Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers
Abstract
:1. Introduction
2. Experimental Setup and Methods
3. Experimental Results and Discussions
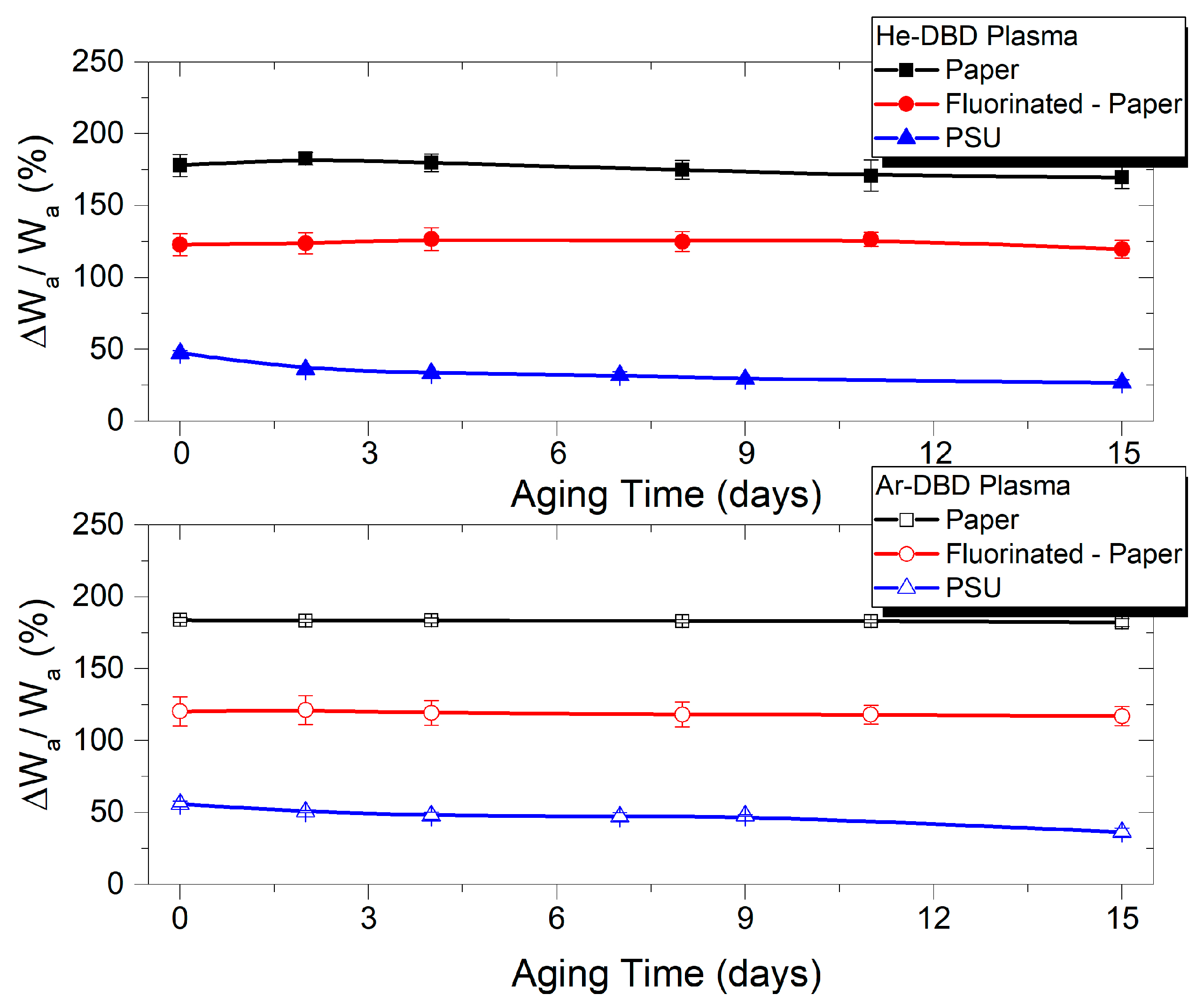
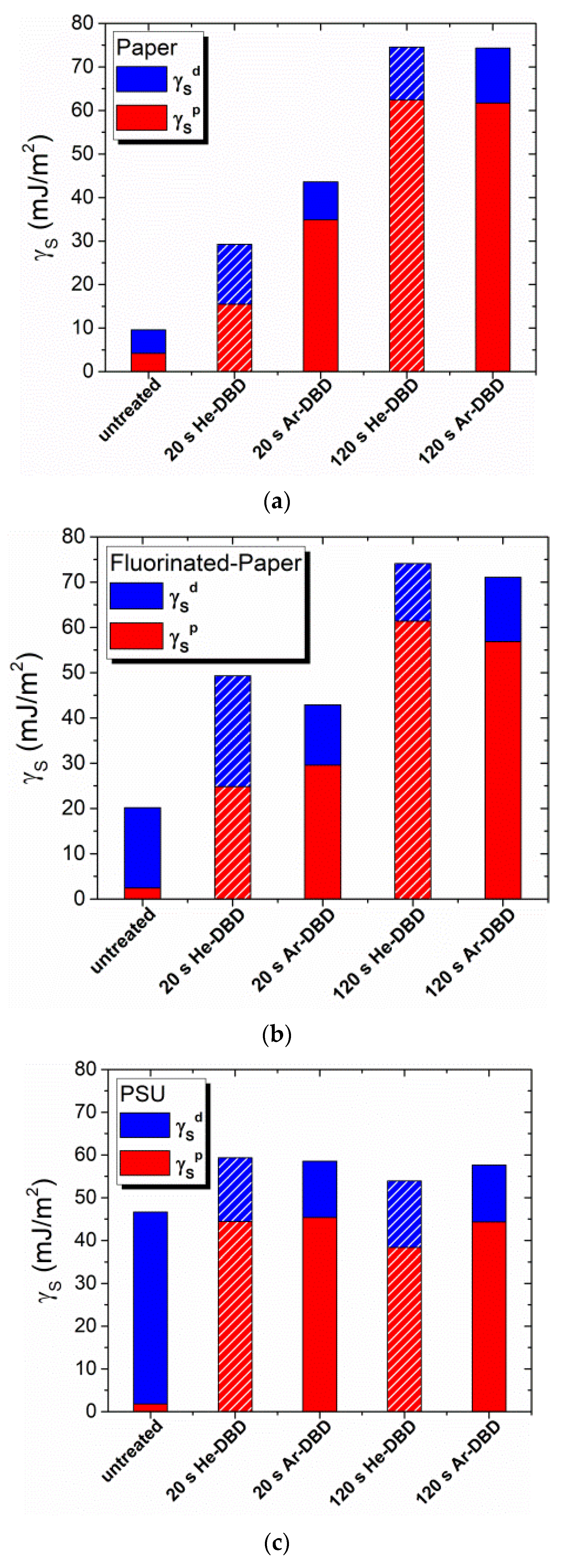
3.1. Contact Angle Measurements
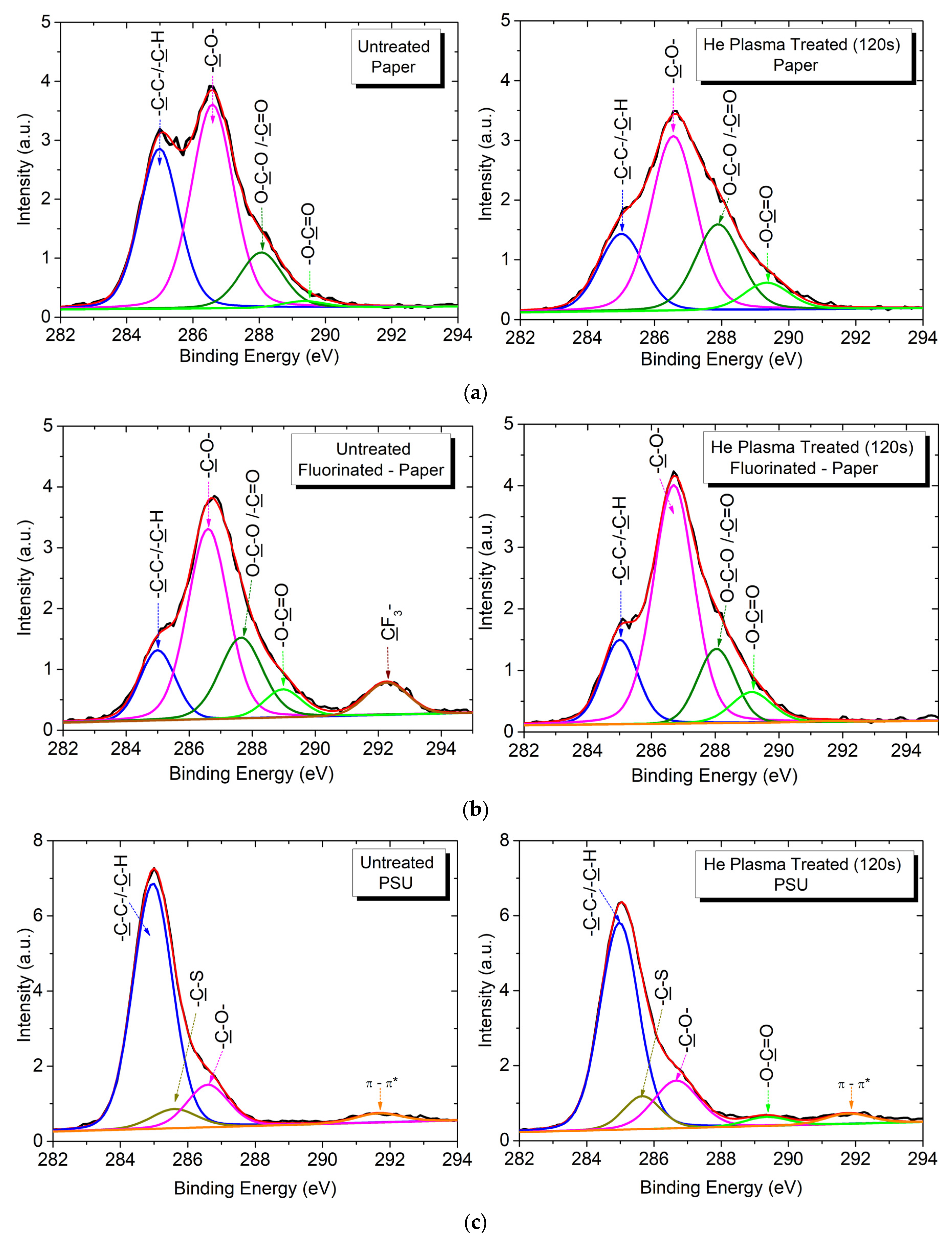
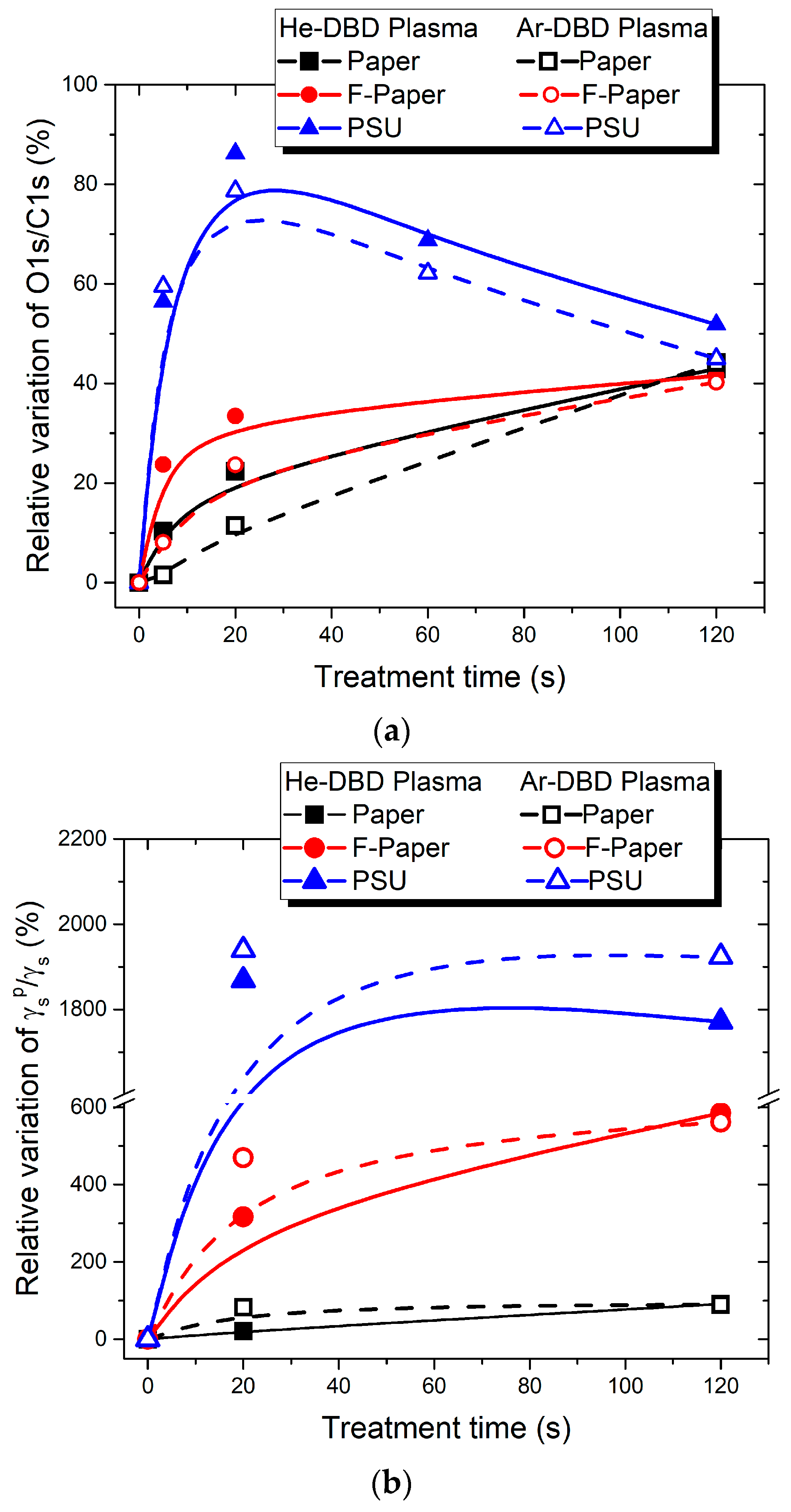
3.2. Surface Chemical Characterization by XPS
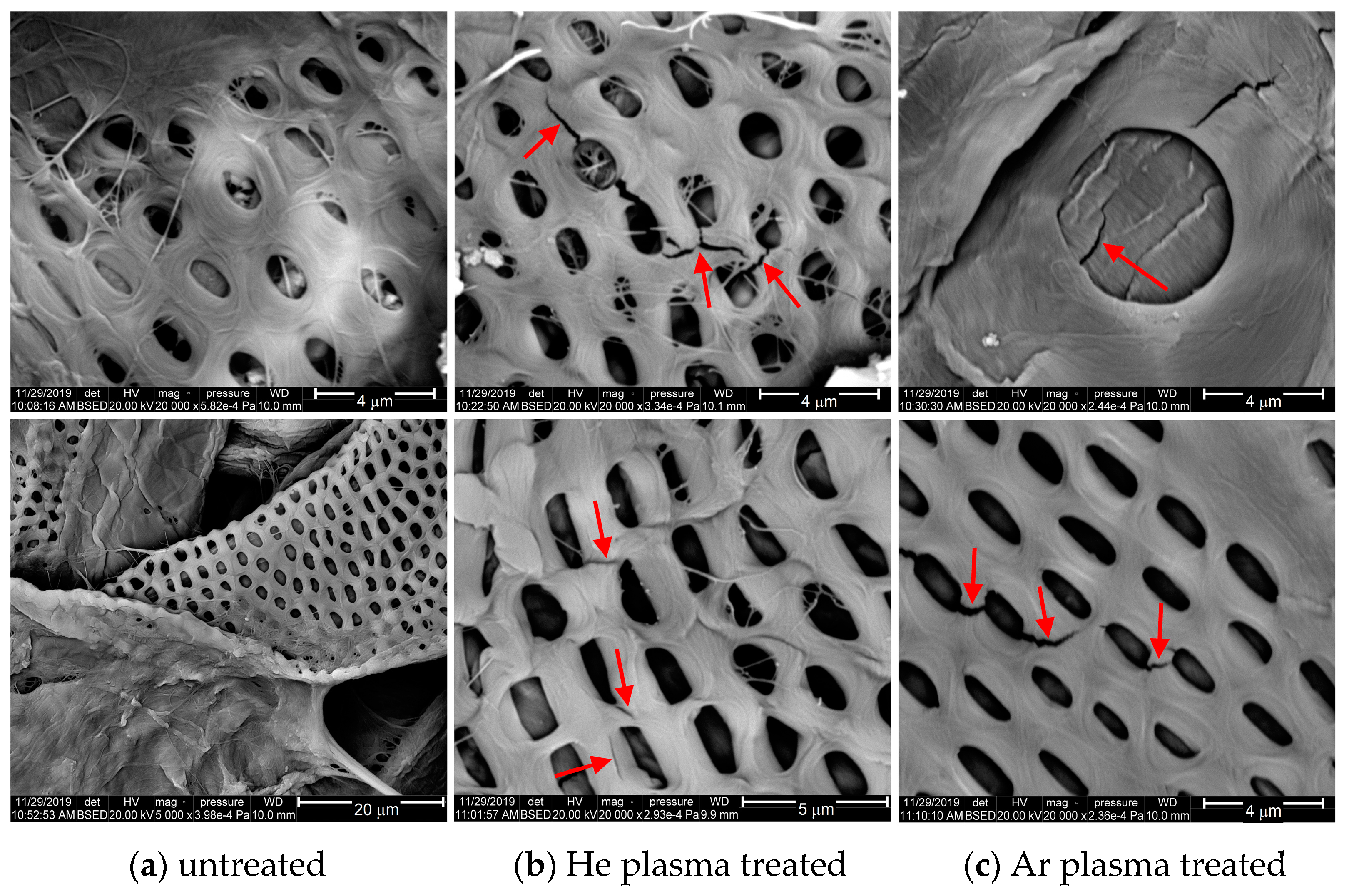
3.3. Surface Morphology Analysis by SEM Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bower, D.I. An Introduction to Polymer Physics; Cambridge University Press: New York, NY, USA, 2002; ISBN 9780511801280. [Google Scholar]
- Obeso, C.G.; Sousa, M.P.; Song, W.; Rodriguez-Pérez, M.A.; Bhushan, B.; Mano, J.F. Modification of Paper Using Polyhydroxybutyrate to Obtain Biomimetic Superhydrophobic Substrates. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 51–55. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Hladnik, A.; Dolenc, J.; Zule, J.; Milosevic, S.; Krstulovic, N.; Klanjšek-Gunde, M.; Hauptmann, N. Modification of Ink-Jet Paper by Oxygen-Plasma Treatment. J. Phys. D Appl. Phys. 2007, 40, 3689–3696. [Google Scholar] [CrossRef]
- Vohrer, U.; Trick, I.; Bernhardt, J.; Oehr, C.; Brunner, H. Plasma Treatment—An Increasing Technology for Paper Restoration? Surf. Coat. Technol. 2001, 142–144, 1069–1073. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Liu, W.; Guo, Y.; Chu, W. Plasma Treatment of Paper for Protein Immobilization on Paper-Based Chemiluminescence Immunodevice. Biosens. Bioelectron. 2016, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, C.; Zhang, C.; Yu, W.; Yang, Q.; Shao, T. Nano-Sized Composite Improving the Insulating Performance of Insulating Paper Using Low-Temperature Plasmas. Nanotechnology 2021, 32, 185704. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Ricard, A. The Production of Active Plasma Species for Surface Treatments. J. Phys. D Appl. Phys. 1997, 30, 2261–2269. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of Atmospheric Pressure Non-Equilibrium Plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- Šimor, M.; Creyghton, Y. Treatment of Polymer Surfaces with Surface Dielectric Barrier Discharge Plasmas. In Atmospheric Pressure Plasma Treatment of Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 27–81. [Google Scholar]
