Synthesizing Polyurethane Using Isosorbide in Primary Alcohol Form, and Its Biocompatibility Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
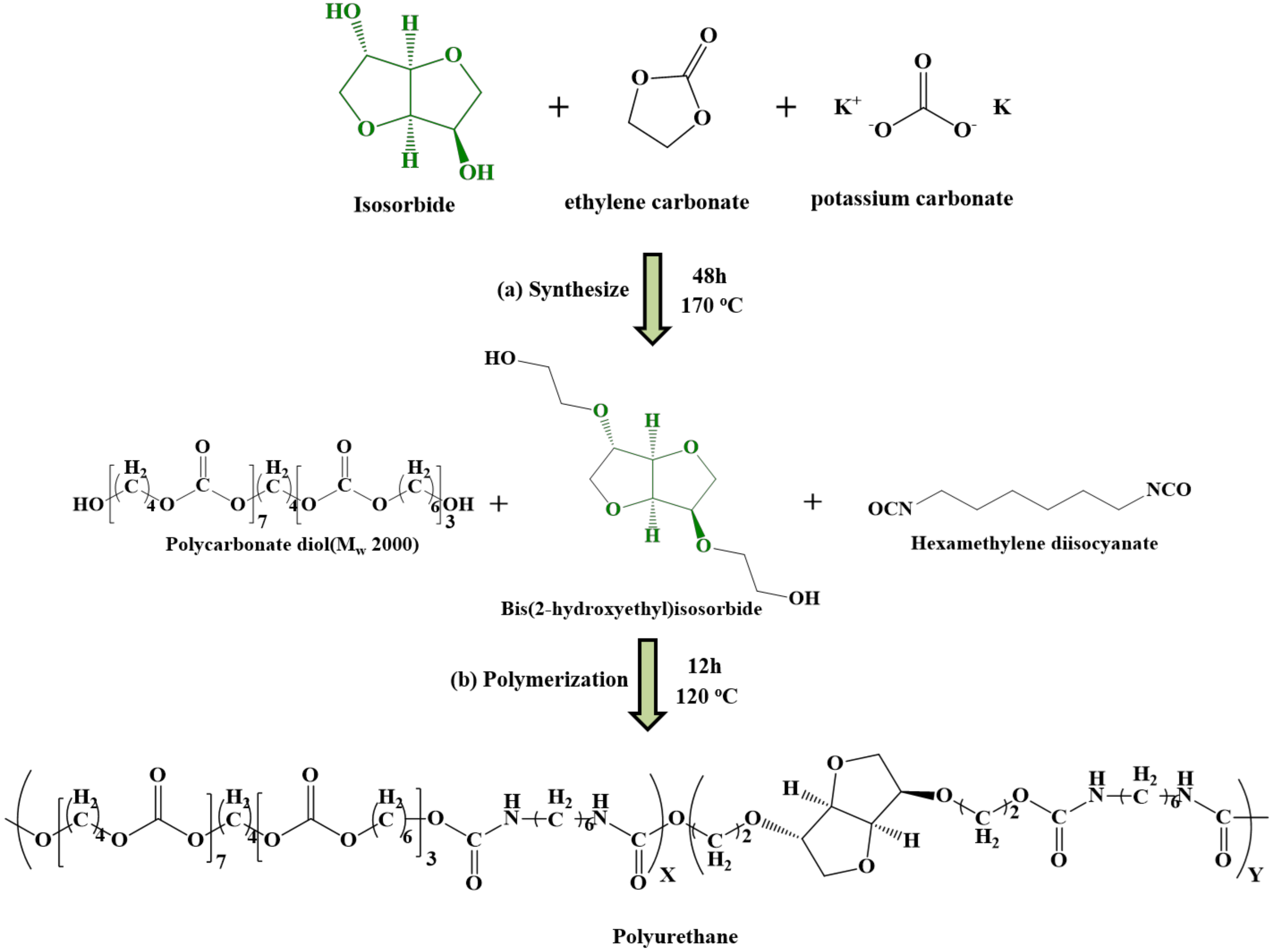
2.2. Synthesis of Bis(2-Hydroxyethyl)Isosorbide (BHIS)
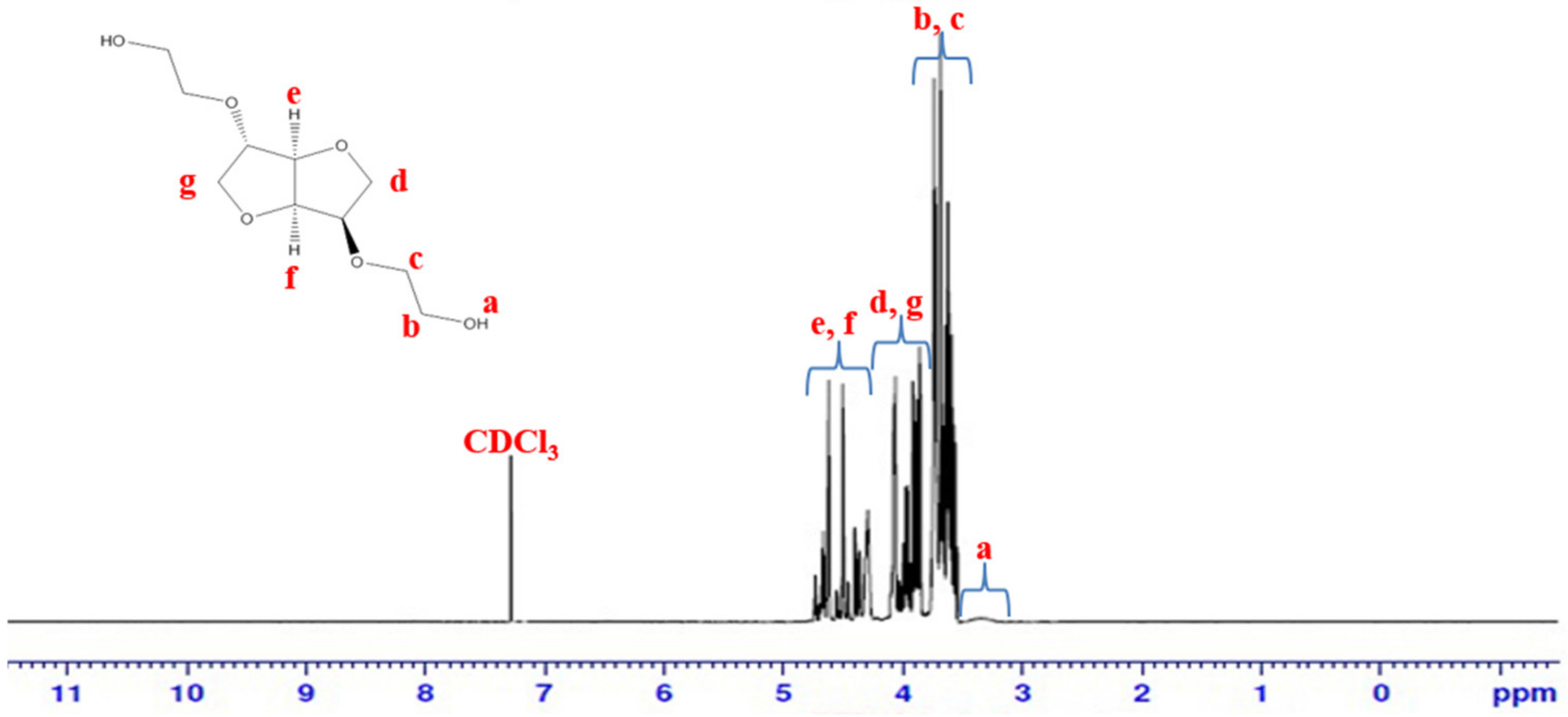
2.3. Characterization of BHIS
2.4. Synthesis of PUs
2.5. Preparation of PU Films
2.6. Characterization of PU Series
2.7. Mechanical Properties
2.8. Degradation Test
2.9. Cell Culture and Cell Viability
2.10. Immunocytochemistry
3. Results and Discussion
3.1. Synthesis and Characterization of BHIS
3.2. Preparation of PUs
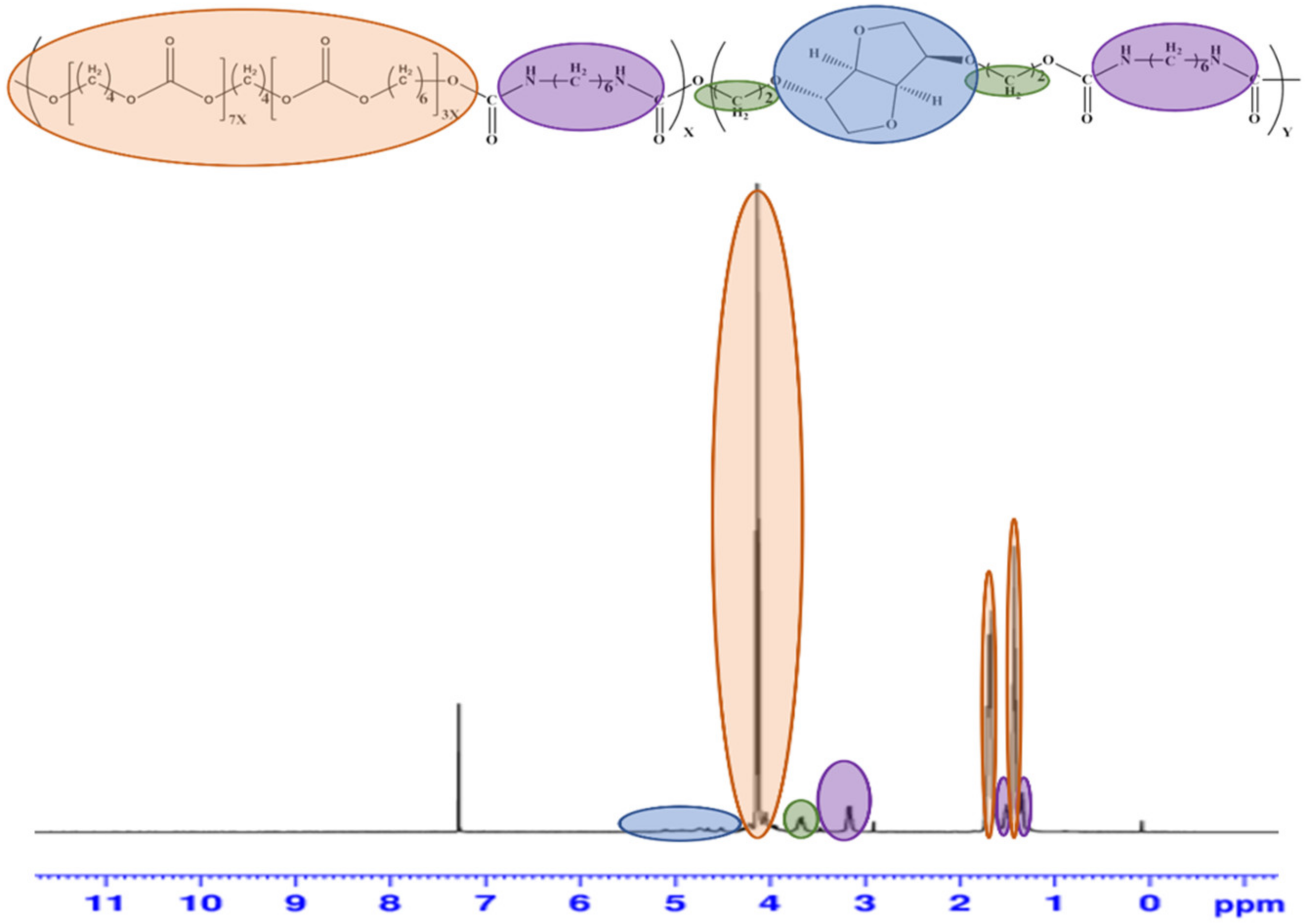
3.3. Characterization of PUs by 1H-NMR
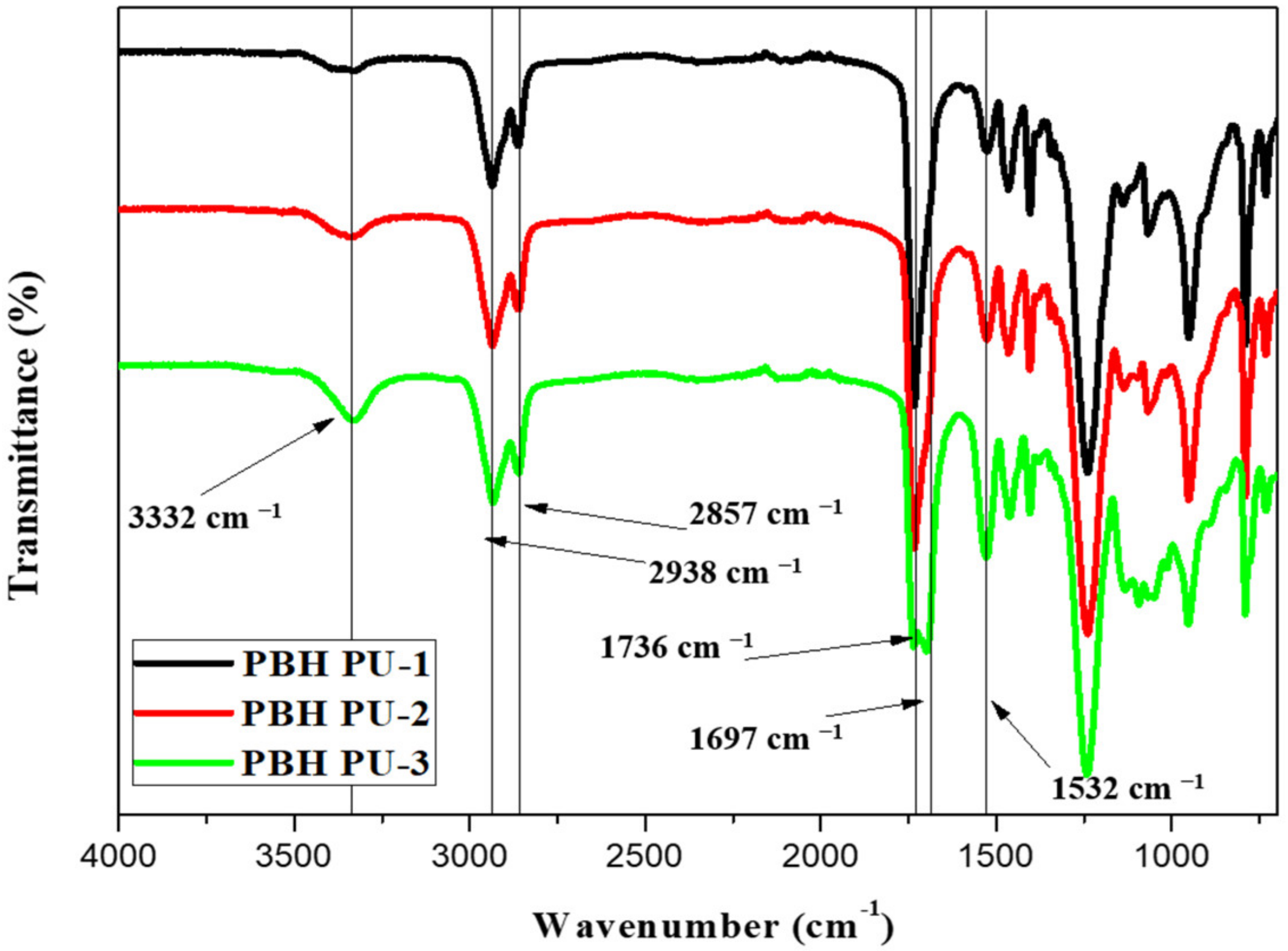
3.4. Characterization of PUs by FT-IR Spectroscopy
3.5. Thermal Properties
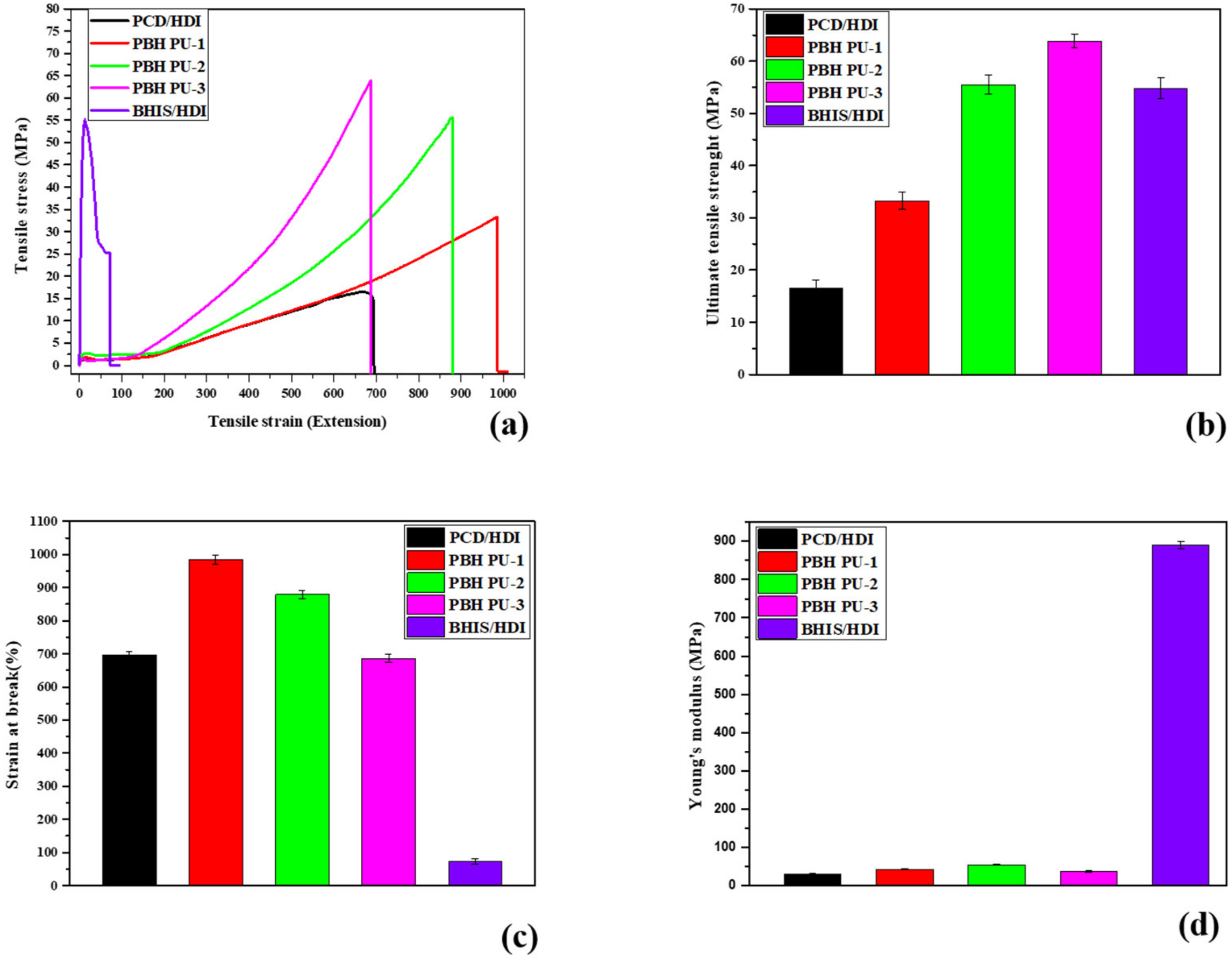
3.6. Mechanical Properties
3.7. Measurement of Contact Angle
3.8. Degradation Test Results
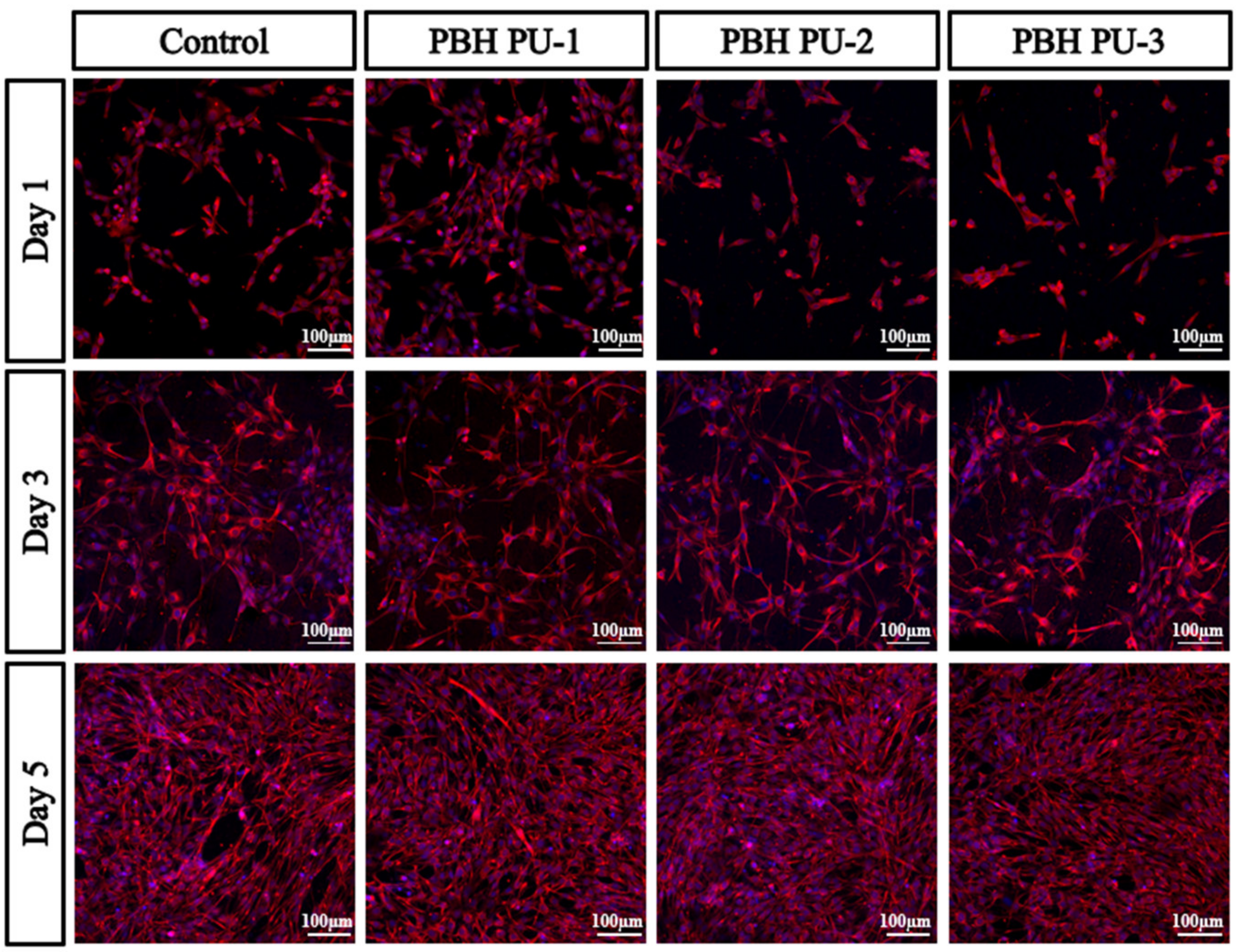
3.9. MTT Assay of Cultured Human Bone Marrow Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications-A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Ferkl, P.; Krskova, I.; Kosek, J. Evolution of Mass Distribution in Walls of Rigid Polyurethane Foams. Chem. Eng. Sci. 2018, 176, 50–58. