Rheological Assessment of Oil-Xanthan Emulsions in Terms of Complex, Storage, and Loss Moduli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Crude Oil
2.1.2. Emulsifying Agent
2.1.3. Xanthan Gums
2.2. Methods
2.2.1. Rheometer
2.2.2. Dynamic Test
3. Results and Discussion
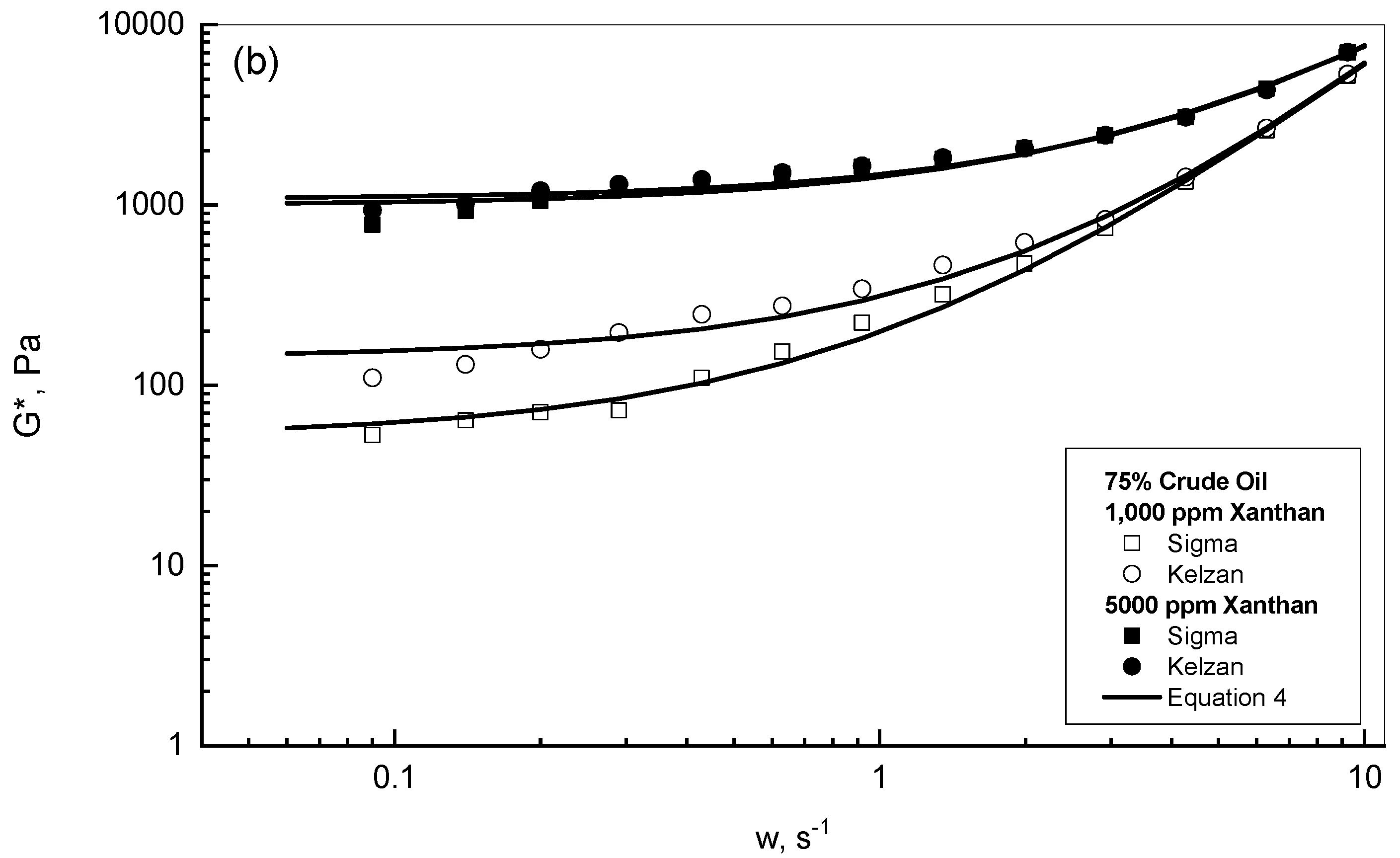
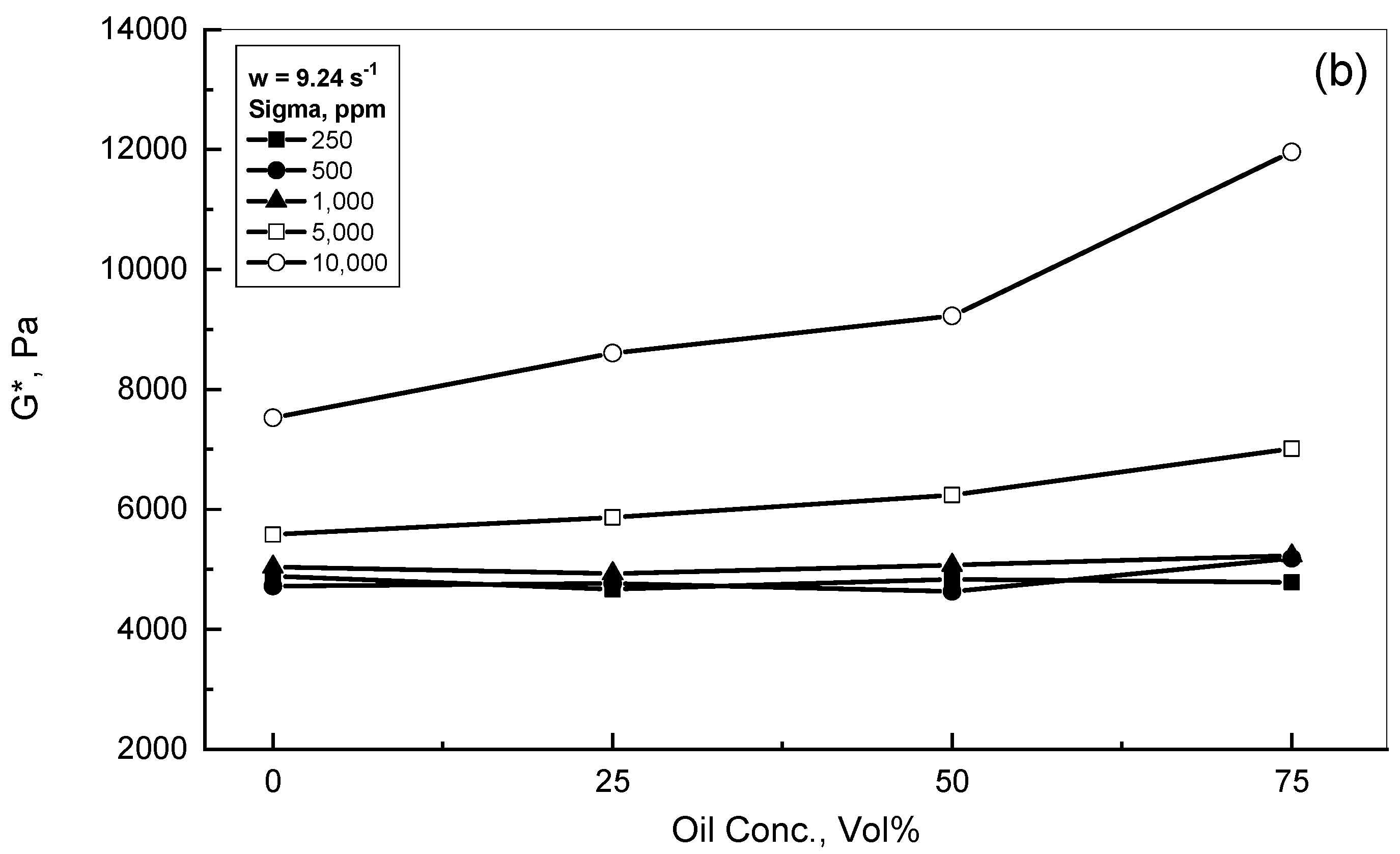
3.1. Complex Modulus Behavior of the Crude Oil-Xanthan Emulsions
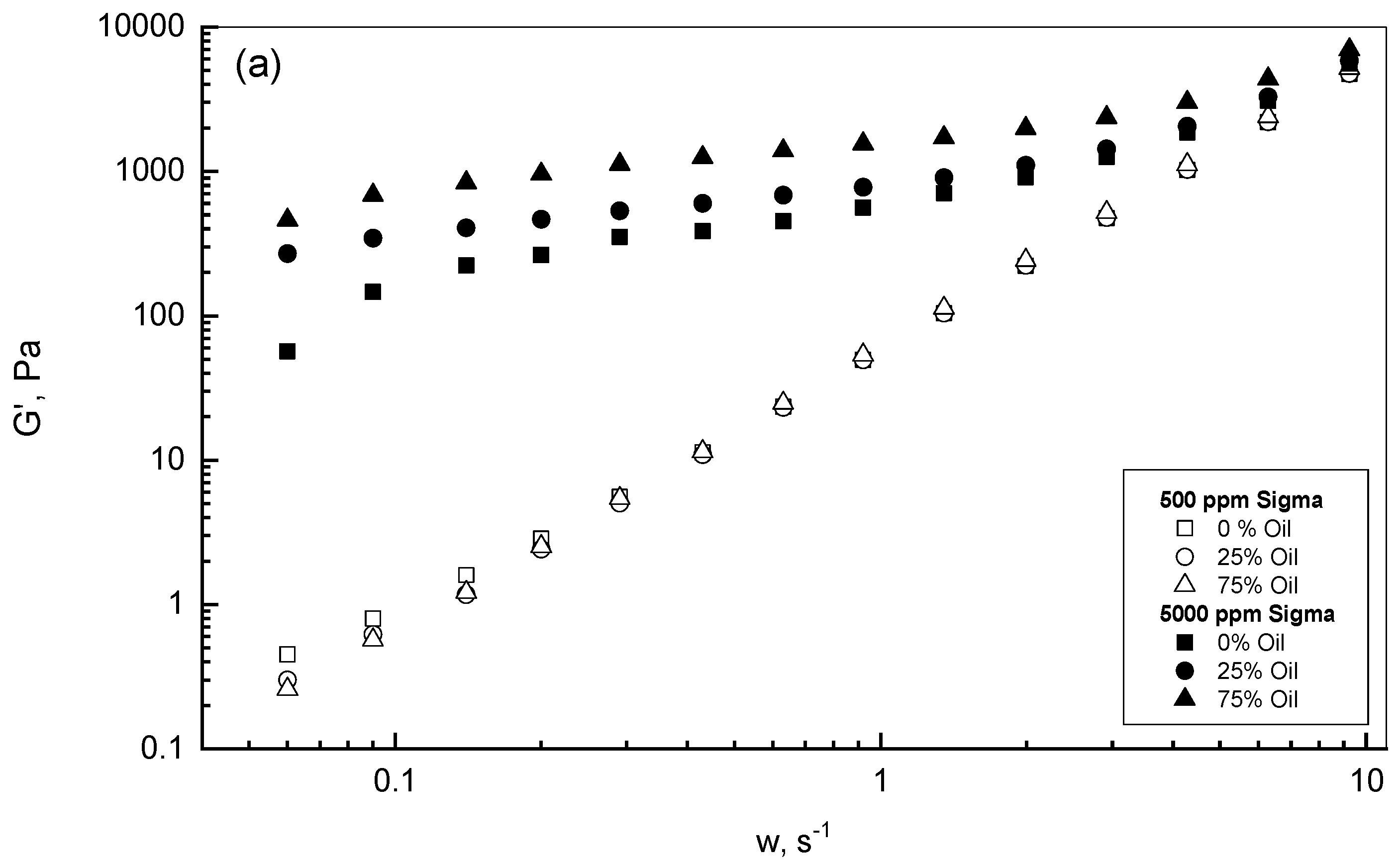
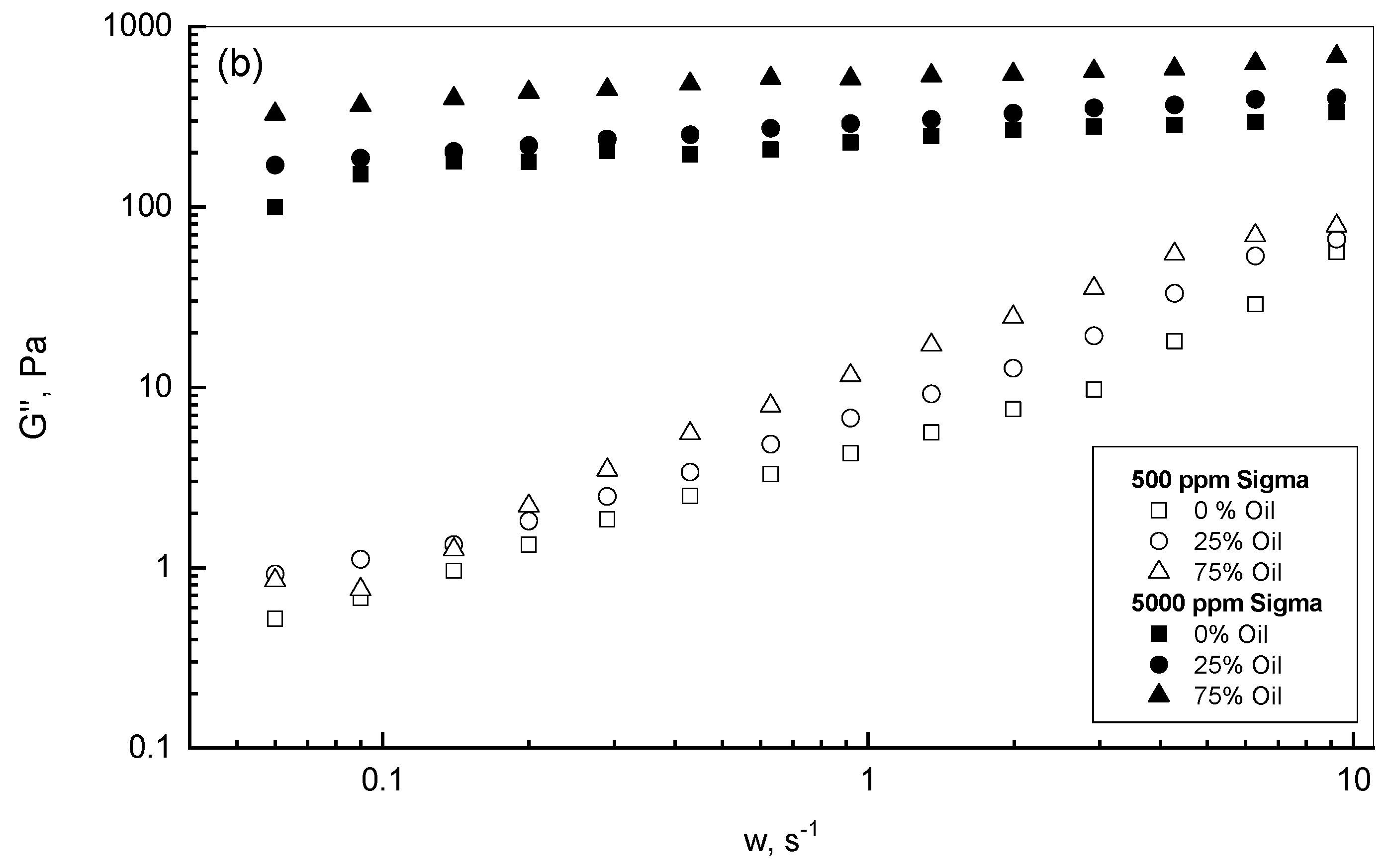
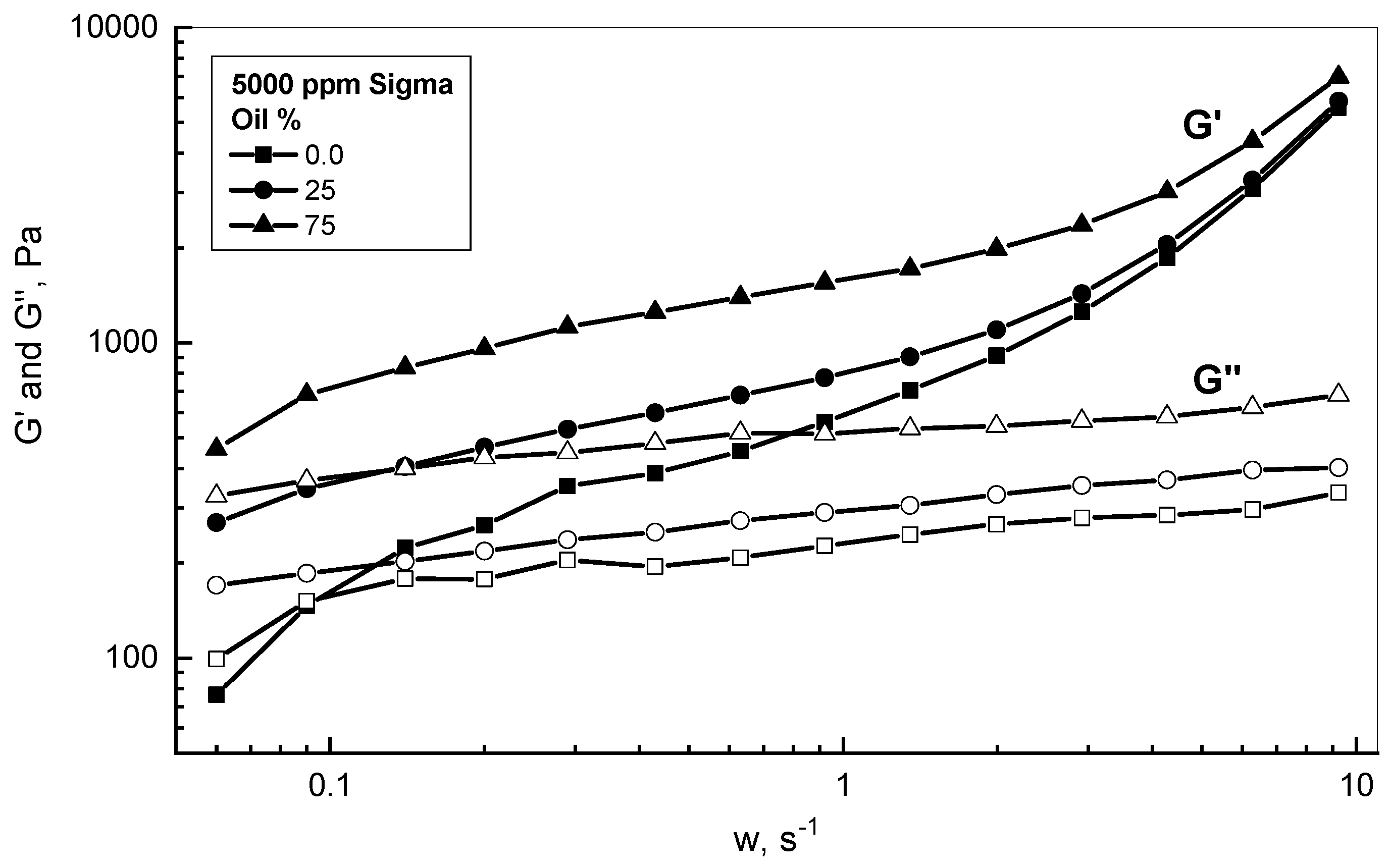
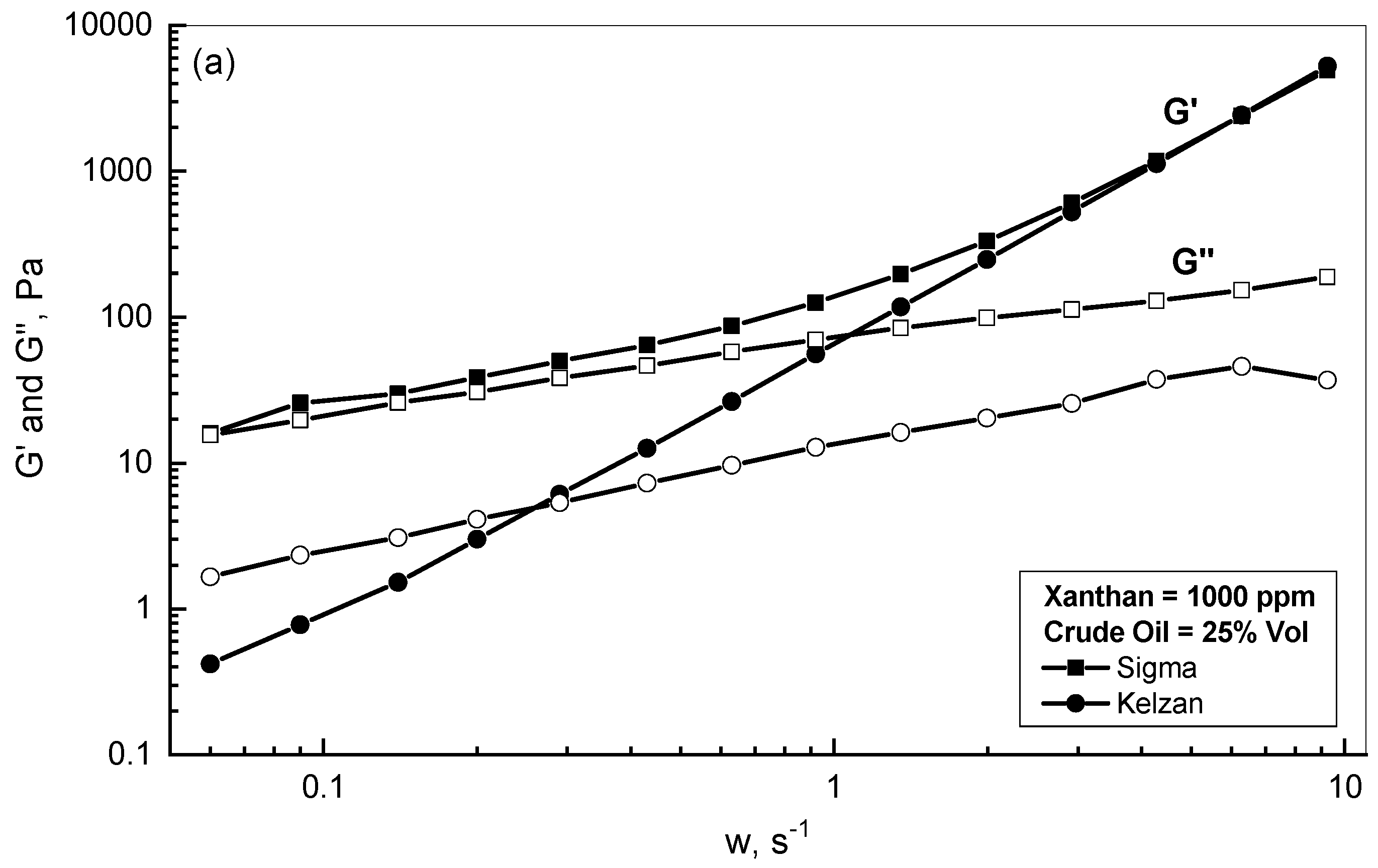
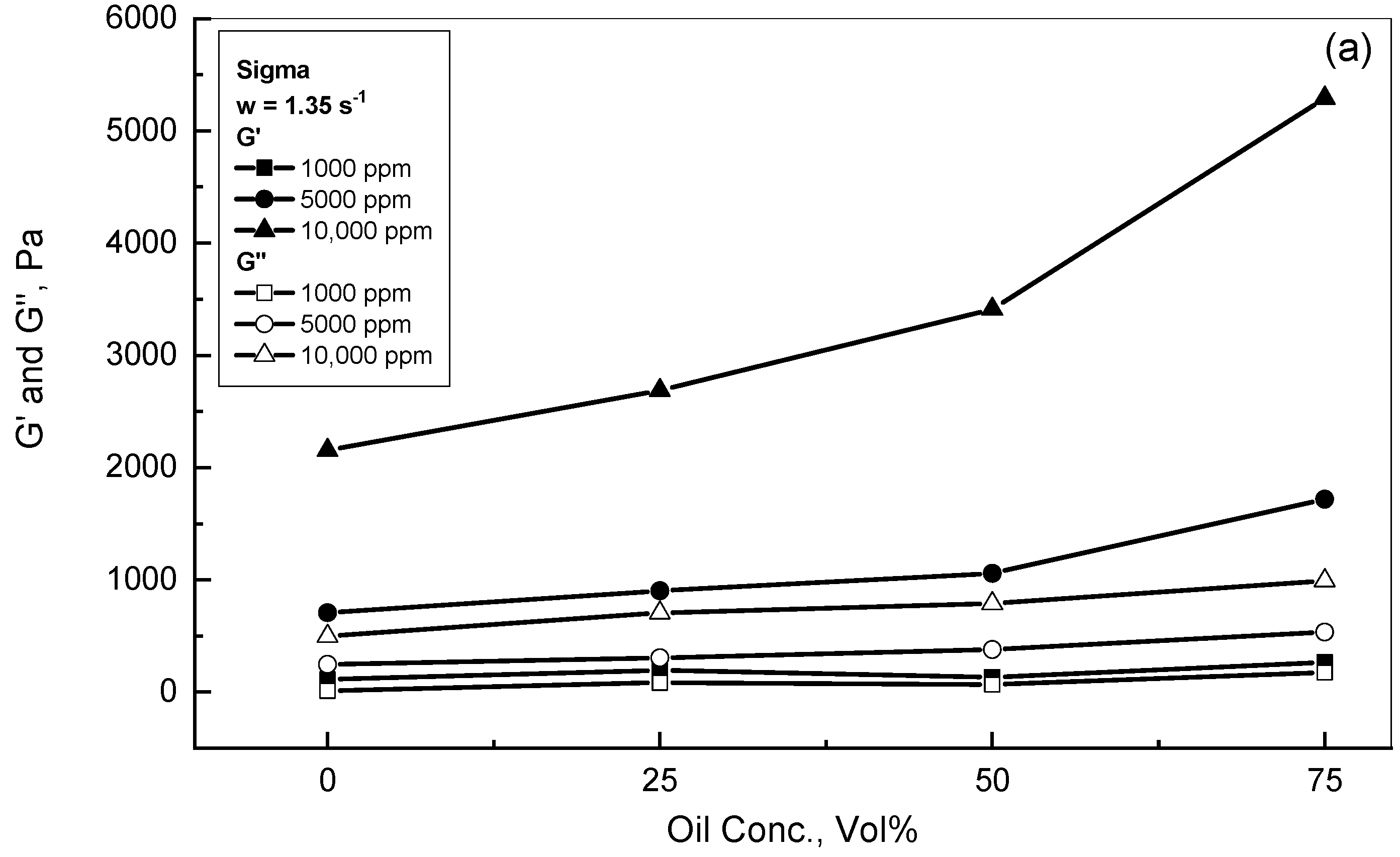
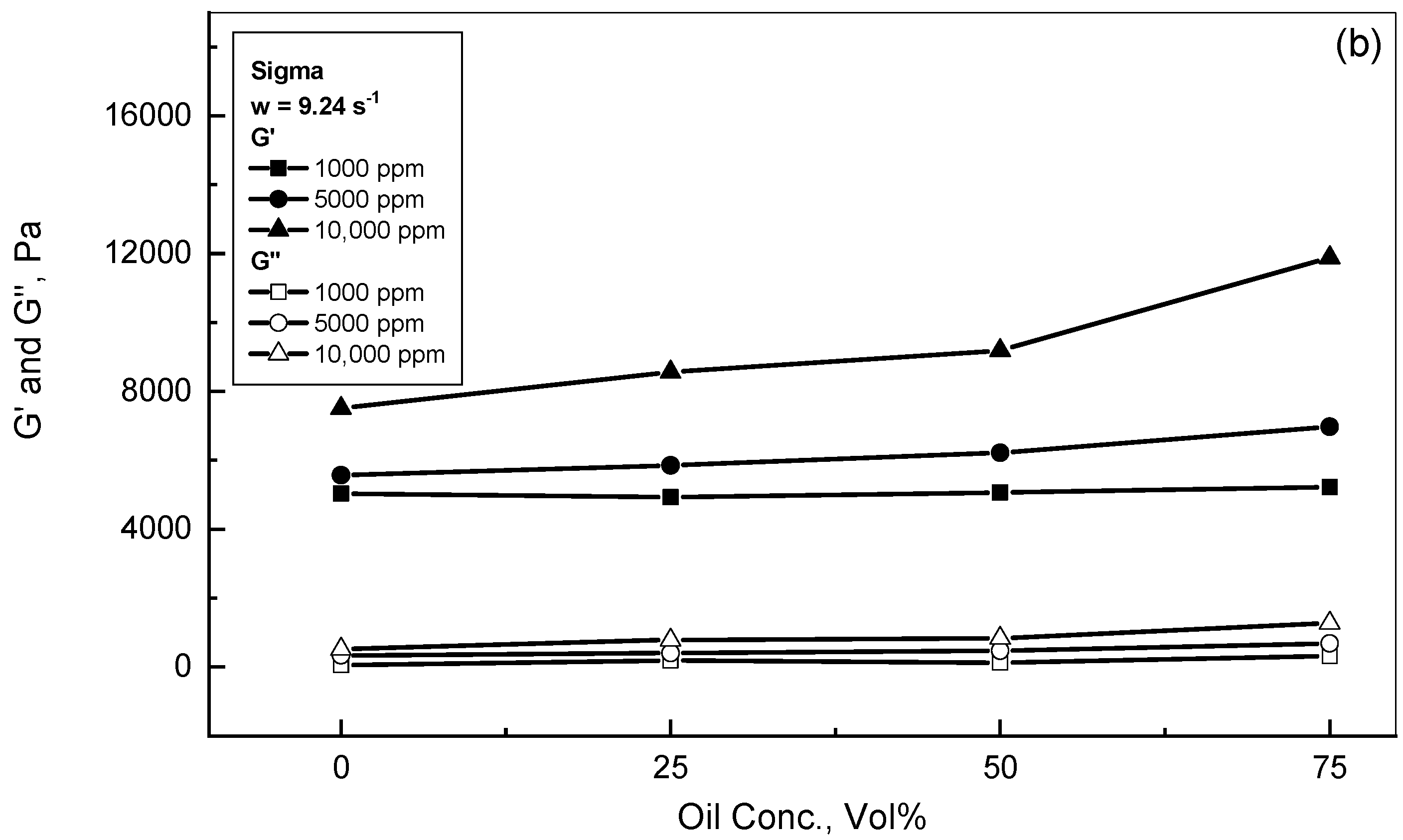
3.2. Crude Oil-Xanthan Emulsion Viscoelastic Behavior in Terms of G′ and G″
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arjmand, O.; Kalbasi, M.; Roostaei, A.R. Experimental Study of Chemical Flooding Using New Chemical Component to Enhance Oil Recovery. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 3056–3061. [Google Scholar]
- Wang, Z.; Le, X.; Zhang, C. The Role of Matching Relationship between Polymer Injection Parameters and Reservoirs in Enhanced Oil Recovery. J. Pet. Sci. Eng. 2013, 111, 139–143. [Google Scholar] [CrossRef]
- Wei, P.; Pu, W.; Sun, L.; Li, D.; Ji, X. Alkyl Polyglucosides Stabilized Foam for Gas Controlling in High-Temperature and High-Salinity Environments. J. Ind. Eng. Chem. 2018, 60, 143–150. [Google Scholar] [CrossRef]
