Recent Challenges and Trends of Polyhydroxyalkanoate Production by Extremophilic Bacteria Using Renewable Feedstocks
Abstract
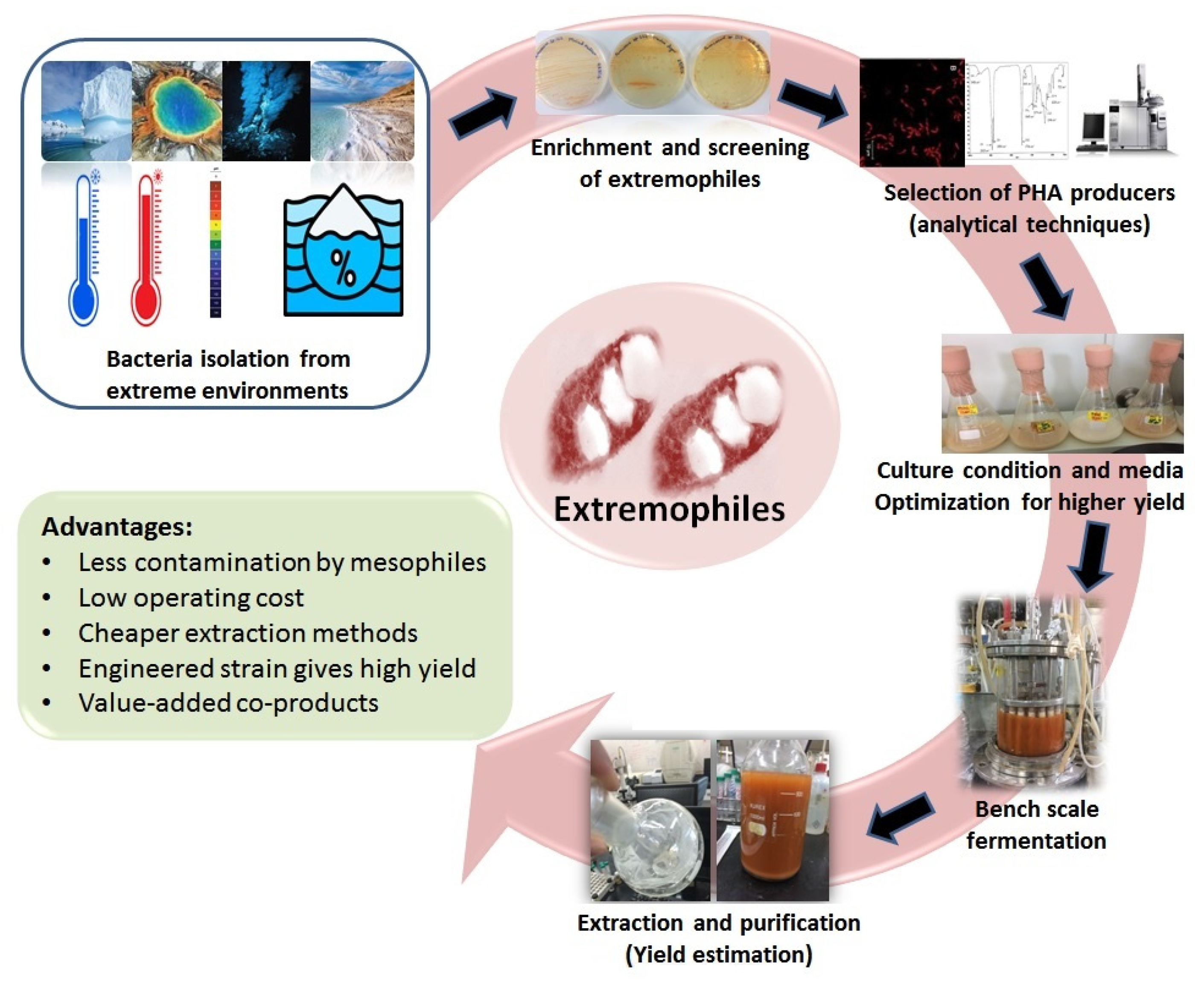
:1. Introduction
2. Bacteria from Extreme Habitats as PHA Producers
3. Effects of Renewable Feedstocks on PHA Production by Extremophiles
3.1. Waste Oils
3.2. Crude Glycerol
3.3. Cheese Whey and Cheese Whey Mother Liquor
3.4. Other Waste Materials as Substrate
4. Properties of PHA Produced from Waste Substrates by Extremophiles
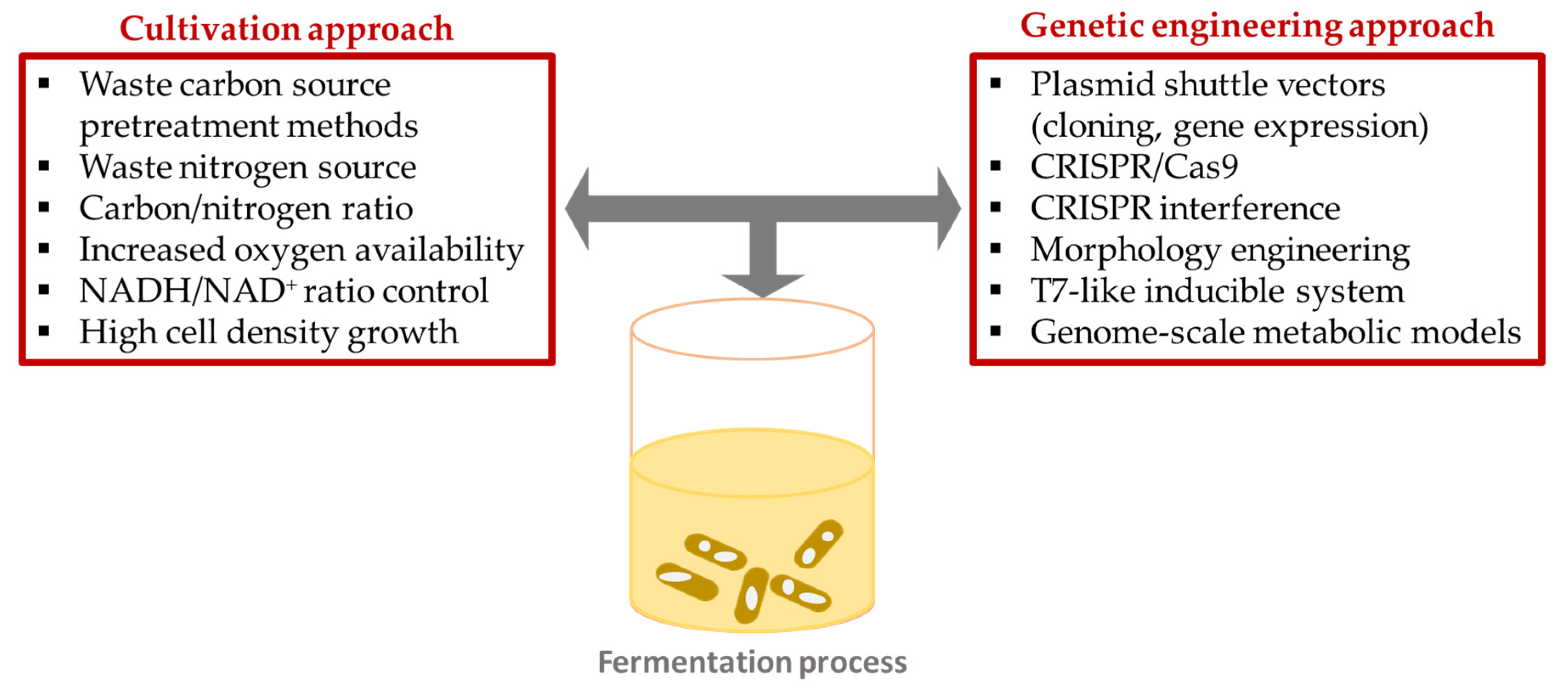
5. Strategies to Enhance PHA Production from Renewable Feedstocks
5.1. Cultivation Approach
| Bacteria | Carbon Source | Cultivation Mode and Extremophile Conditions | PHA Concentration (g/L) | References |
|---|---|---|---|---|
| NaCl concentration | ||||
| Salinivibrio sp. M318 | Mixture of waste fish oil and glycerol | Fed-batch bioreactor cultivation; 30 g/L NaCl | 5.17 | [31] |
| Salinivibrio sp. TGB10 | Mixture of waste fish oil and glycerol | Fed-batch bioreactor cultivation; 27.5 g/L NaCl | 27.36 | [61] |
| Effect of waste additives | ||||
| Yangia sp. ND199 | Crude glycerol | Fed-batch cultivations in shaking flasks; 45 g/L NaCl | 8.3 | [62] |
| Crude glycerol + fructose corn syrup | 20.3 | |||
| Effect of waste feedstock dilution | ||||
| Halomonas halophila | Hydrolyzed spent coffee ground | Batch cultivation; 66 g/L NaCl | 2.17 | [14] |
| Hydrolyzed spent coffee ground 2× diluted | 0.27 | |||
| Hydrolyzed corn stover | nd | |||
| Hydrolyzed corn stover 2× diluted | 0.82 | |||
| Effect of genetic engineering | ||||
| Halomonas campaniensis LS21 wild type | Alkaline seawater | Open fed continuous cultivation; 27 g/L NaCl | 0.91 | [39] |
| Halomonas campaniensis LS21 recombinant | Alkaline seawater | Open fed continuous cultivation; 27 g/L NaCl | 2.77 | |
| Waste feedstock treatment | ||||
| Halomonas sp. YLGW01 | Activated carbon–treated crude glycerol | Fed-batch fermentation; C/N ratio of 10:1 (%) (v/v) | 10.5 | [52] |
| Activated carbon–non treated crude glycerol | Fed-batch fermentation; C/N ratio of 10:1 (%) (v/v) | 8.0 | ||
5.2. Genetic Engineering Approach
6. Challenges of PHA Production by Extremophiles from Waste Carbon Sources
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wu, Q.; Chen, J.C.; Chen, G.Q. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates. Metab. Eng. 2014, 26, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, S.; Singh, D. Microbial polyhydroxyalkanoates from extreme niches: Bioprospection status, opportunities and challenges. Int. J. Biol. Macromol. 2020, 147, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Vicente, D.; Proença, D.N.; Morais, P.V. The Role of Bacterial Polyhydroalkanoate (PHA) in a Sustainable Future: A Review on the Biological Diversity. Int. J. Environ. Res. Public Health 2023, 20, 2959. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Mukhopadhyay, A.; Petzold, C.J.; Keasling, J.D.; Martín, H.G. Synthetic and systems biology for microbial production of commodity chemicals. npj Syst. Biol. Appl. 2016, 2, 16009. [Google Scholar] [CrossRef]
- Zhu, D.; Adebisi, W.A.; Ahmad, F.; Sethupathy, S.; Danso, B.; Sun, J. Recent Development of Extremophilic Bacteria and Their Application in Biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 483. [Google Scholar] [CrossRef]
