On the Effect of Non-Thermal Atmospheric Pressure Plasma Treatment on the Properties of PET Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymeric Film and Chemicals
2.2. Pretreatment of PET Film with Non-Thermal Atmospheric Pressure Plasma
2.3. Controlled Biodegradation Test in Soil under Aerobic Conditions
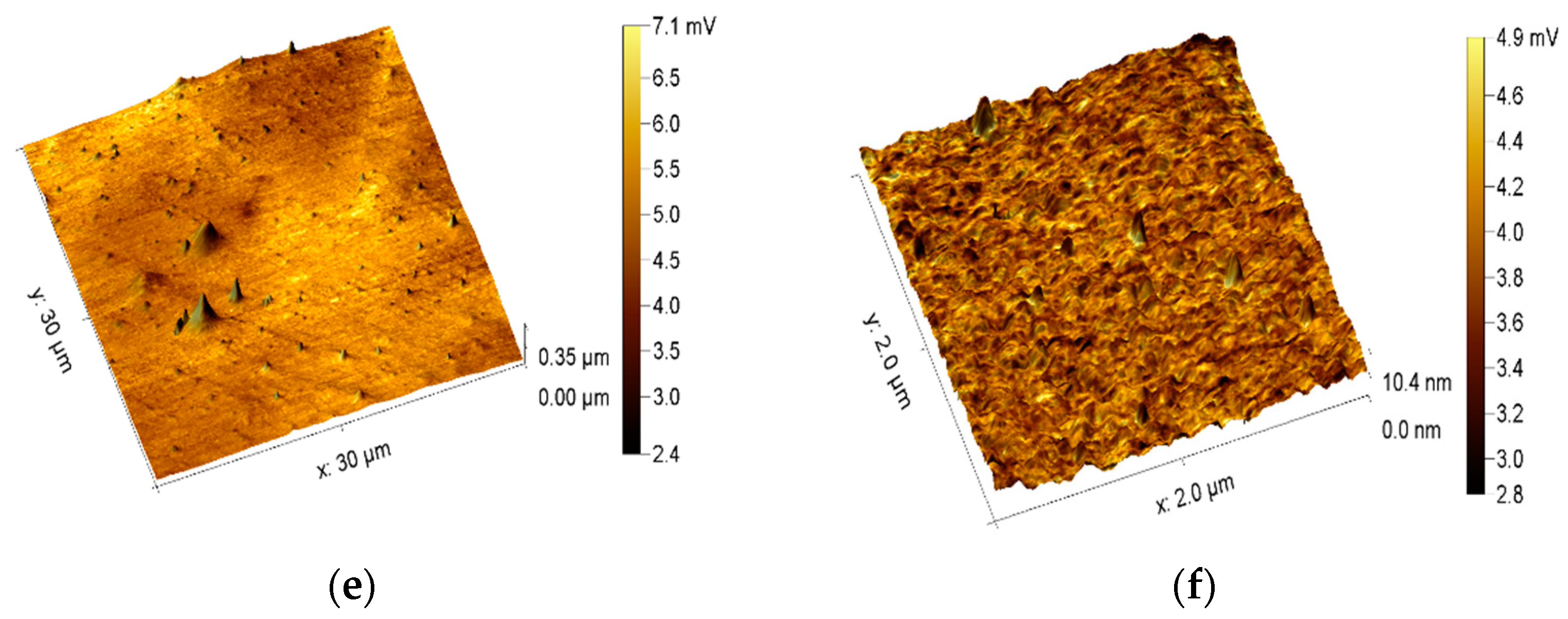
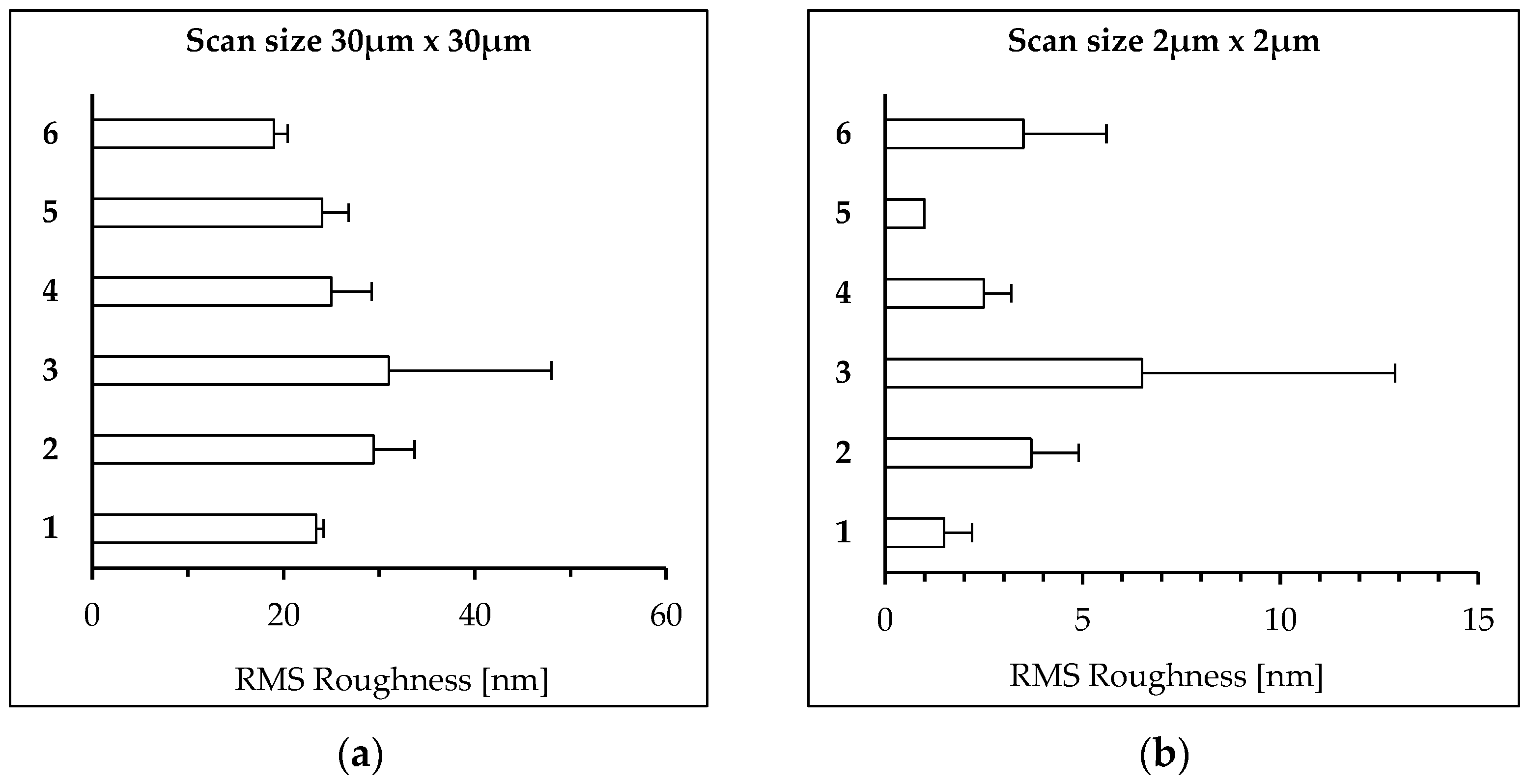
2.4. AFM Surface Analysis
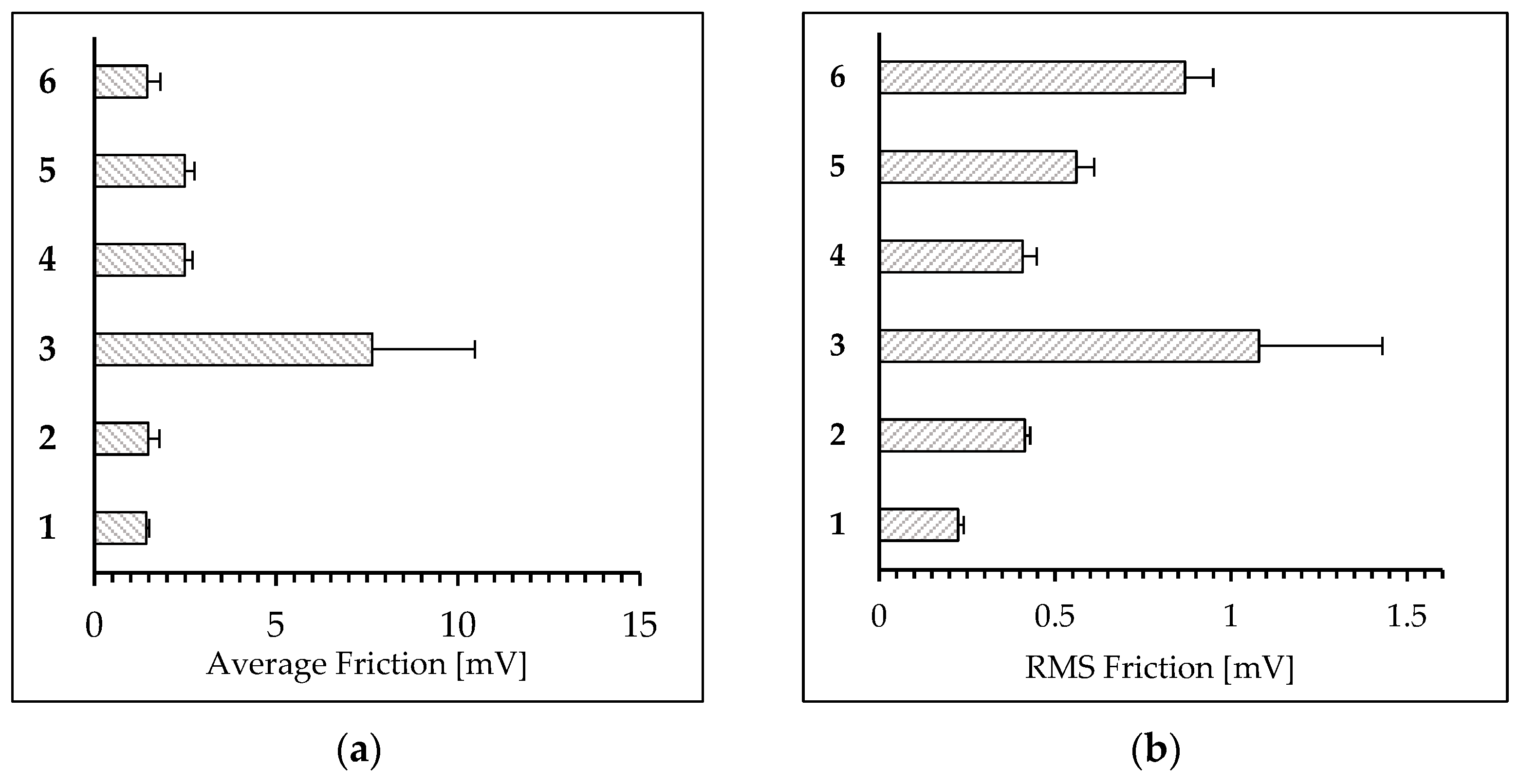
2.5. LFM Surface Mapping
2.6. Measurements of Number and Weight Average Molecular Weights
2.7. Fourier Transformed Infrared Spectroscopy (FTIR) Analysis
2.8. Differential Scanning Calorimetry (DSC) Analysis
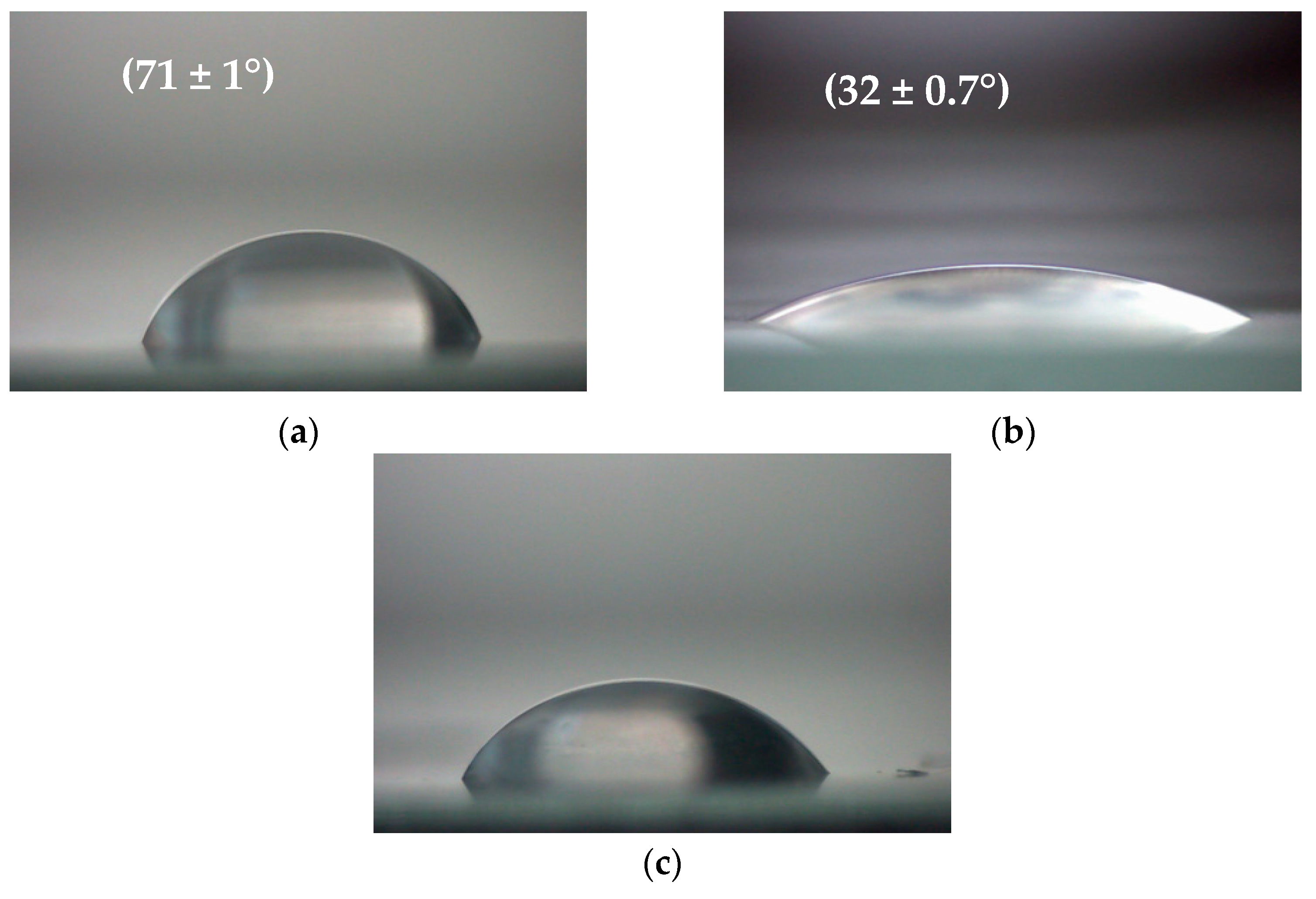
2.9. Wettability Measurements of Polymer Films
2.10. Tests of Dielectric Properties of Polymer Films
2.11. Formation of Biofilm on PET Film Surface
2.12. Scanning Electron Microscopy Analysis (SEM)
2.13. Statistical Analysis
3. Results
3.1. Analysis of Surface Morphology
3.2. Changes in Polymer Film Wettability
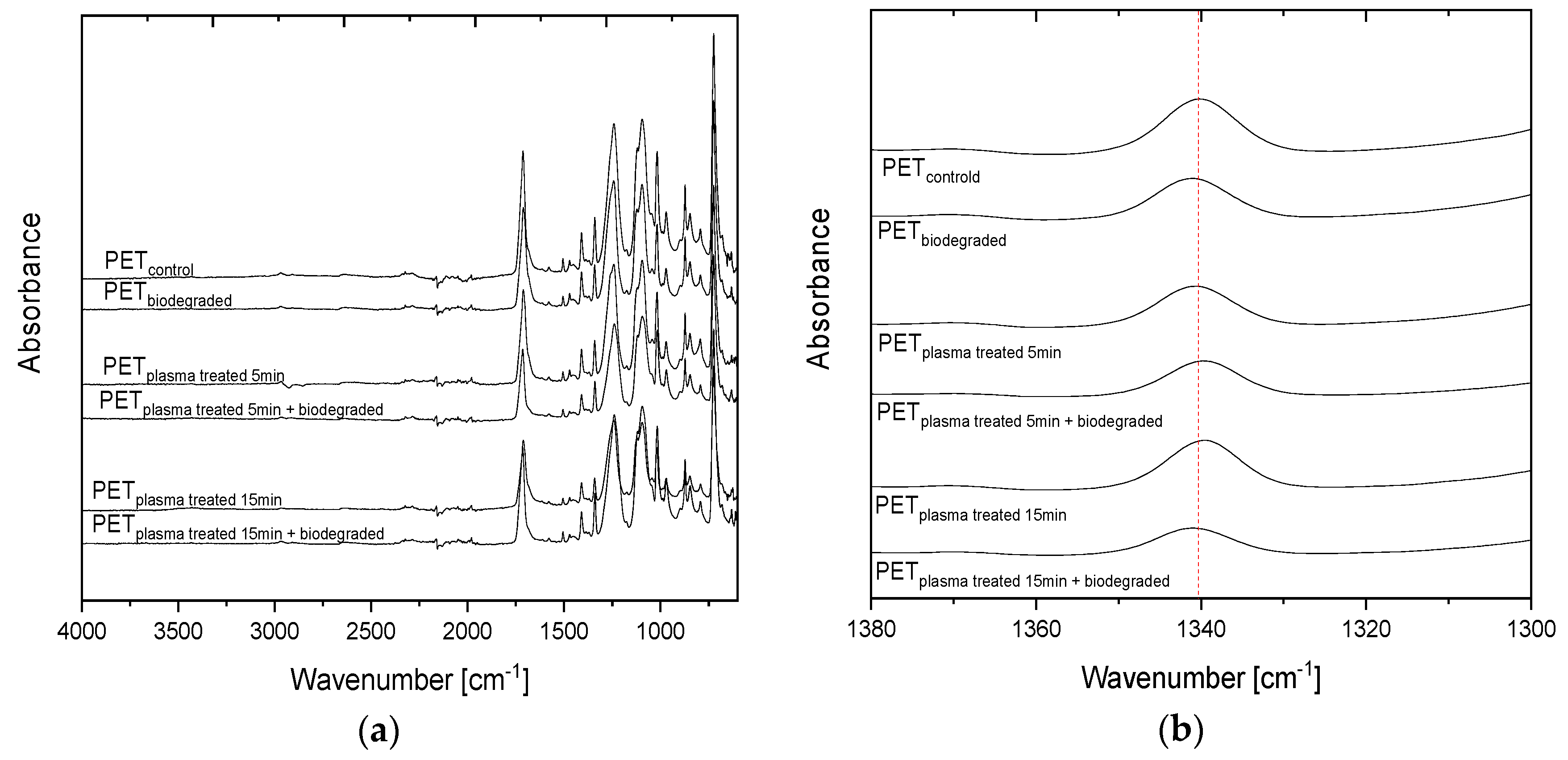
3.3. Analysis of Changes in Chemical Properties of PET Films
3.4. Analysis of Electrical Changes of PET Films
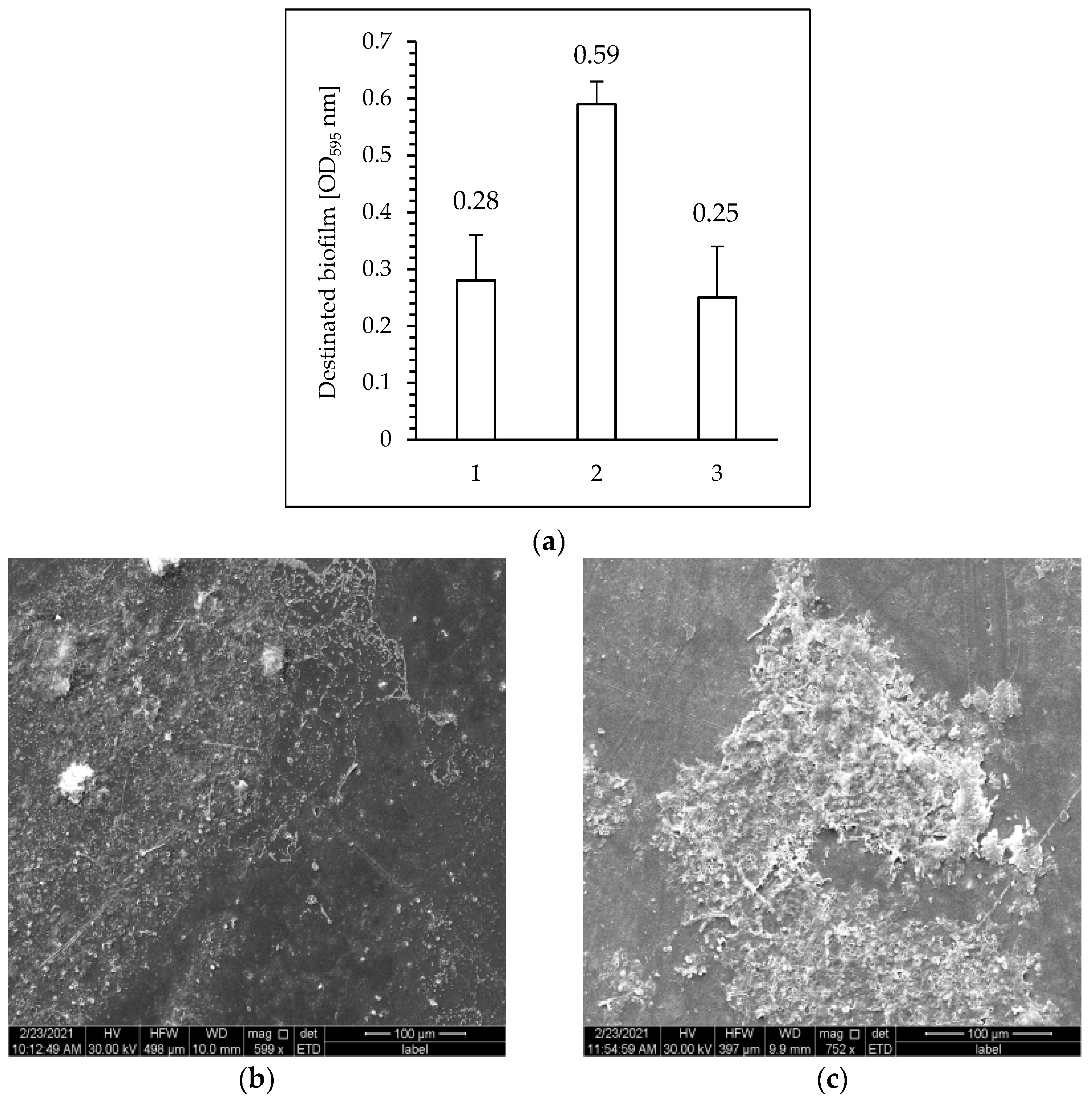
3.5. Analysis of the Effectiveness of Biofilm Development on the Surface of PET Films
3.6. The Effect of Plasma Treatment on the Biodegradability of PET Film