- Shiran, B.S.; Skauge, A. Enhanced Oil Recovery (EOR) by Combined Low Salinity Water/Polymer Flooding. Energy Fuels 2013, 27, 1223–1235. [Google Scholar] [CrossRef]
- Wu, Y.F.; Mahmoudkhani, A.; Watson, P.; Fenderson, T.; Nair, M. Development of New Polymers with Better Performance Under Conditions of High Temperature and High Salinity. In Proceedings of the SPE EOR Conference at Oil and GasWest Asia, SPE 155653, Muscat, Oman, 16–18 April 2012. [Google Scholar]
- Ghannam, M. Emulsion Flow Behavior of Crude Oil-Alcoflood Polymers. J. Chem. Jpn. 2003, 36, 35–44. [Google Scholar] [CrossRef]
- Sherman, P. Encyclopedia of Emulsion Technology; Becher, P., Ed.; Dekker: New York, NY, USA, 1983; Volume 1. [Google Scholar]
- Plegue, T.H.; Frank, S.G.; Fruman, D.H.; Zakin, J.L. Viscosity and Colloidal Properties of Concentrated Crude Oil-in-Water Emulsions. J. Colloid Interface Sci. 1986, 114, 88–105. [Google Scholar] [CrossRef]
- Pal, R.; Rhodes, E. Viscosity/Concentration Relationships for Emulsions. J. Rheol. 1989, 33, 1021–1045. [Google Scholar] [CrossRef]
- Princen, H.M. Rheology of Foams and Highly Concentrated Emulsions: I. Elastic Properties and Yield Stress of a Cylindrical Model System. J. Colloid Interface Sci. 1983, 91, 160–175. [Google Scholar] [CrossRef]
- Princen, H.M. Rheology of Foams and Highly Concentrated Emulsions. II. Experimental Study of the Yield Stress and Wall Effects for Concentrated Oil-in-Water Emulsions. J. Colloid Interface Sci. 1985, 105, 150–171. [Google Scholar] [CrossRef]
