Physical, Morphological, and Rheological Properties of Agglomerated Milk Protein Isolate Powders: Effect of Binder Type and Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fluidized-Bed Agglomeration Process (FBAP)
2.3. Particle Size Distribution (PSD)
2.4. Flowability, Cohesiveness, and Porosity Measurements
2.5. Water-Binding Capacity Measurement
2.6. Wettability Measurement
2.7. Solubility Measurement
2.8. Scanning Electron Microscopy (SEM)
2.9. Sample Preparation for Rheological Properties
2.10. Rheological Properties
2.11. Statistical Analysis
3. Results
3.1. PSD Analysis
3.2. Flowability, Cohesiveness, and Porosity (ε)
3.3. Water-Holding Properties
3.4. Morphology
3.5. Rheological Properties
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kneifel, W.; Seiler, A. Water-holding properties of milk protein products-A review. Food Struct. 1993, 12, 3. [Google Scholar]
- Augustin, M.A.; Clarke, P.T. Dry milk ingredients. In Dairy Ingredients for Food Processing; Blackwell Publishing, Ltd.: Ames, IA, USA, 2011; pp. 141–159. [Google Scholar]
- Lagrange, V.; Whitsett, D.; Burris, C. Global market for dairy proteins. J. Food Sci. 2015, 80, A16–A22. [Google Scholar] [CrossRef]
- Wu, S.; Fitzpatrick, J.; Cronin, K.; Miao, S. The effect of pH on the wetting and dissolution of milk protein isolate powder. J. Food Eng. 2019, 240, 114–119. [Google Scholar] [CrossRef]
- Ji, J.; Cronin, K.; Fitzpatrick, J.; Maguire, P.; Zhang, H.; Miao, S. The structural modification and rehydration behaviours of milk protein isolate powders: The effect of granule growth in the high shear granulation process. J. Food Eng. 2016, 189, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hazlett, R.; Schmidmeier, C.; O’mahony, J.A. Approaches for improving the flowability of high-protein dairy powders post spray drying—A review. Powder Technol. 2021, 388, 26–40. [Google Scholar] [CrossRef]