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Ebrahimi, M. Superhydrophobic modified-polyurethane coatings for bushing of power transformers: From material to fabrication, mechanical and electrical properties. Prog. Org. Coat. 2018, 123, 134–137. [Google Scholar] [CrossRef]
- Ding, H.; Wang, J.; Wang, C.; Chu, F. Synthesis of a novel phosphorus and nitrogen-containing bio-based polyols and its application in flame retardant polyurethane sealant. Polym. Degrad. Stab. 2016, 124, 43–50. [Google Scholar] [CrossRef]
- Tirri, T.; Aubert, M.; Wilén, C.E.; Pfaendner, R.; Hoppe, H. Novel Tetrapotassium Azo Diphosphonate (INAZO) as Flame Retardant for Polyurethane Adhesives. Polym. Degrad. Stab. 2013, 95, 630–636. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M.; Barmar, M. Highly Stretchable Nanoalginate Based Polyurethane Elastomers. Carbohydr. Polym. 2013, 95, 630–636. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2022, 5, 143–158. [Google Scholar] [CrossRef]
- Chen, J.; Dong, R.; Ge, J.; Guo, B.; Ma, P.X. Biocompatible, Biodegradable, and Electroactive Polyurethane-Urea Elastomers with Tunable Hydrophilicity for Skeletal Muscle Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 28273–28285. [Google Scholar] [CrossRef]