- Pappas, D. Status and Potential of Atmospheric Plasma Processing of Materials. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 020801. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New Developments in Surface Functionalization of Polymers Using Controlled Plasma Treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Fridman, A. Organic and Polymer Plasma Chemistry. In Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008; pp. 589–675. ISBN 9780521847353. [Google Scholar]
- Ghobeira, R.; Esbah Tabaei, P.S.; Morent, R.; De Geyter, N. Chemical Characterization of Plasma-Activated Polymeric Surfaces via XPS Analyses: A Review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Tuominen, M.; Teisala, H.; Aromaa, M.; Stepien, M.; Mäkelä, J.M.; Saarinen, J.J.; Toivakka, M.; Kuusipalo, J. Creation of Superhydrophilic Surfaces of Paper and Board. J. Adhes. Sci. Technol. 2014, 28, 864–879. [Google Scholar] [CrossRef]
- Pawlat, J.; Terebun, P.; Kwiatkowski, M.; Diatczyk, J. RF Atmospheric Plasma Jet Surface Treatment of Paper. J. Phys. D Appl. Phys. 2016, 49, 374001. [Google Scholar] [CrossRef]
- Cornelius, C.; Saquing, C.; Venditti, R.; McCord, M.; Bourham, M. The Effect of Atmospheric Pressure Plasma on Paper and Pulps. Bioresources 2017, 12, 8199–8216. [Google Scholar] [CrossRef]
- Holc, M.; Karlovits, I.; Ravnjak, D.; Palatinus, A.; Junkar, I. Influence of Gaseous Plasma Treatment on Functional Properties of Coated Papers. Wood Res. 2020, 65, 423–436. [Google Scholar] [CrossRef]
- Sawangrat, C.; Thipchai, P.; Kaewapai, K.; Jantanasakulwong, K.; Suhr, J.; Wattanachai, P.; Rachtanapun, P. Surface Modification and Mechanical Properties Improvement of Bamboo Fibers Using Dielectric Barrier Discharge Plasma Treatment. Polymers 2023, 15, 1711. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Vizireanu, S.; Nicolae, C.A.; Frone, A.N.; Casarica, A.; Carpen, L.G.; Dinescu, G. Treatment of Nanocellulose by Submerged Liquid Plasma for Surface Functionalization. Nanomaterials 2018, 8, 467. [Google Scholar] [CrossRef]