- Yu, L.P.; Wu, F.Q.; Chen, G.Q. Next-generation industrial biotechnology-transforming the current industrial biotechnology into competitive processes. Biotechnol. J. 2019, 14, e1800437. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Jiang, X.R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Fact. 2020, 19, 86. [Google Scholar] [CrossRef]
- Kourilova, X.; Pernicova, I.; Vidlakova, M.; Krejcirik, R.; Mrazova, K.; Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Obruca, S. Biotechnological Conversion of Grape Pomace to Poly(3-hydroxybutyrate) by Moderately Thermophilic Bacterium Tepidimonas taiwanensis. Bioengineering 2021, 8, 141. [Google Scholar] [CrossRef]
- Guleria, S.; Singh, H.; Sharma, V.; Bhardwaj, N.; Arya, S.K.; Puri, S.; Khatri, M. Polyhydroxyalkanoates production from domestic waste feedstock: A sustainable approach towards bio-economy. J. Clean. Prod. 2022, 340, 130661. [Google Scholar] [CrossRef]
- Von Hegner, I. Extremophiles: A special or general case in the search for extra-terrestrial life? Extremophiles 2020, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Yadav, B.; Tyagi, R.D.; Drogui, P. A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresour. Technol. 2021, 341, 125900. [Google Scholar] [CrossRef]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the promising poly (3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Jun, H.B.; Kim, B.S. Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain LL1. Int. J. Biol. Macromol. 2018, 107, 2552–2558. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, B.S. Paracoccus sp. strain LL1 as a single cell factory for the conversion of waste cooking oil to polyhydroxyalkanoates and carotenoids. Appl. Food Biotechnol. 2019, 6, 53–60. [Google Scholar]
- Cui, B.; Huang, S.; Xu, F.; Zhang, R.; Zhang, Y. Improved productivity of poly (3-hydroxybutyrate)(PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Appl. Microbiol. Biotechnol. 2015, 99, 6009–6019. [Google Scholar] [CrossRef]
- Giedraitytė, G.; Kalėdienė, L. Purification and characterization of polyhydroxybutyrate produced from thermophilic Geobacillus sp. AY 946034 strain. Chemija 2015, 26, 38–45. [Google Scholar]
- Kourilova, X.; Pernicova, I.; Sedlar, K.; Musilova, J.; Sedlacek, P.; Kalina, M.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates (PHA) by a thermophilic strain of Schlegelella thermodepolymerans from xylose rich substrates. Bioresour. Technol. 2020, 315, 123885. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Zhang, Y.; Xu, F. Isolation and characterization of a thermophilic Bacillus shackletonii K5 from a biotrickling filter for the production of polyhydroxybutyrate. J. Environ. Sci. 2014, 26, 1453–1462. [Google Scholar] [CrossRef]
- Methe, B.A.; Nelson, K.E.; Deming, J.W.; Momen, B.; Melamud, E.; Zhang, X.; Moult, J.; Madupu, R.; Nelson, W.C.; Dodson, R.J.; et al. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 2005, 102, 10913–10918. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Morisaki, K.; Tomizawa, S.; Ohtani, M.; Demura, T.; Miyazaki, M.; Nogi, Y.; Deguchi, S. Synthesis of poly-and oligo (hydroxyalkanoate) s by deep-sea bacteria, Colwellia spp., Moritella spp., and Shewanella spp. Polym. J. 2013, 45, 1094–1100. [Google Scholar] [CrossRef]