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.; Neal, M.A. Applications and societal benefits of plastics. Philos Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Siracusa, V. Microbial degradation of synthetic biopolymers waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Nkwachukwu, O.I.; Chima, C.H.; Ikenna, A.O.; Albert, L. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. Int. J. Ind. Chem. 2013, 4, 34. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021, 759, 1435362. [Google Scholar] [CrossRef]
- Improving Plastics Management: Trends, Policy Responses, and the Role of International Cooperation and Trade. 2018. Available online: https://www.oecd.org/environment/waste/policy-highlights-improving-plastics-management.pdf (accessed on 14 August 2023).
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef]
- Report.pdf. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/global-plastics-market (accessed on 1 June 2023).
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Belinger, A.; Dap, S.; Naudé, N. Influence of the dielectric thickness on the homogeneity of a diffuse dielectric barrier discharge in air. J. Phys. D Appl. Phys. 2022, 55, 465201. [Google Scholar] [CrossRef]
- Neretti, G.; Popoli, A.; Scaltriti, S.G.; Cristofolini, A. Real time power control in a high voltage power supply for dielectric Barrier discharge reactors: Implementation strategy and load thermal analysis. Electronics 2022, 11, 1536. [Google Scholar] [CrossRef]
- Emmons, D.J.; Weeks, D.E. Steady-state model of an argon-helium high-pressure radio frequency dielectric barrier discharge. IEEE Trans. Plasma Sci. 2020, 48, 2715–2722. [Google Scholar] [CrossRef]
- Shaygani, A.; Adamiak, K. Mean model of the dielectric barrier discharge plasma actuator including photoionization. J. Phys. Appl. Phys. 2023, 56, 055203. [Google Scholar] [CrossRef]
- Hua, W.; Fukagata, K. Near-surface electron transport and its influence on the discharge structure of nanosecond-pulsed dielectric-barrier-discharge under different electrode polarities. Phys. Plasmas 2019, 26, 013514. [Google Scholar] [CrossRef]
- Sato, S.; Furukawa, H.; Komuro, A.; Takahashi, M.; Ohnishi, N. Successively accelerated ionic wind with integrated dielectric-barrier-discharge plasma actuator for low-voltage operation. Sci. Rep. 2019, 9, 5813. [Google Scholar] [CrossRef] [PubMed]
- Colonna, G.; Pintassilgo, C.D.; Pegoraro, F.; Cristofolini, A.; Popoli, A.; Neretti, G.; Gicquel, A.; Duigou, O.; Bieber, T.; Hassouni, K.; et al. Theoretical and experimental aspects of non-equilibrium plasmas in different regimes: Fundamentals and selected applications. Eur. Phys. J. D 2021, 75, 183. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Saifutdinova, A.A.; Saifutdinov, A.I.; Gainullina, S.V.; Timerkaev, B.A. Modeling the parameters of an atmospheric pressure dielectric barrier discharge controlled by the shape of the applied voltage. IEEE Trans. Plasma Sci. 2022, 50, 1144–1156. [Google Scholar] [CrossRef]