- Wu, S.; Fitzpatrick, J.; Cronin, K.; Maidannyk, V.; Miao, S. Effects of spraying surfactants in a fluidised bed on the rehydration behaviour of milk protein isolate powder. J. Food Eng. 2020, 266, 109694. [Google Scholar] [CrossRef]
- Lee, D.; Min, G.; Roh, W.; Yoo, B. Effect of various types of sugar binder on the physical properties of gum powders prepared via fluidized-bed agglomeration. Foods 2021, 10, 1387. [Google Scholar] [CrossRef]
- Szulc, K.; Lenart, A. Effect of agglomeration on flowability of baby food powders. J. Food Sci. 2010, 75, E276–E284. [Google Scholar] [CrossRef]
- Szulc, K.; Lenart, A. Surface modification of dairy powders: Effects of fluid-bed agglomeration and coating. Int. Dairy J. 2013, 33, 55–61. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Agglomerated xanthan gum powder used as a food thickener: Effect of sugar binders on physical, microstructural, and rheological properties. Powder Technol. 2020, 362, 301–306. [Google Scholar] [CrossRef]
- Dhanalakshmi, K.; Ghosal, S.; Bhattacharya, S. Agglomeration of food powder and applications. Crit. Rev. Food Sci. Nutr. 2011, 51, 432–441. [Google Scholar] [CrossRef]
- Rayo, L.M.; E Carvalho, L.C.; Sardá, F.A.; Dacanal, G.C.; Menezes, E.W.; Tadini, C.C. Production of instant green banana flour (Musa cavendischii, var. Nanicão) by a pulsed-fluidized bed agglomeration. LWT-Food Sci. Technol. 2015, 63, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Cronin, K.; Fitzpatrick, J.; Fenelon, M.; Miao, S. Effects of fluid bed agglomeration on the structure modification and reconstitution behaviour of milk protein isolate powders. J. Food Eng. 2015, 167, 175–182. [Google Scholar] [CrossRef]
- Sikand, V.; Tong, P.S.; Roy, S.; Rodriguez-Saona, L.E.; Murray, B.A. Solubility of commercial milk protein concentrates and milk protein isolates. J. Dairy Sci. 2011, 94, 6194–6202. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Yoo, B. Cellulose derivatives agglomerated in a fluidized bed: Physical, rheological, and structural properties. Int. J. Biol. Macromol. 2021, 181, 232–240. [Google Scholar] [CrossRef]