- Rogala, M.M.; Gawor, J.; Gromadka, R.; Kowalczyk, M.; Grzesiak, J. Biodiversity and habitats of polar region polyhydroxyalkanoic acid-producing bacteria: Bioprospection by popular screening methods. Genes 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Darnal, S.; Kumar, S.; Kumar, S.; Singh, D. Bioprocess for co-production of polyhydroxybutyrate and violacein using Himalayan bacterium Iodobacter sp. PCH194. Bioresour. Technol. 2021, 319, 124235. [Google Scholar] [CrossRef] [PubMed]
- Eronen-Rasimus, E.; Hultman, J.; Hai, T.; Pessi, I.S.; Collins, E.; Wright, S.; Laine, P.; Viitamäki, S.; Lyra, C.; Thomas, D.N.; et al. Sea-ice bacteria Halomonas sp. strain 363 and Paracoccus sp. strain 392 produce multiple types of poly-3-hydroxyalkaonoic acid (PHA) storage polymers at low temperature. Appl. Environ. Microbiol. 2021, 87, e00929-21. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Jin, J.O.; Choi, I.; Kim, M. Recent trends of biotechnological production of polyhydroxyalkanoates from C1 carbon sources. Front. Bioeng. Biotechnol. 2023, 10, 907500. [Google Scholar] [CrossRef]
- Eberly, J.O.; Ely, R.L. Photosynthetic accumulation of carbon storage compounds under CO2 enrichment by the thermophilic cyanobacterium Thermosynechococcus elongatus. J. Ind. Microbiol. Biotechnol. 2012, 39, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—The case for thermophilic bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- Rizki, W.O.S.; Ratnaningsih, E.; Hertadi, R. Production of poly-(R)-3-hydroxybutyrate from halophilic bacterium Salinivibrio sp. utilizing palm oil mill effluent as a carbon source. Biocatal. Agric. Biotechnol. 2023, 47, 102558. [Google Scholar] [CrossRef]
- Sangkharak, K.; Prasertsan, P. The production of polyhydroxyalkanoate by Bacillus licheniformis using sequential mutagenesis and optimization. Biotech. Bioprocess Eng. 2013, 18, 272–279. [Google Scholar] [CrossRef]
- Van Thuoc, D.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Kucera, D.; Nebesarova, J.; Kalina, M.; Novackova, I.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates on waste frying oil employing selected Halomonas strains. Bioresour. Technol. 2019, 292, 122028. [Google Scholar] [CrossRef]
- Arcila-Echavarría, D.C.; Lu-Chau, T.A.; Gómez-Vanegas, N.A.; Peñuela-Vasquez, M.; Marsiglia-López, D.E. Optimization of nutritional and operational conditions for producing PHA by the halophilic bacterium Halomonas boliviensis from oil palm empty fruit bunch and gluten hydrolysates. Waste Biomass Valorization 2022, 13, 1589–1597. [Google Scholar] [CrossRef]
- Satoh, Y.; Tajima, K.; Nakamoto, S.; Xuerong, H.; Matsushima, T.; Ohshima, T.; Kawano, S.; Erata, T.; Dairi, T.; Munekata, M. Isolation of a thermotolerant bacterium producing medium-chain-length polyhydroxyalkanoate. J. Appl. Microbiol. 2011, 111, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Mishra, S.K.; Shethia, B.; Pancha, I.; Jain, D.; Mishra, S. Isolation of promising bacterial strains from soil and marine environment for polyhydroxyalkanoates (PHAs) production utilizing Jatropha biodiesel byproduct. Int. J. Biol. Macromol. 2010, 47, 283–287. [Google Scholar] [CrossRef]