- Patel, D.K.; Gupta, V.; Dwivedi, A.; Pandey, S.K.; Aswal, V.K.; Rana, D.; Maiti, P. Biomaterials Using Diamine Modified Graphene Grafted Polyurethane. Polymer 2016, 106, 109–119. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Y.; Tang, L.; Hong, Y. Low-Initial-Modulus Biodegradable Polyurethane Elastomers for Soft Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 2169–2180. [Google Scholar] [CrossRef]
- Acik, G.; Kamaci, M.; Altinkok, C.; Karabulut, H.F.; Tasdelen, M.A. Synthesis and Properties of Soybean Oil-Based Biodegradable Polyurethane Films. Prog. Org. Coat. 2018, 123, 261–266. [Google Scholar] [CrossRef]
- Xiao, R.; Grinstaff, M.W. Chemical Synthesis of Polysaccharides and Polysaccharide Mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Wiecinska, P.; Mizerski, T.; Szafran, M. Monoacryloyl Esters of Carbohydrates: Synthesis, Polymerization and Application in Ceramic Technology. Carbohydr. Polym. 2014, 111, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Battegazzore, D.; Bocchini, S.; Nicola, G.; Martini, E.; Frache, A. Isosorbide, a Green Plasticizer for Thermoplastic Starch That Does Not Retrogradate. Carbohydr. Polym. 2015, 119, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.-P. Polymers From Renewable 1,4:3,6-dianhydrohexitols (Isosorbide, Isomannide and Isoidide): A Review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Fieche, I.G.; Huchette, I.M. Isosorbide—Preparation, Properties and Chemistry. Starch-Starke 1986, 38, 26–30. [Google Scholar]