- Carr, R.L. Classifying flow properties of solids. Chem. Eng. 1965, 1, 69–72. [Google Scholar]
- Hausner, H.H. Friction conditions in a mass of metal powder. Int. J. Powder Metall. 1967, 13, 7–13. [Google Scholar]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chem. 2009, 114, 127–131. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Ghosal, S.; Indira, T.; Bhattacharya, S. Agglomeration of a model food powder: Effect of maltodextrin and gum Arabic dispersions on flow behavior and compacted mass. J. Food Eng. 2010, 96, 222–228. [Google Scholar] [CrossRef]
- Dyankova, S.; Doneva, M.; Todorov, Y.; Terziyska, M. Determination of particle size distribution and analysis of a natural food supplement on pectin base. IOSR J. Pharm. 2016, 6, 1–8. [Google Scholar]
- Turchiuli, C.; Eloualia, Z.; El Mansouri, N.; Dumoulin, E. Fluidised bed agglomeration: Agglomerates shape and end-use properties. Powder Technol. 2005, 157, 168–175. [Google Scholar] [CrossRef]
- Chever, S.; Méjean, S.; Dolivet, A. Agglomeration during spray drying: Physical and rehydration properties of whole milk/sugar mixture powders. LWT-Food Sci. Technol. 2017, 83, 33–41. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Agglomeration of galactomannan gum powders: Physical, rheological, and structural characterizations. Carbohydr. Polym. 2021, 256, 117599. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, H.; Dirim, S.N. Application of the agglomeration process on spinach juice powders obtained using spray drying method. Dry. Technol. 2020, 39, 19–34. [Google Scholar] [CrossRef]
- Kim, E.H.J.; Chen, X.D.; Pearce, D. Surface characterization of four industrial spray-dried dairy powders in relation to chemical composition, structure and wetting property. Colloids Surf. B Biointerfaces 2002, 26, 197–212. [Google Scholar] [CrossRef]
- Atalar, I.; Yazici, F. Effect of different binders on reconstitution behaviors and physical, structural, and morphological properties of fluidized bed agglomerated yogurt powder. Dry. Technol. 2019, 37, 1656–1664. [Google Scholar] [CrossRef]