- Lim, M.T.; Zulkifli, A.Z.S.; Jayapalan, K.K.; Chin, O. Development of a dimensionless parameter for characterization of dielectric barrier discharge devices with respect to geometrical features. Plasma Sci. Technol. 2017, 19, 095402. [Google Scholar] [CrossRef]
- Štěpánová, V.; Šrámková, P.; Sihelník, S.; Stupavská, M.; Jurmanová, J.; Kováčik, D. The Effect of ambient air plasma generated by coplanar and volume dielectric barrier discharge on the surface characteristics of polyamide foils. Vacuum 2021, 183, 109887. [Google Scholar] [CrossRef]
- Homola, T.; Krumpolec, R.; Zemánek, M.; Kelar, J.; Synek, P.; Hoder, T.; Černák, M. An array of micro-hollow surface dielectric barrier discharges for large-area atmospheric-pressure surface treatments. Plasma Chem. Plasma Process. 2017, 37, 1149–1163. [Google Scholar] [CrossRef]
- Azimi, H.; Tavakoli, M.; Sharifian, M. Effect of dielectric barrier discharge (DBD) plasma treatment on the polypropylene film in presence of air and nitrogen at atmospheric pressure. Adv. Appl. NanoBio-Technol. 2021, 2, 41–48. [Google Scholar]
- Pekárek, S.; Mikeš, J.; Červenka, M.; Hanuš, O. Air supply mode effects on ozone production of surface dielectric barrier discharge in a cylindrical configuration. Plasma Chem. Plasma Process. 2021, 41, 779–792. [Google Scholar] [CrossRef]