- Pantazaki, A.A.; Papaneophytou, C.P.; Pritsa, A.G.; Liakopoulou-Kyriakides, M.; Kyriakidis, D.A. Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem. 2009, 44, 847–853. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Marciniak, P.; Moraczewski, K.; Rytlewski, P.; Czaplicki, S.; Zadernowska, A. Cheese whey mother liquor as dairy waste with potential value for polyhydroxyalkanoate production by extremophilic Paracoccus homiensis. Sustain. Mater. Technol. 2022, 33, e00449. [Google Scholar] [CrossRef]
- Szacherska, K.; Moraczewski, K.; Rytlewski, P.; Czaplicki, S.; Ciesielski, S.; Oleskowicz-Popiel, P.; Mozejko-Ciesielska, J. Polyhydroxyalkanoates production from short and medium chain carboxylic acids by Paracoccus homiensis. Sci. Rep. 2022, 12, 7263. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Ling, C.; Yang, T.; Chen, X.; Chen, Y.; Deng, H.; Wu, Q.; Chen, J.; Chen, G.Q. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels 2014, 7, 108. [Google Scholar] [CrossRef]
- Sheu, D.S.; Chen, W.M.; Yang, J.Y.; Chang, R.C. Thermophilic bacterium Caldimonas taiwanensis produces poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzym. Microb. Technol. 2009, 44, 289–294. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, B.S. Valorization of polyhydroxyalkanoates production process by co-synthesis of value-added products. Bioresour. Technol. 2018, 269, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Yootoum, A.; Jantanasakulwong, K.; Rachtanapun, P.; Moukamnerd, C.; Chaiyaso, T.; Pumas, C.; Tanadchangsaeng, N.; Watanabe, M.; Fukui, T.; Insomphun, C. Characterization of newly isolated thermotolerant bacterium Cupriavidus sp. CB15 from composting and its ability to produce polyhydroxyalkanoate from glycerol. Microb. Cell Factories 2023, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of high quality polyhydroxyalkanoate co- and terpolyesters for potential medical application by the archaeon Haloferax mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Kourilova, X.; Novackova, I.; Koller, M.; Obruca, S. Evaluation of mesophilic Burkholderia sacchari, thermophilic Schlegelella thermodepolymerans and halophilic Halomonas halophila for polyhydroxyalkanoates production on model media mimicking lignocellulose hydrolysates. Bioresour. Technol. 2021, 325, 124704. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.B.; Tian, L.; Pu, N.; Li, Z.J. Efficient production of poly-3-hydroxybutyrate from acetate and butyrate by halophilic bacteria Salinivibrio spp. TGB4 and TGB19. Int. J. Biol. Macromol. 2022, 221, 1365–1372. [Google Scholar] [CrossRef]
- Yin, J.; Yang, J.; Yu, X.; Chen, T.; He, S. Enhanced poly (3-hydroxybutyrateco-3-hydroxyvalerate) production from high-concentration propionate by a novel halophile Halomonas sp. YJ01: Detoxification of the 2-methylcitrate cycle. Bioresour. Technol. 2023, 388, 129738. [Google Scholar] [CrossRef] [PubMed]
- Naitam, M.G.; Tomar, G.S.; Pushpad, U.; Singh, S.; Kaushik, R. Halophilic bacteria mediated poly-β-hydroxybutyrate production using paddy straw as a substrate. Bioresour. Technol. Rep. 2022, 17, 100915. [Google Scholar] [CrossRef]
- Andhalkar, V.V.; Foong, S.Y.; Kee, S.H.; Lam, S.S.; Chan, Y.H.; Djellabi, R.; Bhubalan, K.; Medina, F.; Constantí, M. Integrated biorefinery design with techno-economic and life cycle assessment tools in polyhydroxyalkanoates processing. Macromol. Mater. Eng. 2023, 2300100. [Google Scholar] [CrossRef]