- Shi, C.; Ma, H.; Wo, Z.; Zhang, X. Superhydrophobic Modification of the Surface of Cellulosic Materials Based on Honeycomb-like Zinc Oxide Structures and Their Application in Oil–Water Separation. Appl. Surf. Sci. 2021, 563, 150291. [Google Scholar] [CrossRef]
- Lenfeld, P.; Brdlík, P.; Borůvka, M.; Běhálek, L.; Habr, J. Effect of Radiation Crosslinking and Surface Modification of Cellulose Fibers on Properties and Characterization of Biopolymer Composites. Polymers 2020, 12, 3006. [Google Scholar] [CrossRef]
- Pykönen, M.; Sundqvist, H.; Kaukoniemi, O.-V.; Tuominen, M.; Lahti, J.; Fardim, P.; Toivakka, M. Ageing Effect in Atmospheric Plasma Activation of Paper Substrates. Surf. Coat Technol. 2008, 202, 3777–3786. [Google Scholar] [CrossRef]
- Skácelová, D.; Kováčik, D.; Homola, T.; Čech, J.; Černák, M. Surface Modification of Paper and Paperboards Using Atmospheric Pressure Plasmas. In Atmospheric Pressure Plasmas: Processes, Technology and Applications; Parker, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2016; pp. 227–236. [Google Scholar]
- Galmiz, O.; Tucekova, Z.K.; Kelar, J.; Zemanek, M.; Stupavska, M.; Kovacik, D.; Cernak, M. Effect of Atmospheric Pressure Plasma on Surface Modification of Paper. AIP Adv. 2019, 9, 105013. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and Thermal Properties of Native Cellulose. In Cellulose—Fundamental Aspects; InTech: Houston, TX, USA, 2013. [Google Scholar]
- Ansell, M.P.; Mwaikambo, L.Y. The Structure of Cotton and Other Plant Fibres. In Handbook of Textile Fibre Structure; Elsevier: Amsterdam, The Netherlands, 2009; pp. 62–94. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Ida, S. PES (Poly(Ether Sulfone)), Polysulfone. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1528–1534. [Google Scholar]