- Rose, M.; Palkovits, R. Isosorbide as a Renewable Platform Chemical for Versatile Applications—Quo Vadis? ChemSusChem 2012, 5, 167–176. [Google Scholar] [CrossRef]
- Galbis, J.A.; de Garcia García-Martín, M.; de Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef]
- Beach, E.S.; Weeks, B.R.; Stern, R.; Anastas, P.T. Plastics Additives and Green Chemistry. Pure Appl. Chem. 2013, 85, 1611–1624. [Google Scholar] [CrossRef]
- Hammami, N.; Majdoub, M.; Habas, J.-P. Structure-Properties Relationships in Isosorbide-Based Polyacetals: Influence of linear or Cyclic Architecture on Polymer Physicochemical Properties. Eur. Polym. J. 2017, 93, 795–804. [Google Scholar] [CrossRef]
- Darroman, E.; Durand, N.; Boutevin, B.; Caillol, S. New Cardanol/Sucrose Epoxy Blends for Biobased Coatings. Prog. Org. Coat. 2015, 83, 47–54. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Z.; Zhang, L.; Tang, Z.; Zhang, R.; Zhu, J. Isosorbide Dioctoate as a “Green” Plasticizer for Poly(Lactic Acid). Mater. Des. 2016, 91, 262–268. [Google Scholar] [CrossRef]
- Cakić, S.M.; Špírková, M.; Ristić, I.S.; B-Simendić, J.K.; M-Cincović, M.; Poręba, R. The Waterborne Polyurethane Dispersions Based on Polycarbonate Diol: Effect of Ionic Content. Mater. Chem. Phys. 2013, 138, 277–285. [Google Scholar] [CrossRef]