- Li, S.; Dang, X.; Yu, X.; Abbas, G.; Zhang, Q.; Cao, L. The application of dielectric barrier discharge non-thermal plasma in VOCs abatement: A review. Chem. Eng. J. 2020, 388, 124275. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Faisal, A.; Hafeez, A.; Javed, F.; Mustafa, M.; Rehman, F. Hydrogen production through water vapors using optimized corona-DBD hybrid plasma micro-reactor. Fuel 2023, 331, 125838. [Google Scholar] [CrossRef]
- Massima Mouele, E.S.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; Fatoba, O.O.; et al. Removal of pharmaceutical residues from water and wastewater using dielectric barrier discharge methods—A review. Int. J. Environ. Res. Public Health 2021, 18, 1683. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric barrier discharge cold atmospheric plasma: Bacterial inactivation mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Caillier, B.; Therese, L.; Belenguer, P.; Guillot, P. Parametric studies of a mercury-free DBD lamp. Plasma 2021, 4, 82–93. [Google Scholar] [CrossRef]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar]
- Liu, H.; Pei, Y.; Xie, D.; Deng, X.; Leng, Y.X.; Jin, Y.; Huang, N. Surface modification of ultra-high molecular weight polyethylene (UHMWPE) by argon plasma. Appl. Surf. Sci. 2010, 256, 3941–3945. [Google Scholar] [CrossRef]
- Ataeefard, M.; Moradian, S.; Mirabedini, M.; Ebrahimi, M.; Asiaban, S. Surface properties of low density polyethylene upon low-temperature plasma treatment with various gases. Plasma Chem. Plasma Process. 2008, 28, 377–390. [Google Scholar] [CrossRef]