- Zhao, Y.-F.; Zhu, L.-P.; Yi, Z.; Zhu, B.-K.; Xu, Y.-Y. Improving the Hydrophilicity and Fouling-Resistance of Polysulfone Ultrafiltration Membranes via Surface Zwitterionicalization Mediated by Polysulfone-Based Triblock Copolymer Additive. J. Memb. Sci. 2013, 440, 40–47. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Modification of Polysulfone Ultrafiltration Membranes by CO2 Plasma Treatment. Desalination 2005, 172, 189–205. [Google Scholar] [CrossRef]
- Eren, E.; Sarihan, A.; Eren, B.; Gumus, H.; Kocak, F.O. Preparation, Characterization and Performance Enhancement of Polysulfone Ultrafiltration Membrane Using PBI as Hydrophilic Modifier. J. Memb. Sci. 2015, 475, 1–8. [Google Scholar] [CrossRef]
- Kassa, S.T.; Hu, C.C.; Keshebo, D.L.; Belle Marie Ang, M.; Lai, J.Y.; Chu, J.P. Surface Modification of High-Rejection Ultrafiltration Membrane with Antifouling Capability Using Activated Oxygen Treatment and Metallic Glass Deposition. Appl. Surf. Sci. 2020, 529, 147131. [Google Scholar] [CrossRef]
- Kim, S.; Cohen, Y.; Moses, K.J.; Sharma, S.; Bilal, M. Polysulfone Surface Nano-Structured with Tethered Polyacrylic Acid. Appl. Surf. Sci. 2019, 470, 411–422. [Google Scholar] [CrossRef]
- Flynn, C.N.; Byrne, C.P.; Meenan, B.J. Surface Modification of Cellulose via Atmospheric Pressure Plasma Processing in Air and Ammonia–Nitrogen Gas. Surf. Coat. Technol. 2013, 233, 108–118. [Google Scholar] [CrossRef]
- Kolářová, K.; Vosmanská, V.; Rimpelová, S.; Švorčík, V. Effect of Plasma Treatment on Cellulose Fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Mikula, M.; Sutý, S.; Jancovicova, V.; Trnovec, B.; Černáková, Ľ.; Šutý, Š.; Jančovičová, V. Stabilization of Paper by Nitrogen Plasma Assisted Application of Chitosan at Atmospheric Pressure. Acta Chim. Slovaca 2009, 2, 62–69. [Google Scholar]
- Chiper, A.; Borcia, G. Argon Versus Helium Dielectric Barrier Discharge for Surface Modification of Polypropylene and Poly(Methyl Methacrylate) Films. Plasma Chem. Plasma Process. 2013, 33, 553–568. [Google Scholar] [CrossRef]
- Chiper, A.S.; Rusu, G.B.; Vitelaru, C.; Mihaila, I.; Popa, G. A Comparative Study of Helium and Argon DBD Plasmas Suitable for Thermosensitive Materials Processing. Rom. J. Phys. 2011, 56, 126–131. [Google Scholar]
- Chiper, A.S. Systematic Investigation of the Pulsed Barrier Discharges in Flowing and Stationary Gas: From Differences to Similarities. Phys Plasmas 2021, 28, 053511. [Google Scholar] [CrossRef]