- Pavličević, J.; Špirkova, M.; Strachota, A.; Szécsényi, K.M.; Lazić, N.; Budinski-Simendić, J. The Influence of Montmorillonite and Bentonite Addition on Thermal Properties of Polyurethanes Based on Aliphatic Polycarbonate Diols. Thermochim. Acta 2010, 509, 73–80. [Google Scholar] [CrossRef]
- Costa, V.; Nohales, A.; Félix, P.; Guillem, C.; Gutiérrez, D.; Gómez, C.M. Structure–property relationships of polycarbonate diol-based polyurethanes as a function of soft segment content and molar mass. J. Appl. Polym. Sci. 2015, 132, 41704. [Google Scholar] [CrossRef]
- Gomez, C.M.; Gutierrez, D.; Asensio, M.; Costa, V.; Nohales, A. Transparent Thermoplastic Polyurethanes Based on Aliphatic Diisocyanates and Polycarbonate Diol. J. Elastom Plast. 2017, 49, 77–95. [Google Scholar] [CrossRef]
- Ammar, M.; Cao, Y.; He, P.; Wang, L.; Chen, J.; Li, H. Efficient Green Route for Hexamethylene-1,6-Diisocyanate Synthesis by Thermal Decomposition of Hexamethylene-1,6-dicarbamate Over Co3O4/ZSM-5 Catalyst: An Indirect Utilization of CO2. Chin. J. Chem. Eng. 2017, 25, 1760–1770. [Google Scholar] [CrossRef]
- Hong, S.M.; Yoon, J.Y.; Cha, J.R.; Ahn, J.; Mandakhbayar, N.; Park, J.H.; Im, J.; Jin, G.; Kim, M.Y.; Knowles, J.C. Hyperelastic, shape-memorable, and ultra-cell-adhesive degradable polycaprolactone-polyurethane copolymer for tissue regeneration. Bioeng. Transl. Med. 2022, 7, e10332. [Google Scholar] [CrossRef]