- Hierro-Iglesias, C.; Chimphango, A.; Thornley, P.; Fernández-Castané, A. Opportunities for the development of cassava waste biorefineries for the production of polyhydroxyalkanoates in Sub-Saharan Africa. Biomass Bioenergy 2022, 166, 106600. [Google Scholar] [CrossRef]
- Amândio, M.S.; Pereira, J.M.; Rocha, J.M.; Serafim, L.S.; Xavier, A.M. Getting value from pulp and paper industry wastes: On the way to sustainability and circular economy. Energies 2022, 15, 4105. [Google Scholar] [CrossRef]
- Rekhi, P.; Goswami, M.; Ramakrishna, S.; Debnath, M. Polyhydroxyalkanoates biopolymers toward decarbonizing economy and sustainable future. Crit. Rev. Biotechnol. 2022, 42, 668–692. [Google Scholar] [CrossRef]
- Kim, B.; Oh, S.J.; Hwang, J.H.; Kim, H.J.; Shin, N.; Bhatia, S.K.; Jeon, J.M.; Yoon, J.J.; Yoo, J.; Ahn, J.; et al. Polyhydroxybutyrate production from crude glycerol using a highly robust bacterial strain Halomonas sp. YLGW01. Int. J. Biol. Macromol. 2023, 236, 123997. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Yang, H.; Liu, C.; Zhang, Z.; Chen, G. Biodegradable polyhydroxyalkanoates production from wheat straw by recombinant Halomonas elongata A1. Int. J. Biol. Macromol. 2021, 187, 675–682. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, S. Efficient production of polyhydroxyalkanoate through halophilic bacteria utilizing algal biodiesel waste residue. Front. Bioeng. Biotechnol. 2021, 9, 624859. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, H.; Li, D.; Nawaz, H.; You, T.; Xu, F. Efficiently unsterile polyhydroxyalkanoate production from lignocellulose by using alkali-halophilic Halomonas alkalicola M2. Bioresour. Technol. 2022, 351, 126919. [Google Scholar] [CrossRef]
- Elain, A.; Le Grand, A.; Corre, Y.M.; Le Fellic, M.; Hachet, N.; Le Tilly, V.; Loulergue, P.; Audic, J.-L.; Bruzaud, S. Valorisation of local agro-industrial processing waters as growth media for polyhydroxyalkanoates (PHA) production. Ind. Crops Prod. 2016, 80, 1–5. [Google Scholar] [CrossRef]
- Rathi, D.N.; Amir, H.G.; Abed, R.M.; Kosugi, A.; Arai, T.; Sulaiman, O.; Hashim, R.; Sudesh, K. Polyhydroxyalkanoate biosynthesis and simplified polymer recovery by a novel moderately halophilic bacterium isolated from hypersaline microbial mats. J. Appl. Microbiol. 2013, 114, 384–395. [Google Scholar] [CrossRef]
- Lemechko, P.; Le Fellic, M.; Bruzaud, S. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) using agro-industrial effluents with tunable proportion of 3-hydroxyvalerate monomer units. Int. J. Biol. Macromol. 2019, 128, 429–434. [Google Scholar] [CrossRef]
- Kulkarni, S.O.; Kanekar, P.P.; Jog, J.P.; Sarnaik, S.S.; Nilegaonkar, S.S. Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int. J. Biol. Macromol. 2015, 72, 784–789. [Google Scholar] [CrossRef]
- Zhang, C.C.; Zhou, C.Z.; Burnap, R.L.; Peng, L. Carbon/nitrogen metabolic balance: Lessons from cyanobacteria. Trends Plant Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef]