- Chiper, A.S. Tailoring the Working Gas Flow to Improve the Surface Modification of Plasma-Treated Polymers. Mater. Lett. 2021, 305, 130832. [Google Scholar] [CrossRef]
- Adam, N.K.; Livingston, H.K. Contact Angles and Work of Adhesion. Nature 1958, 182, 128. [Google Scholar] [CrossRef]
- Good, R.J.; Srivatsa, N.R.; Islam, M.; Huang, H.T.L.; Van Oss, C.J. Theory of the Acid-Base Hydrogen Bonding Interactions, Contact Angles, and the Hysteresis of Wetting: Application to Coal and Graphite Surfaces. J. Adhes. Sci. Technol. 1990, 4, 1–617. [Google Scholar] [CrossRef]
- Good, R.J.; Girifalco, L.A. A Theory for Estimation of Surface and Interfacial Energies, III: Estimation of Surface Energies of Solids from Contact Angle Data. J. Phys. Chem. 1960, 64, 561–565. [Google Scholar] [CrossRef]
- Peršin, Z.; Vesel, A.; Kleinschek, K.S.; Mozetič, M. Characterisation of Surface Properties of Chemical and Plasma Treated Regenerated Cellulose Fabric. Text. Res. J. 2012, 82, 2078–2089. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Andrade, F.K.; Alves, C.; Gama, M. Surface Modification of Bacterial Cellulose by Nitrogen-Containing Plasma for Improved Interaction with Cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Kurniawan, H.; Lai, J.-T.; Wang, M.-J. Biofunctionalized Bacterial Cellulose Membranes by Cold Plasmas. Cellulose 2012, 19, 1975–1988. [Google Scholar] [CrossRef]
- Hopkins, J.; Badyal, J.P.S. XPS and Atomic Force Microscopy of Plasma-Treated Polysulf One. J. Polym. Sci. A Polym. Chem. 1996, 34, 1385–1393. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Paul, D.R.; Arendt, M.; Yuan, Y.; Cabasso, I. Modification of Asymmetric Polysulfone Membranes by Mild Surface Fluorination Part II. Characterizationof the Fluorinated Surface. J. Memb. Sci. 1994, 94, 143–162. [Google Scholar] [CrossRef]
- Matouk, Z.; Torriss, B.; Rincón, R.; Dorris, A.; Beck, S.; Berry, R.M.; Chaker, M. Functionalization of Cellulose Nanocrystal Films Using Non-Thermal Atmospheric –Pressure Plasmas. Appl. Surf. Sci. 2020, 511, 145566. [Google Scholar] [CrossRef]