- Princen, H.M.; Kiss, A.D. Rheology of Foams and Highly Concentrated Emulsions: III. Static Shear Modulus. J. Colloid Interface Sci. 1986, 112, 427–437. [Google Scholar] [CrossRef]
- Han, C.; King, R. Measurements of the Rheological Properties of Concentrated Emulsions. J. Rheol. 1980, 24, 213–237. [Google Scholar] [CrossRef]
- Pal, R. Rheology of Polymer-Thickened Emulsions. J. Rheol. 1992, 36, 1245–1259. [Google Scholar] [CrossRef]
- Sosa-Herrera, M.G.; Berli, C.L.A.; Martinez-Padilla, L.P. Physicochemical and Rheological Properties of Oil-in-Water Emulsions Prepared with Sodium Caseinate/Gellan Gum Mixtures. Food Hydrocoll. 2008, 22, 934–942. [Google Scholar] [CrossRef]
- Tanaka, H.; White, J. Experimental Investigations of Shear and Elongational Flow Properties of Polystyrene Melts Reinforced with Calcium Carbonate, Titanium Dioxide, and Carbon Black. Poly. Eng. Sci. 1980, 20, 949–956. [Google Scholar] [CrossRef]
- Chan, D.; Powell, R.L. Rheology of Suspensions of Spherical Particles in a Newtonian and a non-Newtonian Fluid. J. Non-Newt. Fluid Mech. 1984, 15, 165–179. [Google Scholar] [CrossRef]
- Metzner, A.B. Rheology of Suspensions in Polymeric Liquids. J. Rheol. 1985, 29, 739–775. [Google Scholar] [CrossRef]
- Gupta, R.K.; Seshadri, S.G. Maximum Loading Levels in Filled Liquid Systems. J. Rheol. 1986, 30, 503–508. [Google Scholar] [CrossRef]