- Tao, G.B.; Tan, B.W.; Li, Z.J. Production of polyhydroxyalkanoates by a moderately halophilic bacterium of Salinivibrio sp. TGB10. Int. J. Biol. Macromol. 2021, 186, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Van-Thuoc, D.; Huu-Phong, T.; Minh-Khuong, D.; Hatti-Kaul, R. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by a moderate halophile Yangia sp. ND199 using glycerol as a carbon source. Appl. Biochem. Biotechnol. 2015, 175, 3120–3132. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.W.; Lin, Y.N.; Yi, X.Q.; Yu, Z.X.; Liu, X.; Chen, G.Q. Synthetic biology of extremophiles: A new wave of biomanufacturing. Trends Biotechnol. 2023, 41, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yin, J.; Ye, J.W.; Xiang, R.J.; Ning, Z.Y.; Huang, W.Z.; Chen, G.Q. Promoter Engineering for Enhanced P(3HB- co-4HB) Production by Halomonas bluephagenesis. ACS Synth. Biol. 2018, 7, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Takei, Y.; Azuma, Y. Establishment of genetic tools for genomic DNA engineering of Halomonas sp. KM-1, a bacterium with potential for biochemical production. Microb. Cell Fact. 2022, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, H.M.; Chen, X.; Li, T.; Wu, Q.; Ouyang, Q.; Chen, G.Q. Novel T7-like expression systems used for Halomonas. Metab. Eng. 2017, 39, 128–140. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, X.; Lin, Y.; Zhang, L.; Yuan, Y.; Wang, H.; Winterburn, J.; Wu, F.; Wu, Q.; Ye, J.W.; et al. Engineering an oleic acid-induced system for Halomonas, E. coli and Pseudomonas. Metab. Eng. 2022, 72, 325–336. [Google Scholar] [CrossRef]
- Jiang, X.R.; Yao, Z.H.; Chen, G.Q. Controlling cell volume for efficient PHB production by Halomonas. Metab. Eng. 2017, 44, 30–37. [Google Scholar] [CrossRef]
- Ling, C.; Qiao, G.Q.; Shuai, B.W.; Song, K.N.; Yao, W.X.; Jiang, X.R.; Chen, G.Q. Engineering self-flocculating Halomonas campaniensis for wastewaterless open and continuous fermentation. Biotechnol. Bioeng. 2019, 116, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Acuña, J.M.B.-D.; Bielecka, A.; Häussler, S.; Schobert, M.; Jahn, M.; Wittmann, C.; Jahn, D.; Poblete-Castro, I. Production of medium chain length polyhydroxyalkanoate in metabolic flux optimized Pseudomonas putida. Microb. Cell Fact 2014, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.R.; Wang, H.; Shen, R.; Chen, G.Q. Engineering the bacterial shapes for enhanced inclusion bodies accumulation. Metab. Eng. 2015, 29, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.R.; Chen, G.Q. Morphology engineering of bacteria for bio-production. Biotechnol. Adv. 2016, 34, 435–440. [Google Scholar] [CrossRef]
- Tao, W.; Lv, L.; Chen, G.Q. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb. Cell Fact. 2017, 16, 48. [Google Scholar] [CrossRef]
- Ye, J.; Hu, D.; Che, X.; Jiang, X.; Li, T.; Chen, J.; Zhang, H.M.; Chen, G.Q. Engineering of Halomonas bluephagenesis for low cost production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab. Eng. 2018, 47, 143–152. [Google Scholar] [CrossRef]
- Haque, M.A.; Priya, A.; Hathi, Z.J.; Qin, Z.H.; Mettu, S.; Lin, C.S.K. Advancements and current challenges in the sustainable downstream processing of bacterial polyhydroxyalkanoates. Curr. Opin. Green Sustain. Chem. 2022, 36, 100631. [Google Scholar] [CrossRef]


| Carbon Source | Microorganism | PHA Types | PHA Yield (% CDM) | Fermentation Condition | Remarks | References |
|---|---|---|---|---|---|---|
| Waste oils | ||||||
| Palm oil mill effluent | Salinivibrio sp. | P(3HB) | nd | 15% (v/v) POME, 5% (w/v) NaCl, 0.1% (w/v) yeast, and 0.1% (w/v) ammonium sulfate. | P(3HB) with high thermal stability | [29] |