- Švorčík, V.; Kotál, V.; Siegel, J.; Sajdl, A.; Macková, A.; Hrnatowicz, V. Ablation and water etching of poly(ethylene) modified by argon plasma. Polym. Degrad. Stab. 2007, 92, 1645–1649. [Google Scholar] [CrossRef]
- Gómez-Méndez, L.D.; Moreno-Bayona, D.A.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Vargas, A.; Bogoya, J.M. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLoS ONE. 2018, 13, e0203786. [Google Scholar] [CrossRef]
- Czapka, T.; Maliszewska, I.; Olesiak-Bańska, J. Influence of atmospheric pressure non-thermal plasma on inactivation of biofilm cells. Plasma Chem. Plasma Process. 2018, 38, 1181–1197. [Google Scholar] [CrossRef]
- Mark, H.F.; Bikales, N.M.; Overberger, C.G.; Menges, G. Encyclopedia of Polymer Science and Engineering; John Wiley and Sons: New York, NY, USA, 1985. [Google Scholar]
- IEC 61340-2-3; Methods of test for determining the resistance and resistivity of solid materials used to avoid electrostatic charge accumulation. International Electrotechnical Commission: Geneva, Switzerland, 2016.
- ASTM D150; Standard Test Methods for AC Loss Characteristics and Permittivity (Dielectric Constant) of Solid Electrical Insulation. ASTM International: West Conshohocken, PA, USA, 2022.
- IEC 60243-1; Electric strength of insulating materials—Test methods—Part 1: Tests at power frequencies. International Electrotechnical Commission: Geneva, Switzerland, 2013.