- Poslinski, A.J.; Ryan, M.E.; Gupta, R.K.; Seshadri, S.G.; Frechette, F.J. Rheological Behavior of Filled Polymeric Ssytems I. Yield Stress and Shear-Thinning Effects. J. Rheol. 1988, 32, 703–735. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Gbonhinbor, J.; Onyekonwu, M. Natural Polymer Flow Behaviour in Porous Media for Enhanced Oil Recovery Applications: A Review. J. Petrol. Explor. Prod. Technol. 2018, 8, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterial Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 7, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Vasilieva, N.Y.; Borovkova, V.S.; Fetisova, O.Y.; Issaoui, N.; Malyar, Y.N.; Elsuf’ev, E.V.; Karacharov, A.A.; Skripnikov, A.M.; Miroshnikova, A.V.; et al. Food Xanthan Polysaccharide Sulfation Process with Sulfamic Acid. Foods 2021, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Ryles, R.G. Chemical Stability Limits of Water-Soluble Polymers Used in Oil Recovery Processes. SPE Res. Eng. 1988, 3, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.K.; Ross-Murphy, S.B. Non-Linear Viscoelasticity of Polysaccharide Solutions. 1: Guar Galactomannan Solutions. Int. J. Biol. Macromol. 1987, 9, 250–256. [Google Scholar] [CrossRef]
- Sutherland, I.W. Extracellular Polysaccharides. In Biotechnology; Rehm, H.J., Reed, G., Eds.; VCH: Weinheim, Germany, 1996; Volume 6. [Google Scholar]
- Rodd, A.B.; Dunstan, D.E.; Boger, D.V. Characterisation of Xanthan Gum Solutions Using Dynamic Light Scattering and Rheology. Carbohydr. Polym. 2000, 42, 159–174. [Google Scholar] [CrossRef]