| Palm oil mill effluent | Bacillus licheniformis M2–12 | P(3HB) | 88.7 | pH 7, 45 °C | PHA production using cheap raw materials | [30] |
| Waste fish oil and glycerol | Salinivibrio sp. M318 | P(3HB-co-3HV) | 51.5 | pH 6.5, 30 °C, 0.5 g/L KH2PO4, 600 rpm | Higher biomass of 69.1 g/L was obtained after 78 h in fed -batch culture. | [31] |
| Waste frying oil | Halomonas hydrothermalis | P(3HB) | 61.98 | pH 7.5, 30 °C, 40 g/L NaCl, 300 rpm | Supplementation of valerate led to an HV content of 50.15 mol% | [32] |
| Halomonas neptunia | P(3HB) | 55.71 | pH 7.5, 30 °C, 60 g/L NaCl, 300 rpm | Supplementation of n-propanol led to an HV content of 29.5 mol% | ||
| Waste cooking oil | Paracoccus sp. LL1 | P(3HB-co-3HV) | 30.89 | pH 7.5, 30 °C, 1.0 g/L (NH4)2SO4, 0.1% Tween-80, 300 rpm, 2.5 vvm | Batch culture with 3.24 g/L biomass and 0.89 mg/L carotenoids | [16] |
| Oil Palm Empty Fruit Bunch | Halomonas boliviensis | P(3HB) | 35.7 | pH 7, 31 °C, 2.5 g/L KH2PO4, 200 rpm | Non-conventional nutrients for cost-effective PHA production | [33] |
| Crude glycerol | ||||||
| Biodiesel fuel by-product | Thermotolerant Pseudomonas sp. strain SG4502 | mcl-PHA | 40.6 | 45 °C, minimal salt media, 160 rpm | PHA production at high temperature | [34] |
| Jatropha biodiesel byproduct | Halomonas hydrothermalis SM-P-3M | P(3HB) | 75.0 | pH 7, 37 °C, 0.4 g/L KH2PO4, 200 rpm | High PHB content by a marine environment isolate | [35] |
| Crude glycerol | Paracoccus sp. LL1 | P(3HB-co-3HV) | 39.3 | pH 7.5, 30 °C, 1.0 g/L (NH4)2SO4, 300 rpm | Cell-retention culture with 24.2 g/L of biomass and 7.14 mg/L of carotenoids | [15] |
| Residues from cheese production | ||||||
| Whey-based media | Thermus thermophilus HB8 | P(3HB-co-3HHp-co-3HN-co-3HU) | 35 | initial phosphate concentration of 50 mM | Novel heteropolymer consisting of both scl- and mcl-PHA | [36] |
| Cheese whey mother liquor | Paracoccus homiensis | P(3HB-co-3HV) | 29.0 | pH 7.6, 28 °C, 3.0 g/L KH2PO4, 110 rpm | Utilization and management of dairy wastes | [37] |
| Acidogenic fermentate of acid whey | 17.0 | pH 5.5, 30 °C, UASB reactor with autocontrols | Valorization of carboxylic acid rich waste streams | [38] | ||
| Other waste materials | ||||||
| Mixed substrates (Kitchen wastes) | Halomonas campaniensis strain LS21 | P(3HB) | 26 | 27 g/L NaCl, pH 10, 37 °C for 65 days | Secreted extracellular enzymes for waste hydrolysis | [39] |
| Recombinant H. campaniensis | P(3HB) | 70 | Secreted enzymes and also maintained the phbCAB plasmid throughout the fermentation process without contamination | |||
| Cassava starch + valerate | Caldimonas Taiwanensis | P(3HB-co-3HV) | 67 | Nitrogen limited conditions (C/N = 30) | PHA production by thermophilic bacteria | [40] |
| Bacteria | Carbon Source | Type of PHA | Mw (kDa) | Mn (kDa) | Tm (°C) | Tg (°C) | Td (°C) | References |
|---|---|---|---|---|---|---|---|---|
| Halomonas sp. YLGW01 | Crude glycerol | P(3HB) | 580.0 | 430.0 | nd | nd | nd | [52] |
| Halomonas elongata P2 | Wheat straw | P(3HB) | nd | nd | 165.0 | nd | nd | [53] |
| Halomonas daqingensis | Algal biodiesel waste residue | P(3HB) | 309.0 | 169.8 | nd | nd | 290.0 | [54] |
| Halomonas ventosae | Algal biodiesel waste residue | P(3HB) | nd | nd | nd | nd | 296.0 | [54] |