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Yasukawa, A.; Kobayashi, Y. Wettability characteristics of poly(ethyleneterephthalate) films treated by atmospheric pressure plasma and ultraviolet excimer light. Polym. J. 2011, 43, 545–551. [Google Scholar] [CrossRef][Green Version]
- Umamaheswari, S.; Murali, M. FTIR spectroscopic study of fungal degradation of poly(ethylene terephthalate) and polystyrene foam. Chem. Eng. 2013, 64, 19159–19164. [Google Scholar]
- Atkinson, J.R.; Hay, J.N.; Biddlestone, F. An investigation of glass formation and physical ageing in poly(ethylene terephthalate) by FT-IR spectroscopy. Polymers 2000, 41, 6965–6968. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Omaníková, L.; Bočkaj, J.; Černák, M.; Plavec, R.; Feranc, J.; Jurkovič, P. Influence of composition and plasma power on properties of film from biodegradable polymer blends. Polymers 2020, 12, 1592. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Mroczka, R.; Łopucki, R. Chitosan/phospholipid coated polyethylene terephthalate (PET) polymer surfaces activated by air plasma. Coll. Surf. A Physicochem. Eng. Aspects. 2017, 532, 155–164. [Google Scholar] [CrossRef]
- Akhavan, K.; Jarvis, K.; Majewski, P. Development of oxidized sulfur polymer films through a combination of plasma polymerization and oxidative plasma treatment. Langmuir 2014, 30, 1444–1454. [Google Scholar] [CrossRef]
- Mertens, M.; Mohr, M.; Brühne, K.; Fecht, H.J.; Łojkowski, M.; Święszkowski, W.; Łojkowski, W. Patterned hydrophobic and hydrophilic surfaces of ultra-smooth nanocrystalline diamond layers. Appl. Surf. Sci. 2016, 390, 526–530. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ul Ahmad, A. Controlled surface wettability by plasma polymer surface modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Evolution of the surface wettability of PET polymer upon treatment with an atmospheric-pressure plasma jet. Polymers 2020, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Mattila-Sandholm, T.; Wirtanen, G. Biofilm formation in the industry: A review. Food Rev. Inter. 1992, 8, 573–603. [Google Scholar] [CrossRef]
- Amoroso, P.F.; Adams, R.J.; Waters, M.G.; Williams, D.W. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: An in vitro study. Clin. Oral Implants Res. 2006, 17, 633–637. [Google Scholar]
- Koseki, H.; Yonekura, A.; Shida, T.; Yoda, I.; Horiuchi, H.; Morinaga, Y.; Yanagihara, K.; Sakoda, H.; Osaki, M.; Tomita, M. Early Staphylococcal biofilm formation on solid orthopaedic implant materials: In vitro study. PLoS ONE 2014, 9, e107588. [Google Scholar] [CrossRef]
- Xu, L.C.; Siedlecki, C.A. Staphylococcus epidermidis adhesion on hydrophobic and hydrophilic textured biomaterial surfaces. Biomed. Mater. 2014, 9, 035003. [Google Scholar] [CrossRef]
- De-la-Pinta, I.; Cobos, M.; Ibarretxe., J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef]
- Seddiki, O.; Harnagea, C.; Levesque, L.; Mantovani, D.; Rosei, F. Evidence of antibacterial activity on titanium surfaces through nanotextures. Appl. Surf. Sci. 2014, 308, 275–284. [Google Scholar] [CrossRef]