- Cuq, B.; Mandato, S.; Jeantet, R.; Saleh, K.; Ruiz, T. Agglomeration/granulation in food powder production. In Handbook of Food Powders; Elsevier: Amsterdam, The Netherlands, 2013; pp. 150–177. [Google Scholar]
- Saluja, A.; Kalonia, D.S. Nature and consequences of protein–protein interactions in high protein concentration solutions. Int. J. Pharm. 2008, 358, 1–15. [Google Scholar] [CrossRef]
- Park, J.; Yoo, B. Particle agglomeration of gum mixture thickeners used for dysphagia diets. J. Food Eng. 2020, 279, 109958. [Google Scholar] [CrossRef]

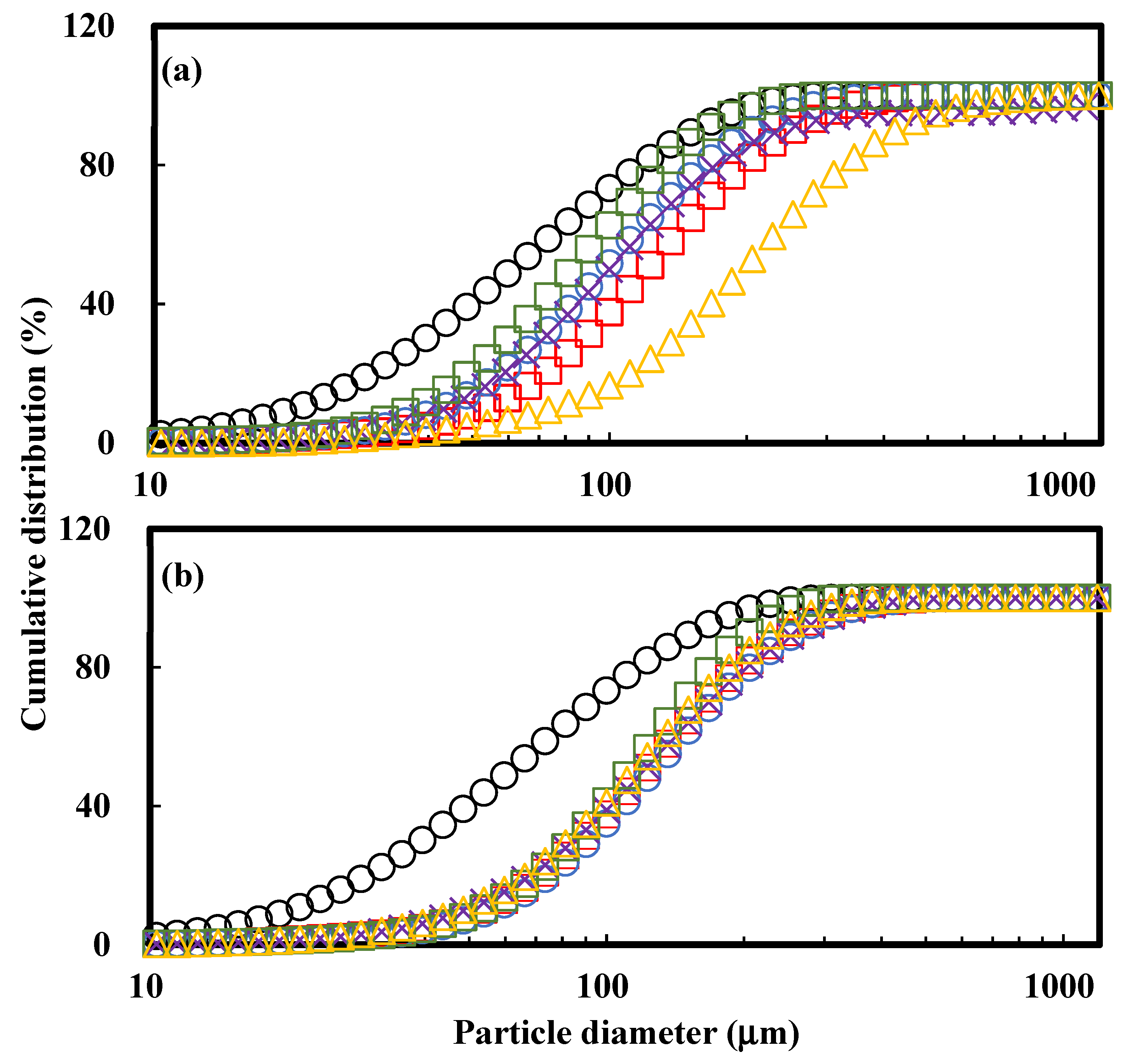
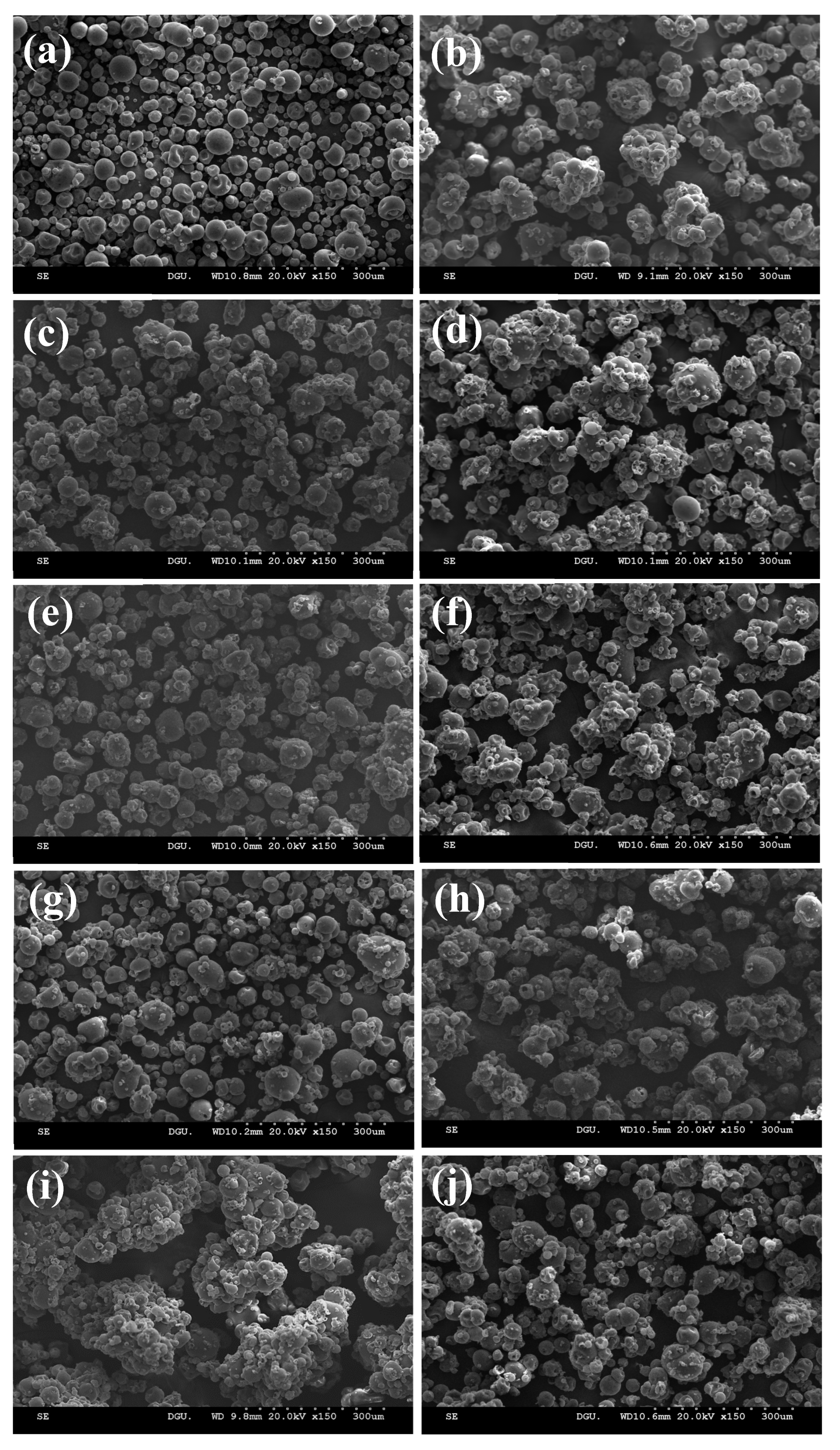
| Sample | Binder Type | Binder Conc. (%) | D10 (mm) | D50 (mm) | D90 (mm) | Span |
|---|---|---|---|---|---|---|
| Raw MPIP | 20.4 ± 0.01 i | 61.4 ± 0.09 g | 152 ± 1.93 g | 2.15 ± 0.03 a | ||
| Agglomerated MPIP | Control (no sugar) | 0 | 53.5 ± 0.14 c | 122 ± 0.35 c | 254 ± 0.73 c | 1.65 ± 0.01 e |
| Sucrose | 10 | 42.7 ± 0.64 g | 97.4 ± 1.78 e | 205 ± 5.40 e | 1.66 ± 0.00 e | |
| 20 | 55.9 ± 0.11 b | 126 ± 0.20 b | 261 ± 0.86 b | 1.63 ± 0.01 f | ||
| Lactose | 10 | 43.6 ± 0.05 f | 99.5 ± 0.29 e | 230 ± 5.42 d | 1.84 ± 0.01 c | |
| 20 | 48.7 ± 0.35 d | 122 ± 1.72 c | 260 ± 2.19 b | 1.74 ± 0.01 d | ||
| Xylitol | 10 | 36.9 ± 0.02 h | 82.3 ± 0.19 f | 164 ± 0.33 f | 1.54 ± 0.01 g | |
| 20 | 52.8 ± 0.06 c | 112 ± 0.32 d | 206 ± 0.30 e | 1.36 ± 0.01 h | ||
| Sorbitol | 10 | 73.6 ± 0.04 a | 192 ± 2.74 a | 432 ± 0.10 a | 1.88 ± 0.01 b | |
| 20 | 47.7 ± 0.25 e | 112 ± 1.82 d | 232 ± 3.42 d | 1.62 ± 0.01 f |
| Sample | Binder Type | Binder Conc. (%) | ρbulk (g/cm3) | ρtapped (g/cm3) | ε (%) | CI (%) | HR |
|---|---|---|---|---|---|---|---|
| Raw MPIP | 0.32 ± 0.00 a | 0.43 ± 0.00 a | 65.5 ± 0.00 i | 26.7 ± 0.29 a | 1.36 ± 0.01 a | ||
| Agglomerated MPIP | Control (no sugar) | 0 | 0.31 ± 0.00 b | 0.39 ± 0.01 c | 76.9 ± 0.00 h | 20.3 ± 0.58 b | 1.26 ± 0.01 c |