- Whistler, R.L.; BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Eagan Press: St. Paul, MN, USA, 1997. [Google Scholar]
- Milas, M.; Rinaudo, M. Conformation Investigation of the Bacterial Polysaccharide Xanthan. Carbohydr. Res. 1979, 76, 189–196. [Google Scholar] [CrossRef]
- Coviello, T.; Kajiwara, K.; Burchard, W.; Dentini, M.; Crescenzi, V. Solution Properties of Xanthan 1. Dynamic and Static Light Scattering From Native and Modified Xanthans in Dilute Solutions. Macromolecules 1986, 19, 2826–2831. [Google Scholar] [CrossRef]
- Morris, E.R. Bacterial Polysaccharides. In Food Polysaccharides and Their Applications; Stephen, A.M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1995. [Google Scholar]
- Sato, T.; Norisuye, T.; Fujita, H. Double-Stranded Helix of Xanthan: Dimensional and Hydrodynamic Properties in 0.1 M Aqueous Sodium Chloride. Macromolecules 1984, 17, 2696–2700. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Esmail, N. Yield Stress Behavior for Crude Oil-Polymer Emulsions. J. Pet. Sci. Eng. 2005, 47, 105–115. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry; Gebrueder Haake GmbH: Karlsruhe, Germany, 2000. [Google Scholar]
- Ghannam, M.T.; Abu-Jdayil, B.; Esmail, N. Experimental Investigation of Crude Oil-Xanthan Emulsions Flow Behavior. J. Pet. Sci. Res. 2014, 3, 1–15. [Google Scholar] [CrossRef]
- Yeon, H.; Zhang, K.; Hyun, B.; Jin, H. Enhanced Oil Recovery Performance and Viscosity Characteristics of Polysaccharide Xanthan Gum Solution. Ind. Eng. Chem. J. 2014, 21, 741–745. [Google Scholar]