- Kawano, T.; Wang, M.-J.; Andou, Y. Surface Modification of a Regenerated Cellulose Film Using Low-Pressure Plasma Treatment with Various Reactive Gases. ACS Omega 2022, 7, 44085–44092. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, K.H.; Cho, K.; Park, C.E. Surface Modification of Polysulfone Ultrafiltration Membrane by Oxygen Plasma Treatment. J. Memb. Sci. 2002, 199, 135–145. [Google Scholar] [CrossRef]
- Vicente, A.T.; Araújo, A.; Gaspar, D.; Santos, L.; Marques, A.C.; Mendes, M.J.; Pereira, L.; Fortunato, E.; Martins, R. Optoelectronics and Bio Devices on Paper Powered by Solar Cells. In Nanostructured Solar Cells; InTech: Houston, TX, USA, 2017. [Google Scholar]
- Inbakumar, S.; Morent, R.; De Geyter, N.; Desmet, T.; Anukaliani, A.; Dubruel, P.; Leys, C. Chemical and Physical Analysis of Cotton Fabrics Plasma-Treated with a Low Pressure DC Glow Discharge. Cellulose 2010, 17, 417–426. [Google Scholar] [CrossRef]
- Calvimontes, A.; Mauersberger, P.; Nitschke, M.; Dutschk, V.; Simon, F. Effects of Oxygen Plasma on Cellulose Surface. Cellulose 2011, 18, 803–809. [Google Scholar] [CrossRef]



| Test Liquid | γL (mJ/m2) | γLd (mJ/m2) | γLp (mJ/m2) |
|---|---|---|---|
| Water (W) | 72.8 | 21.8 | 51.0 |
| Glycerol (G) | 64.0 | 34.0 | 30.0 |
| Treatment Time (s) | Paper | Fluorinated Paper | PSU | |||
|---|---|---|---|---|---|---|
| WGA (°) | GCA (°) | WGA (°) | GCA (°) | WGA (°) | GCA (°) | |
| 0 | 107.4 ± 3.3 | 103 ± 3.0 | 98.8 ± 3.1 | 88.0 ± 3.0 | 83 ± 1.4 | 63.3 ± 1.6 |
| ||||||
| 20 | 75.6 ± 3.3 | 69.5 ±6.5 | 52.3 ±3.2 | 41.0 ± 4.0 | 36.5 ± 2.4 | 32.3 ± 1.3 |
| 120 | 3.9 ± 1.0 | 9.0 ± 1.5 | 5.4 ± 1.0 | 7.5 ± 1.5 | 43.8 ± 1.2 | 38.8 ± 1.4 |
| ||||||
| 20 | 57.4 ± 6.0 | 56.6 ± 7.2 | 57.6 ± 4.1 | 53.4 ± 7.0 | 38.0 ± 1.1 | 35.4 ± 1.7 |
| 120 | 3.5 ± 1.0 | 6.8 ± 2.0 | 15.5 ± 1.5 | 12.1 ± 1.5 | 39.2 ± 1.7 | 36.4 ± 1.0 |
| Treatment Time (s) | γSp/γS | ||
|---|---|---|---|
| Paper | Fluorinated Paper | PSU | |
| 0 | 0.437 | 0.121 | 0.038 |
| |||
| 20 | 0.529 | 0.504 | 0.748 |
| 120 | 0.837 | 0.829 | 0.711 |
| |||
| 20 | 0.799 | 0.689 | 0.775 |
| 120 | 0.830 | 0.800 | 0.769 |