| Sucrose | 10 | 0.31 ± 0.00 b | 0.38 ± 0.00 cd | 77.1 ± 0.00 g | 20.0 ± 0.00 c | 1.25 ± 0.00 d | |
| 20 | 0.28 ± 0.00 e | 0.35 ± 0.00 e | 79.1 ± 0.00 e | 20.0 ± 0.00 c | 1.25 ± 0.00 d | ||
| Lactose | 10 | 0.30 ± 0.01 c | 0.38 ± 0.00 d | 77.1 ± 0.00 g | 21.0 ± 0.82 b | 1.27 ± 0.02 bc | |
| 20 | 0.28 ± 0.00 e | 0.35 ± 0.00 e | 78.2 ± 0.00 f | 21.7 ± 0.58 b | 1.28 ± 0.01 b | ||
| Xylitol | 10 | 0.32 ± 0.00 a | 0.40 ± 0.00 b | 84.0 ± 0.00 c | 20.0 ± 0.00 c | 1.24 ± 0.01 e | |
| 20 | 0.29 ± 0.00 d | 0.35 ± 0.00 e | 86.0 ± 0.00 b | 17.0 ± 0.00 e | 1.20 ± 0.00 f | ||
| Sorbitol | 10 | 0.28 ± 0.00 e | 0.34 ± 0.00 f | 86.5 ± 0.00 a | 15.0 ± 0.00 f | 1.19 ± 0.01 g | |
| 20 | 0.26 ± 0.00 f | 0.32 ± 0.00 g | 80.6 ± 0.00 d | 19.5 ± 0.41 d | 1.24 ± 0.01 e |
| Sample | Binder Type | Binder Conc. (%) | Solubility (%) | Wettability | Water-Binding Capacity (g/g) |
|---|---|---|---|---|---|
| Raw MPIP | 31.1 ± 0.50 i | 0.97 ± 0.03 h | 2.11 ± 0.11 i | ||
| Agglomerated MPIP | Control (no sugar) | 0 | 34.5 ± 0.23 h | 1.29 ± 0.04 c | 2.39 ± 0.07 h |
| Sucrose | 10 | 35.7 ± 0.12 h | 1.04 ± 0.03 g | 2.66 ± 0.01 g | |
| 20 | 39.6 ± 0.20 d | 1.21 ± 0.02 e | 3.01 ± 0.11 e | ||
| Lactose | 10 | 36.5 ± 0.50 g | 1.13 ± 0.03 f | 2.56 ± 0.13 h | |
| 20 | 39.1 ± 0.23 e | 1.25 ± 0.01 d | 2.96 ± 0.04 f | ||
| Xylitol | 10 | 37.7 ± 0.12 f | 1.06 ± 0.01 g | 3.32 ± 0.03 c | |
| 20 | 45.7 ± 0.81 b | 1.20 ± 0.01 e | 3.53 ± 0.08 b | ||
| Sorbitol | 10 | 49.2 ± 0.20 a | 1.76 ± 0.07 a | 3.93 ± 0.18 a | |
| 20 | 41.3 ± 0.46 c | 1.47 ± 0.04 b | 3.19 ± 0.04 d |
| Sample | Binder Type | Binder Conc. (%) | K (Pa·sn) | n | R2 |
|---|---|---|---|---|---|
| Raw MPIP | 1.33 ± 0.01 d | 0.55 ± 0.01 c | 0.99 | ||
| Agglomerated MPIP | Control (no sugar) | 0 | 1.67 ± 0.01 c | 0.55 ± 0.01 c | 0.99 |
| Sucrose | 10 | 1.65 ± 0.04 c | 0.39 ± 0.01 g | 0.99 | |
| 20 | 1.08 ± 0.01 g | 0.57 ± 0.00 b | 0.99 | ||
| Lactose | 10 | 1.35 ± 0.01 d | 0.56 ± 0.00 c | 0.99 | |