- Khan, S.Y.; Yusuf, M.; Sardar, N. Studies on Rheological Behavior of Xanthan Gum Solutions in Presence of Additives. Pet. Petrochem. Eng. J. 2018, 2, 000165. [Google Scholar]
- Jha, P.K.; Mahto, V.; Saxena, V.K. Study the Effect of Polymers on the Stability and Rheological Properties of Oil-in-Water (O/W) Pickering Emulsion Muds. Korea-Aust. Rheol. J. 2018, 30, 127–136. [Google Scholar] [CrossRef]
- Jha, P.K.; Saxena, V.K.; Yatirajula, S.K.; Kumar, A. Impact of Natural Polymer (Xanthan Gum) and Bentonite Clay on the Development of Oil-In-Water (O/W) Emulsion Drilling Fluids. Int. J. Innov. Technol. Explor. Eng. 2021, 10, 129–136. [Google Scholar] [CrossRef]
- Aomari, N.; Gaudu, R.; Cabioc, F.; Omari, A. Rheology of Water in Crude Oil Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 139, 13–20. [Google Scholar] [CrossRef]
- Li, W.H.; Du, H.; Chen, G.; Yeo, S.H. Experimental Investigation of Creep and Recovery Behaviors of Magnetorheological Fluids. Mater. Sci. Eng. A 2002, 333, 368–376. [Google Scholar] [CrossRef]
- Dolz, M.; Hernández, M.J.; Delegido, J. Creep and Recovery Experimental Investigation of Low Oil Content Food Emulsions. Food Hydrocoll. 2008, 22, 421–427. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Effect of Surface Character of Filler Particles on Rheology of Heat-Set Whey Protein Emulsion Gels. Colloids Surf. B Biointerfaces 1999, 12, 373–381. [Google Scholar] [CrossRef]
