E., Mateo-Martí, E., & Ruiz-Bermejo, M. (2023). Multivariate Analysis Applied to Microwave-Driven Cyanide Polymerization: A Statistical View of a Complex System. Polymers, 15(2), 410. https://doi.org/10.3390/polym15020410