| 20 | 1.11 ± 0.01 f | 0.59 ± 0.01 a | 0.99 | ||
| Xylitol | 10 | 2.22 ± 0.02 a | 0.42 ± 0.00 f | 0.99 | |
| 20 | 1.28 ± 0.01 e | 0.53 ± 0.01 d | 0.99 | ||
| Sorbitol | 10 | 1.72 ± 0.01 b | 0.47 ± 0.02 e | 0.99 | |
| 20 | 1.35 ± 0.06 d | 0.51 ± 0.01 e | 0.99 |
| Sample | Binder Type | Binder Conc. (%) | G′ (Pa) | G′′ (Pa) | tan δ |
|---|---|---|---|---|---|
| Raw MPIP | 1.24 ± 0.08 i | 3.17 ± 0.19 i | 2.57 ± 0.16 a | ||
| Agglomerated MPIP | Control (no sugar) | 0 | 3.31 ± 0.20 e | 4.27 ± 0.17 e | 1.29 ± 0.03 e |
| Sucrose | 10 | 3.78 ± 0.27 d | 5.21 ± 0.28 d | 1.38 ± 0.03 d | |
| 20 | 2.59 ± 0.10 f | 3.95 ± 0.09 f | 1.53 ± 0.02 c | ||
| Lactose | 10 | 2.58 ± 0.13 f | 3.89 ± 0.11 f | 1.51 ± 0.04 c | |
| 20 | 2.06 ± 0.03 h | 3.65 ± 0.07 g | 1.77 ± 0.02 b | ||
| Xylitol | 10 | 7.12 ± 0.30 a | 6.56 ± 0.25 a | 0.92 ± 0.03 g | |
| 20 | 2.26 ± 0.26 g | 3.45 ± 0.17 h | 1.54 ± 0.10 c | ||
| Sorbitol | 10 | 6.54 ± 0.10 b | 6.26 ± 0.19 b | 0.96 ± 0.04 g | |
| 20 | 5.08 ± 0.22 c | 6.04 ± 0.22 c | 1.19 ± 0.10 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Yoo, B. Physical, Morphological, and Rheological Properties of Agglomerated Milk Protein Isolate Powders: Effect of Binder Type and Concentration. Polymers 2023, 15, 411. https://doi.org/10.3390/polym15020411
Jeong Y, Yoo B. Physical, Morphological, and Rheological Properties of Agglomerated Milk Protein Isolate Powders: Effect of Binder Type and Concentration. Polymers. 2023; 15(2):411. https://doi.org/10.3390/polym15020411
Chicago/Turabian StyleJeong, Yulim, and Byoungseung Yoo. 2023. "Physical, Morphological, and Rheological Properties of Agglomerated Milk Protein Isolate Powders: Effect of Binder Type and Concentration" Polymers 15, no. 2: 411. https://doi.org/10.3390/polym15020411
APA StyleJeong, Y., & Yoo, B. (2023). Physical, Morphological, and Rheological Properties of Agglomerated Milk Protein Isolate Powders: Effect of Binder Type and Concentration. Polymers, 15(2), 411. https://doi.org/10.3390/polym15020411