- Pal, R. Viscoelastic Properties of Polymer-Thickened Oil-in-Water Emulsions. Chem. Eng. Sci. 1996, 51, 3299–3305. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Furuta, H.; Corredig, M. Study of the Role of the Carbohydrate and Protein Moieties of Soy Soluble Polysaccharides in their Emulsifying Properties. J. Agric. Food Chem. 2004, 52, 5506–5512. [Google Scholar] [CrossRef]
- Shao, P.; Qiu, Q.; Chen, H.; Zhu, J.; Sun, P. Physicochemical Stability of Curcumin Emulsions Stabilized by Ulva Fasciata. Polysaccharide under Different Metallic Ions. Int. J. Biol. Macromol. 2017, 105, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin Extracted from Persimmon Peel: A Physicochemical Characterization and Emulsifying Properties Evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Xiong, L.; Hu, X.; Liu, X.; Tong, Z. Network Chain Density and Relaxation of In Situ Synthesized Polyacrylamide/Hectorite Clay Nanocomposite Hydrogels with Ultrahigh Tensibility. Polymer 2008, 49, 5064–5071. [Google Scholar] [CrossRef]
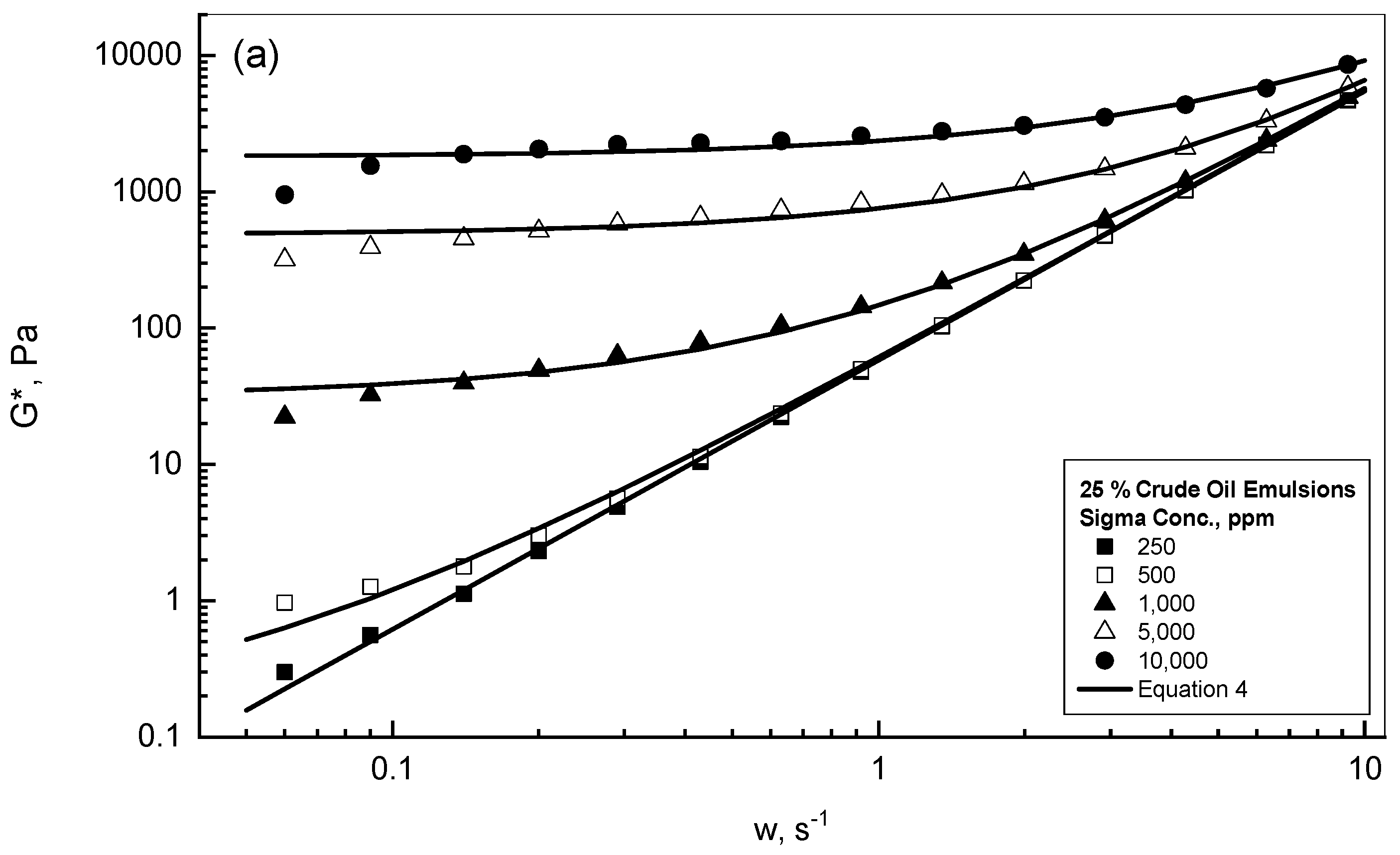
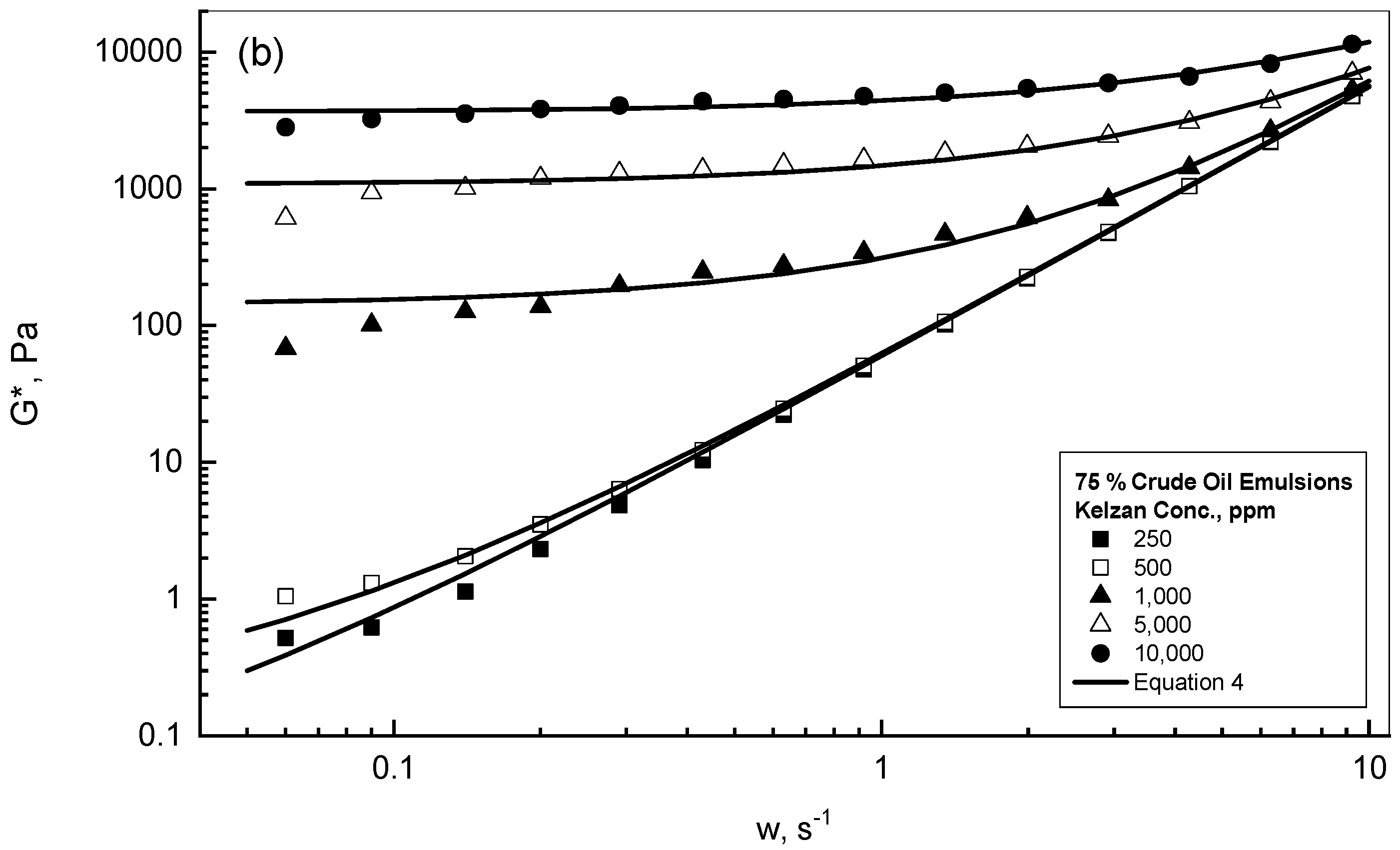
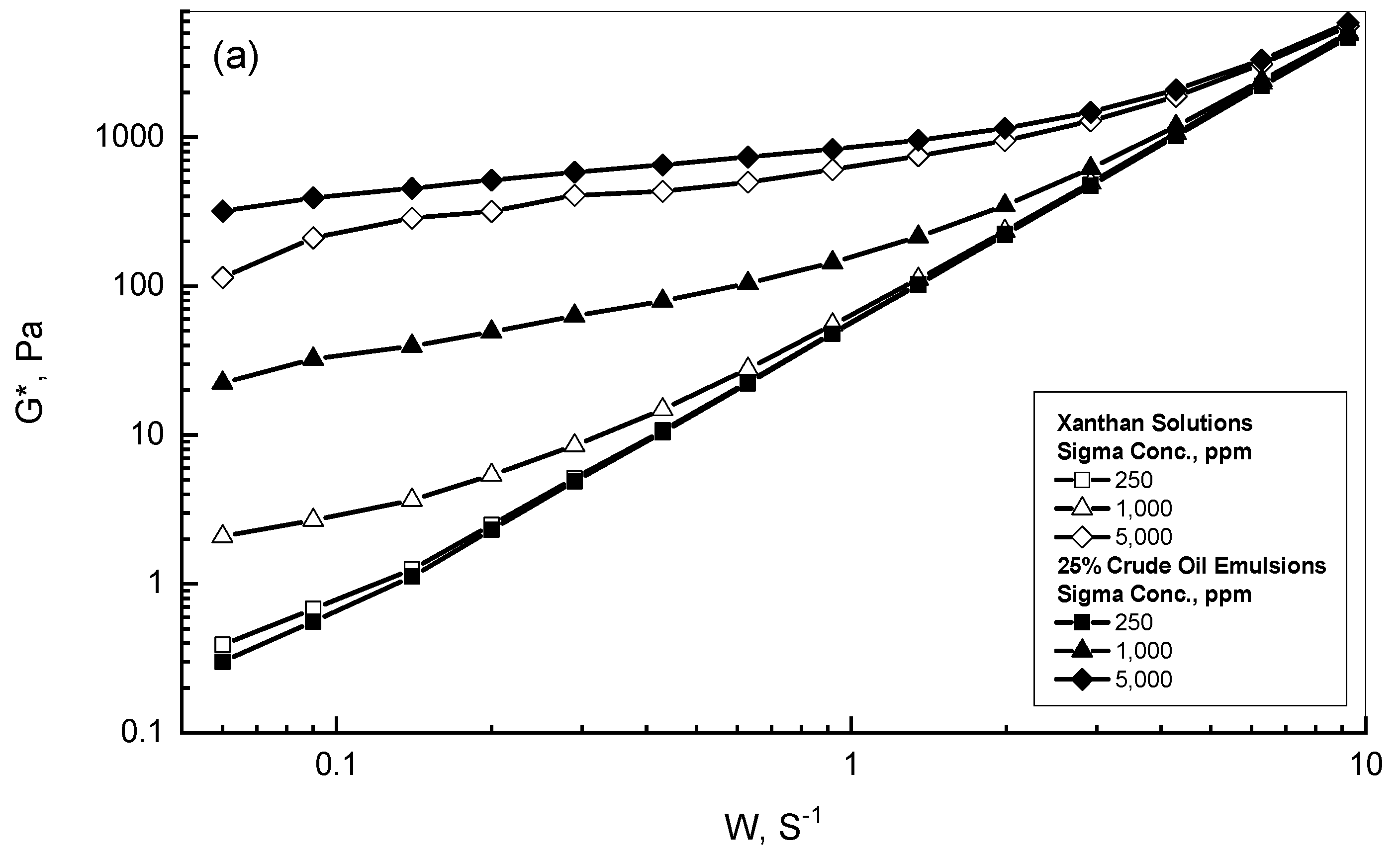


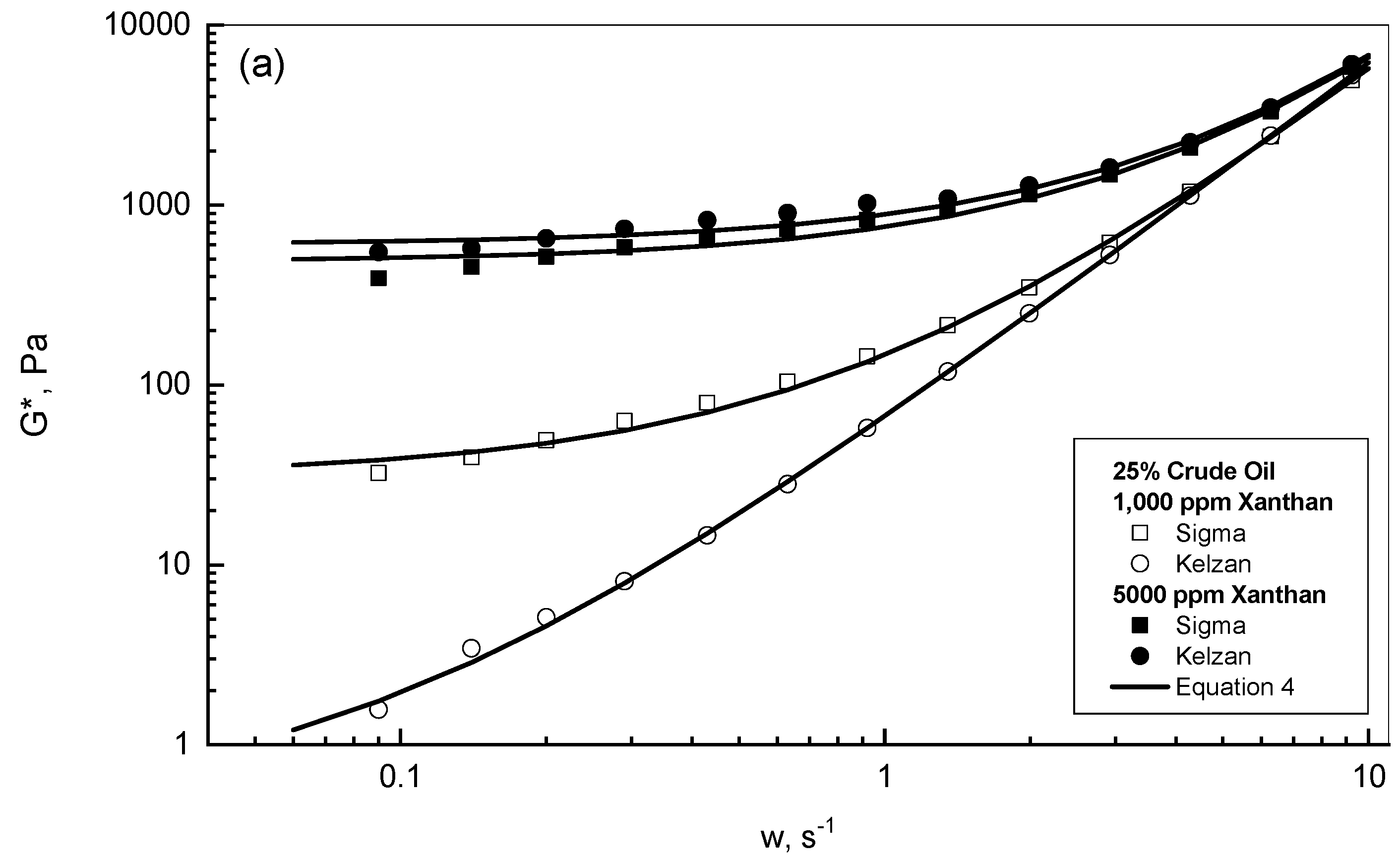


| Gum Conc., ppm | 25% Crude Oil | 75% Crude Oil |
|---|---|---|
| 1000 | 0.2 | 0.3 |
| 5000 | 0.4 | 0.6 |
| 10,000 | 0.9 | 1.0 |
| (a) 25% Crude Oil-Sigma Emulsions | ||||
| Sigma Conc., ppm | Go | B | n | r2 |