- Singh, A.V.; Galluzzi, M.; Borghi, F.; Indrieri, M.; Vyas, V.; Podestà, A.; Gade, W.N. Interaction of bacterial cells with cluster-assembled nanostructured titania surfaces: An Atomic Force Microscopy study. J. Nanosci. Nanotechnol. 2013, 13, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.D. Microbial colonization of polymeric materials for space applications and mechanisms of biodeterioration: A review. Int. Biodeter. Biodegr. 2007, 59, 170–179. [Google Scholar] [CrossRef]
- Han, Y.-N.; Wei, M.; Han, F.; Fang, C.; Wang, D.; Zhong, Y.-J.; Guo, C.-L.; Shi, X.-Y.; Xie, Z.-K.; Li, F.-M. Greater biofilm formation and increased biodegradation of polyethylene film by a microbial consortium of Arthrobacter sp. and Streptomyces sp. Microorganisms 2020, 8, 1979. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Takehana, T.; Yoshida, S.; Hiraga, K.; Oda, K. Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades PET. Int. J. Syst. Evol. Microbiol. 2016, 66, 2813–2818. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.H.; Hur, H.G. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Janczak, K.; Dąbrowska, G.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int. Biodeterior. Biodegrad. 2020, 155, 1–10. [Google Scholar] [CrossRef]


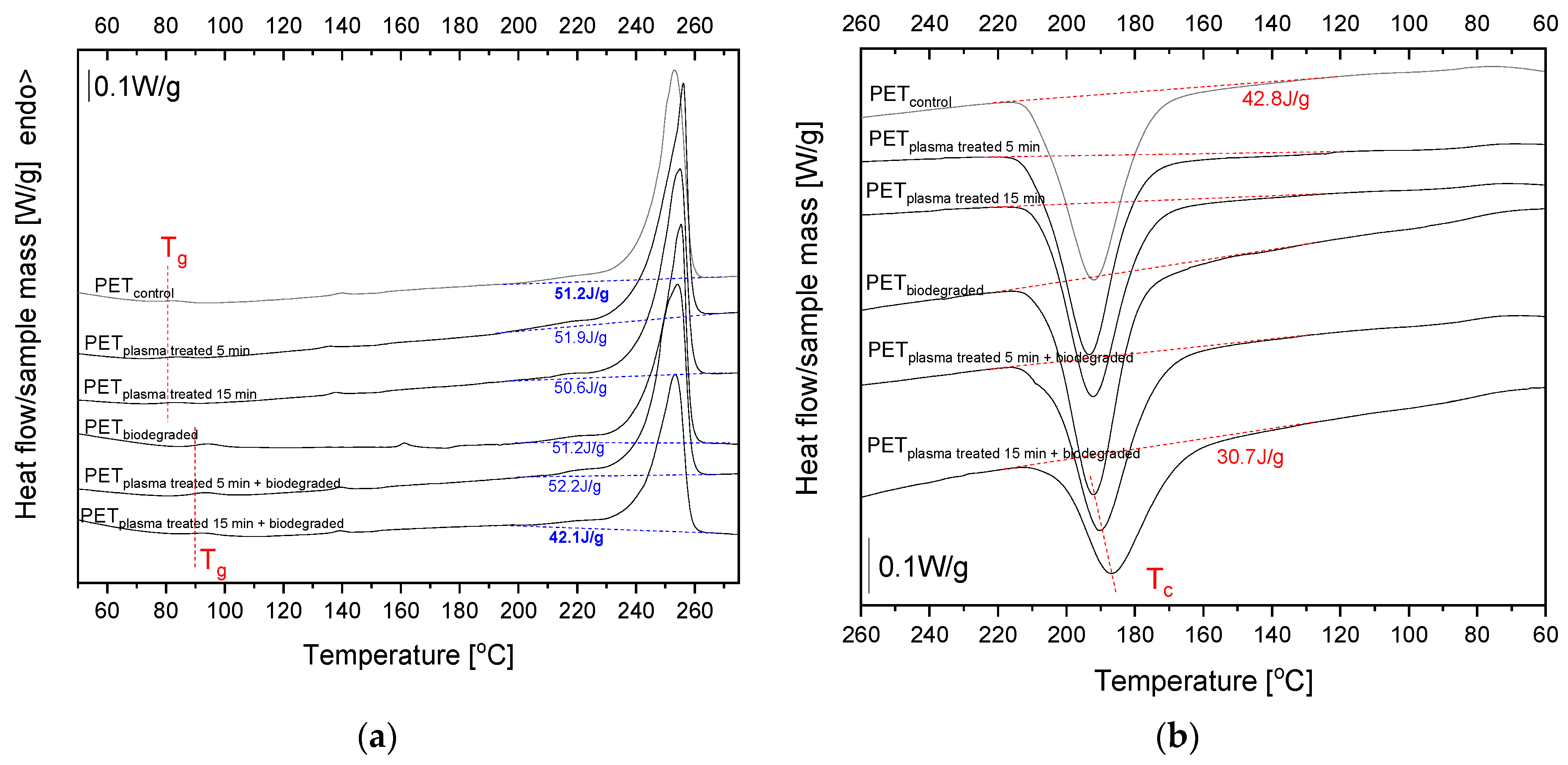
| Sample | 1st Heating | Cooling | |||||
|---|---|---|---|---|---|---|---|
| Tg [°C] | Tm [°C] | ΔHm [J/g] | Xc [%] | Tc [°C] | ΔHc [J/g] | Tg [°C] | |
| PETcontrol | 79.7 | 253.1 | 51.2 | 41.0 | 191.9 | 42.8 | 84.5 |
| PETplasma 5min | 80.3 | 256.0 | 51.9 | 41.5 | 193.4 | 42.8 | 81.0 |
| PETplasma 15min | 79.3 | 254.9 | 50.6 | 40.5 | 192.4 | 41.0 | 81.7 |
| PETbiodegraded | 90.7 | 255.2 | 51.2 | 41.0 | 192.2 | 43.3 | 82.0 |
| PETplasma 5min + biodegraded | 89.8 | 254.1 | 52.2 | 41.8 | 190.2 | 40.4 | 82.4 |
| PETplasma 15min + biodegraded | 90.2 | 253.3 | 42.1 | 33.7 | 186.7 | 30.7 | 88.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliszewska, I.; Gazińska, M.; Łojkowski, M.; Choińska, E.; Nowinski, D.; Czapka, T.; Święszkowski, W. On the Effect of Non-Thermal Atmospheric Pressure Plasma Treatment on the Properties of PET Film. Polymers 2023, 15, 4289. https://doi.org/10.3390/polym15214289
Maliszewska I, Gazińska M, Łojkowski M, Choińska E, Nowinski D, Czapka T, Święszkowski W. On the Effect of Non-Thermal Atmospheric Pressure Plasma Treatment on the Properties of PET Film. Polymers. 2023; 15(21):4289. https://doi.org/10.3390/polym15214289
Chicago/Turabian StyleMaliszewska, Irena, Małgorzata Gazińska, Maciej Łojkowski, Emilia Choińska, Daria Nowinski, Tomasz Czapka, and Wojciech Święszkowski. 2023. "On the Effect of Non-Thermal Atmospheric Pressure Plasma Treatment on the Properties of PET Film" Polymers 15, no. 21: 4289. https://doi.org/10.3390/polym15214289
APA StyleMaliszewska, I., Gazińska, M., Łojkowski, M., Choińska, E., Nowinski, D., Czapka, T., & Święszkowski, W. (2023). On the Effect of Non-Thermal Atmospheric Pressure Plasma Treatment on the Properties of PET Film. Polymers, 15(21), 4289. https://doi.org/10.3390/polym15214289