| Functional Groups | Assignment | Binding Energy (eV) | |
|---|---|---|---|
| Paper/Fluorinated Paper | PSU | ||
| carbon–hydrogen –C–C–, –C–H | C1 | C1 | 285.0 |
| carbon–sulphur –C–S | - | C2 | 285.6 |
| carbon–oxygen –C–O– | C3 | C3 | 286.5 |
| carbonyl –C=O/O–C–O | C4 | - | 288.0 |
| carboxyl –O–C=O | C5 | C5 | 289.0 |
| π−π* shake-up | - | C6 | ~291.8 |
| 2 fluorine–carbon –CF2– and 3 fluorine–carbon –CF3 | -/C7 | - | ~292.3 |
| Treatment Time (s) | Total Atomic Content (at. %) | Atomic Composition of the Carbon Groups C1s (at. %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | Si2p | O1s/C1s | –C–C–; –C–H | –C–O– | –C=O/ O–C–O | –O–C=O | O/C | |
| Binding Energy (eV) | |||||||||
| 285.0 ± 0.1 | 286.5 ± 0.1 | 288.0 ± 0.2 | 289.0 ± 0.2 | ||||||
| 0 | 66.0 | 33.1 | 0.9 | 0.50 | 35.7 ± 0.2 | 49.3 ± 0.5 | 13.3 ± 0.3 | 1.7 ± 0.1 | 1.8 |
| |||||||||
| 5 | 63.8 | 35.3 | 0.9 | 0.55 | 26.4 ± 0.4 | 48.8 ± 1.3 | 20.3 ± 0.6 | 4.5 ± 0.1 | 2.8 |
| 20 | 61.3 | 37.6 | 1.1 | 0.61 | 24.7 ± 0.8 | 49.5 ± 1.1 | 21.5 ± 0.3 | 4.3 ± 0.1 | 3.1 |
| 120 | 57.6 | 41.3 | 1.1 | 0.72 | 21.4 ± 0.5 | 47.3 ± 1.2 | 23.8 ± 1.0 | 7.5 ± 0.2 | 3.7 |
| |||||||||
| 5 | 65.6 | 33.4 | 1.0 | 0.51 | 30.1 ± 0.4 | 49.5 ± 0.4 | 16.1 ± 0.1 | 4.3 ± 0.6 | 2.3 |
| 20 | 63.5 | 35.5 | 1.0 | 0.56 | 26.0 ± 0.3 | 48.2 ± 0.3 | 20.6 ± 0.2 | 5.2 ± 0.4 | 2.9 |
| 120 | 57.4 | 41.5 | 1.1 | 0.72 | 19.4 ± 0.4 | 50.3 ± 0.4 | 20.8 ± 0.2 | 9.5 ± 0.1 | 4.2 |
| Treatment Time (s) | Total Atomic Content (at. %) | Atomic Composition of the Carbon Species C1s (at. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | F1s | O1s/C1s | –C–C–; –C–H | –C–O– | –C=O/ O–C–O | –O–C=O | –CF2–/ –CF3 | O/C | |
| Binding Energy (eV) | ||||||||||
| 285.0 ± 0.1 | 286.5 ± 0.1 | 288.0 ± 0.2 | 289.0 ± 0.2 | ~292.3 | ||||||
| 0 | 53.2 | 27.0 | 19.8 | 0.51 | 18.0 ± 3.7 | 43.1 ± 3.4 | 22.8 ± 1.5 | 7.9 ± 1.5 | 8.2 ± 2.3 | 2.8 |
| ||||||||||
| 5 | 53.2 | 33.4 | 13.4 | 0.63 | 19.3 ± 1.1 | 49.1 ± 0.2 | 21.6 ± 3.0 | 6.4 ± 2.0 | 3.6 ± 0.3 | 3.4 |
| 20 | 57.0 | 38.6 | 4.4 | 0.68 | 19.3 ± 0.7 | 56.0 ± 1.7 | 18.1 ± 2.2 | 5.2 ± 1.0 | 1.4 ± 0.3 | 3.8 |
| 120 | 57.5 | 41.3 | 1.2 | 0.72 | 19.2 ± 0.1 | 59.3 ± 0.1 | 17.4 ± 2.1 | 4.1 ± 2.0 | 0 | 4.2 |