| 250 | 0.0 | 61.415 | 1.973 | 0.999 |
| 500 | 0.12 | 56.117 | 1.998 | 0.999 |
| 103 | 25.952 | 29.767 | 2.117 | 0.999 |
| 5000 | 204.866 | 8.781 | 2.325 | 0.997 |
| 10,000 | 16,884.92 | 587.493 | 1.541 | 0.975 |
| (b) 75% Crude Oil-Kelzan emulsions | ||||
| Kelzan Conc., ppm | Go | B | n | r2 |
| 250 | 0.025 | 59.708 | 1.983 | 0.999 |
| 500 | 0.15 | 57.450 | 1.994 | 0.999 |
| 103 | 65.988 | 10.116 | 2.366 | 0.999 |
| 5000 | 1359.302 | 41.616 | 1.936 | 0.984 |
| 10,000 | 1,068,503 | 31,003 | 1.182 | 0.968 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghannam, M.T.; Selim, M.Y.E.; Zekri, A.Y.; Esmail, N. Rheological Assessment of Oil-Xanthan Emulsions in Terms of Complex, Storage, and Loss Moduli. Polymers 2023, 15, 470. https://doi.org/10.3390/polym15020470
Ghannam MT, Selim MYE, Zekri AY, Esmail N. Rheological Assessment of Oil-Xanthan Emulsions in Terms of Complex, Storage, and Loss Moduli. Polymers. 2023; 15(2):470. https://doi.org/10.3390/polym15020470
Chicago/Turabian StyleGhannam, Mamdouh Taha, Mohamed Y. E. Selim, Abdulrazag Y. Zekri, and Nabil Esmail. 2023. "Rheological Assessment of Oil-Xanthan Emulsions in Terms of Complex, Storage, and Loss Moduli" Polymers 15, no. 2: 470. https://doi.org/10.3390/polym15020470
APA StyleGhannam, M. T., Selim, M. Y. E., Zekri, A. Y., & Esmail, N. (2023). Rheological Assessment of Oil-Xanthan Emulsions in Terms of Complex, Storage, and Loss Moduli. Polymers, 15(2), 470. https://doi.org/10.3390/polym15020470