- Park, H.S.; Gong, M.S.; Knowles, J.C. Catalyst-free synthesis of high elongation degradable polyurethanes containing varying ratios of isosorbide and polycaprolactone: Physical properties and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 281–294. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kang, M.-S.; Knowles, J.C.; Gong, M.-S. Synthesis of bio-based thermoplastic polyurethane elastomers containing isosorbide and polycarbonate diol and their biocompatible properties. J. Biomater. Appl. 2015, 30, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Nils, V.S.; Tamara, S.; Stefan, N. Dual Catalytic Ring-Opening Polymerization of Ethylene Carbonate for the Preparation of Degradable PEG. Biomacromolecules 2020, 21, 2661–2669. [Google Scholar]
- Owji, N.; Aldaadaa, A.; Cha, J.-R.; Shakouri, T.; Garcia-Gareta, E.; Kim, H.-W.; Knowles, J.C. Synthesis, Characterization, and 3D Printing of an Isosorbide-Based, Light-Curable, Degradable Polymer for Potential Application in Maxillofacial Reconstruction. ACS Biomater. Sci. Eng. 2020, 6, 2578–2587. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, D.W.; Ryu, H.; Song, G.S.; Lee, D.S. Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules 2019, 24, 1061. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Ding, J.; Wang, Z.; Zhang, H.; Xie, J.; Wang, Y.; Hong, L.; Mao, Z.; Gao, J.; Gao, C. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials 2020, 232, 119703. [Google Scholar] [CrossRef]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent advances in shape memory soft materials for biomedical applications. Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef]
- Blache, H.; Méchin, F.; Rousseau, A.; Fleury, E.; Pascault, J.-P.; Alcouffe, P.; Jacquel, N.; Saint-Loup, R. New bio-based thermoplastic polyurethane elastomers from isosorbide and rapeseed oil derivatives. Ind. Crop. Prod. 2018, 121, 303–312. [Google Scholar] [CrossRef]
- Zhu, Y.; Durand, M.; Molinier, V.; Aubry, J.-M. Isosorbide as a novel polar head derived from renewable resources. Application to the design of short-chain amphiphiles with hydrotropic properties. Green Chem. 2018, 10, 532–540. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, S.C. Effect of chemical structure on the biodegradation of polyurethanes under composting conditions. Polym. Degrad. Stab. 1998, 62, 343. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]





| Polyurethanes | HDI | PCD | BHIS | Yield (%) |
|---|---|---|---|---|
| mole ratio/weight (g) | ||||
| PCD/HDI | 1/(10) | 1/(118.913) | 0/(0) | 95.9 |
| PBH PU-1 | 1/(10) | 0.8/(95.131) | 0.2/(2.786) | 96.8 |