| Halomonas hydrothermalis | Waste frying oil | P(3HB) | 253.6 | 216.8 | nd | nd | nd | [32] |
| Halomonas alkalicola M2 | Bamboo powder | P(3HB) | 390.0 | 613.0 | 163.0 | nd | 269.0 | [55] |
| Salinivibrio sp. M318 | Waste fish oil + glycerol | P(3HB) | 410.0 | 300.0 | 170 | 4.0 | nd | [31] |
| Halomonas sp. i4786 | Leguminous Processing Water | P(3HB) | 677.5 | 644.5 | 166.9 | −0.2 | nd | [56] |
| Fruit Processing Water | P(3HB) | 588.0 | 518.5 | 172.4 | −5.0 | nd | ||
| Halomonas sp. SK5 | Oil palm trunk | P(3HB) | 165.0 | 827.0 | nd | nd | nd | [57] |
| Paracoccus homiensis | Carboxylic acids-rich stream | P(98.3% HB-co-1.7% HV) | nd | nd | 165.4 (155.0) | 2.5 | 253.8 | [38] |
| Paracoccus homiensis | Cheese whey mother liquor | P(39.41% HB-co-60.59% HV) | nd | nd | 167.7 (158.2) | 2.3 | 280.0 | [37] |
| Halomonas sp. SF2003 | Agro-industrial effluent/valeric acid | P(65% HB-co-35% HV) | 536.8 | 389.0 | 149.7 (166.0) | −11.7 | nd | [58] |
| Halomonas campisalis MCMB-1027 | Bagasse extract | P(94.4% HB-co-5.6% HV) | 139.4 | 838.5 | 168.9 | nd | nd | [59] |
| Salinivibrio sp. M318 | Waste fish oil + glycerol + sodium valerate | P(75.3% HB-co-24.7% 3HV) | 530.0 | 310.0 | 139.0 | −4.7 | nd | [31] |
| Bacteria | Genetic Engineering Strategy | Benefits | References |
|---|---|---|---|
| Halomonas bluephagenesis TD01 | Promoter engineering | 80% of P(3HB-co-4HB) in CDM under non-sterile fed-batch fermentation | [64] |
| Halomonas sp. KM-1 | CRISPR/Cas9 system for genome deletion and integration | disruption of the pyrF gene | [65] |
| Halomonas sp. TD01 | T7-like system for overexpression of the cell-elongation cassette (minCD genes) | 100-fold increase in cell lengths and high levels of P(3HB) production (up to 92% of CDM) | [66] |
| Halomonas bluephagenesis | Ligand-induced system for the control of minCD, and monomer precursor 4-hydroxybutyrate-CoA (4HB-CoA) synthesis pathway | over 10 g/L of P(3HB) accumulated by elongated cell sizes, and 6 g/L of P(3HB-co-9.57 mol% 4HB) copolymer | [67] |
| Halomonas campaniensis LS21 | Temperature-responsible plasmid expression system for inactivation of mreB and ftsZ genes | controllable expanding cell volumes for PHA granules, up to 80% P(3HB) yield | [68] |
| Halomonas campaniensis LS21 | Deletion of the etf operon encoding two subunits of an electron transfer flavoprotein for reduction of downstream cost associated with continuous centrifugation | Most microbial cells flocculated and precipitated to the bottom of the bioreactor within 1 min after stopping the aeration and agitation. | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Możejko-Ciesielska, J.; Ray, S.; Sankhyan, S. Recent Challenges and Trends of Polyhydroxyalkanoate Production by Extremophilic Bacteria Using Renewable Feedstocks. Polymers 2023, 15, 4385. https://doi.org/10.3390/polym15224385
Możejko-Ciesielska J, Ray S, Sankhyan S. Recent Challenges and Trends of Polyhydroxyalkanoate Production by Extremophilic Bacteria Using Renewable Feedstocks. Polymers. 2023; 15(22):4385. https://doi.org/10.3390/polym15224385
Chicago/Turabian StyleMożejko-Ciesielska, Justyna, Subhasree Ray, and Shivangi Sankhyan. 2023. "Recent Challenges and Trends of Polyhydroxyalkanoate Production by Extremophilic Bacteria Using Renewable Feedstocks" Polymers 15, no. 22: 4385. https://doi.org/10.3390/polym15224385