| ||||||||||
| 5 | 54.7 | 30.0 | 15.3 | 0.55 | 17.2 ± 2.1 | 54.3 ± 3.6 | 16.5 ± 3.1 | 3.6 ± 0.9 | 8.4 ± 0.1 | 2.9 |
| 20 | 52.9 | 33.2 | 13.9 | 0.63 | 18.9 ± 0.3 | 44.4 ± 1.8 | 26.0 ± 0.9 | 7.0 ± 0.3 | 3.7 ± 0.4 | 3.4 |
| 120 | 56.2 | 40.0 | 3.8 | 0.73 | 17.2 ± 0.8 | 56.3 ± 0.6 | 21.0 ± 0.7 | 5.5 ± 0.5 | 0 | 4.8 |
| Treatment Time (s) | Total Atomic Content (at. %) | Atomic Composition of the Carbon Species C1s (at. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | S2p | O1s/C1s | –C–C–; –C–H | –C–S | –C–O– | –O–C=O | π−π* Shake-Up | O/C | |
| Binding Energy (eV) | ||||||||||
| 285.0 ± 0.1 | 285.6 ± 0.1 | 286.5 ± 0.1 | 289.0 ± 0.2 | ~291.8 | ||||||
| 0 | 80.9 | 16.4 | 2.7 | 0.20 | 75.2 | 7.1 | 14.3 | - | 3.4 | 0.17 |
| ||||||||||
| 5 | 74.1 | 23.5 | 2.4 | 0.32 | 65.4 | 6.9 | 21.7 | 3.6 | 2.4 | 0.34 |
| 20 | 71.0 | 26.8 | 2.2 | 0.38 | 58.6 | 9.5 | 21.9 | 7.1 | 2.9 | 0.41 |
| 60 | 72.2 | 24.7 | 3.1 | 0.34 | 61.5 | 11.6 | 18.0 | 4.9 | 4.0 | 0.30 |
| 120 | 74.4 | 22.9 | 2.7 | 0.31 | 63.1 | 12.1 | 17.7 | 3.6 | 3.5 | 0.27 |
| ||||||||||
| 5 | 73.6 | 23.8 | 2.6 | 0.32 | 59.6 | 10.9 | 20.3 | 5.8 | 3.4 | 0.35 |
| 20 | 71.5 | 25.9 | 2.6 | 0.36 | 59.0 | 10.3 | 21.4 | 6.5 | 2.8 | 0.39 |
| 60 | 73.0 | 24.0 | 3.0 | 0.33 | 61.5 | 11.0 | 20.0 | 3.2 | 4.3 | 0.30 |
| 120 | 75.2 | 22.1 | 2.7 | 0.29 | 62.1 | 12.8 | 18.5 | 3.2 | 3.4 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiper, A.S.; Borcia, G. Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers. Polymers 2023, 15, 4172. https://doi.org/10.3390/polym15204172
Chiper AS, Borcia G. Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers. Polymers. 2023; 15(20):4172. https://doi.org/10.3390/polym15204172
Chicago/Turabian StyleChiper, Alina Silvia, and Gabriela Borcia. 2023. "Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers" Polymers 15, no. 20: 4172. https://doi.org/10.3390/polym15204172
APA StyleChiper, A. S., & Borcia, G. (2023). Stable Surface Modification by Cold Atmospheric-Pressure Plasma: Comparative Study on Cellulose-Based and Synthetic Polymers. Polymers, 15(20), 4172. https://doi.org/10.3390/polym15204172