| PBH PU-2 | 1/(10) | 0.5/(59.457) | 0.5/(6.964) | 97.5 |
| PBH PU-3 | 1/(10) | 0.2/(23.783) | 0.8/(11.142) | 97.8 |
| BHIS/HDI | 1/(10) | 0/(0) | 1/(13.928) | 98.1 |
| Polymer | Hydroxyl Numbers (mg KOH/g) | Targeted Mn (g/mol) |
|---|---|---|
| BHIS | 478.51 | 234.47 |
| Polyurethanes | Mw 1 | Mn 2 | PDI 3 |
|---|---|---|---|
| PBH PU-1 | 152,941 | 136,874 | 1.117 |
| PBH PU-2 | 150,318 | 130,845 | 1.149 |
| PBH PU-3 | 154,181 | 137,501 | 1.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-M.; Kwon, H.-J.; Lee, C.-W. Synthesizing Polyurethane Using Isosorbide in Primary Alcohol Form, and Its Biocompatibility Properties. Polymers 2023, 15, 418. https://doi.org/10.3390/polym15020418
Hong S-M, Kwon H-J, Lee C-W. Synthesizing Polyurethane Using Isosorbide in Primary Alcohol Form, and Its Biocompatibility Properties. Polymers. 2023; 15(2):418. https://doi.org/10.3390/polym15020418
Chicago/Turabian StyleHong, Suk-Min, Hyuck-Jin Kwon, and Chil-Won Lee. 2023. "Synthesizing Polyurethane Using Isosorbide in Primary Alcohol Form, and Its Biocompatibility Properties" Polymers 15, no. 2: 418. https://doi.org/10.3390/polym15020418
APA StyleHong, S.-M., Kwon, H.-J., & Lee, C.-W. (2023). Synthesizing Polyurethane Using Isosorbide in Primary Alcohol Form, and Its Biocompatibility Properties. Polymers, 15(2), 418. https://doi.org/10.3390/polym15020418








