Pickering Emulsions Stabilized by Conjugated Zein-Soybean Polysaccharides Nanoparticles: Fabrication, Characterization and Functional Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zein/SSPS Conjugates
2.3. Preparation of Zein/SSPS Conjugated Nanoparticles (ZSP)
2.4. Characterization of Zein/SSPS Conjugated Nanoparticles (ZSP)
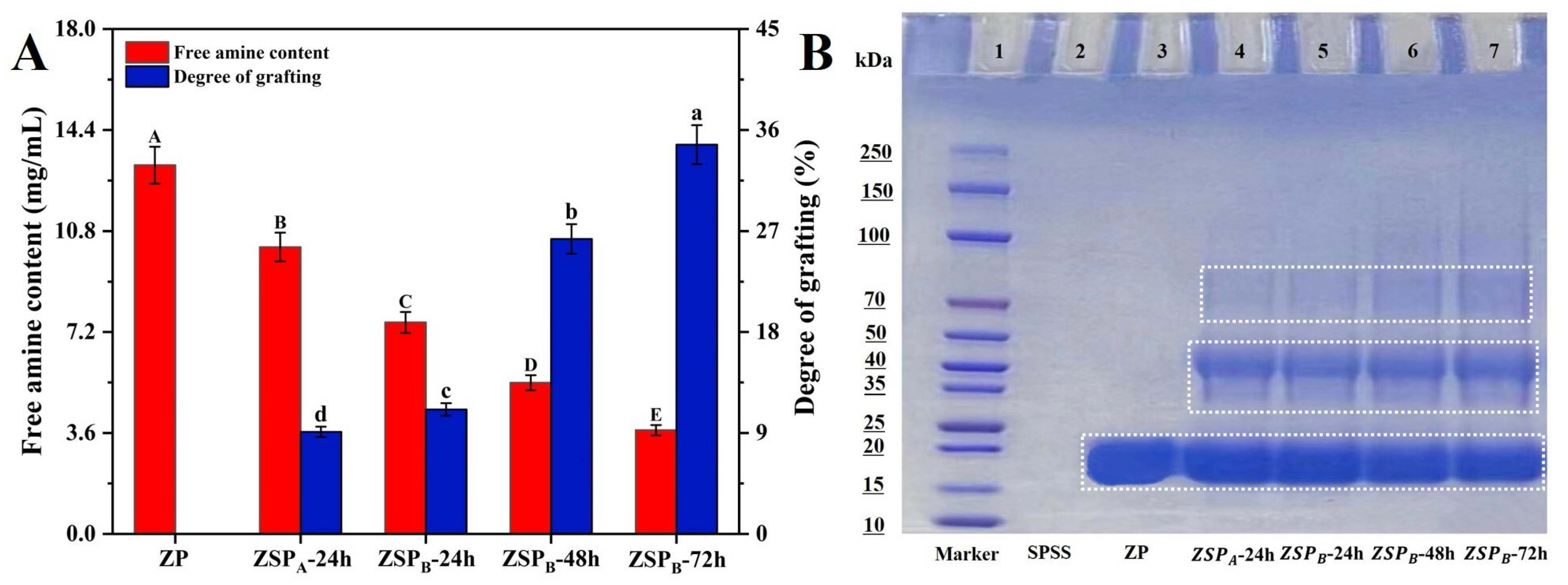
2.4.1. Determination of Free Amine Content and Degree of Grafting (DG)
2.4.2. SDS-PAGE
2.4.3. FTIR Analysis
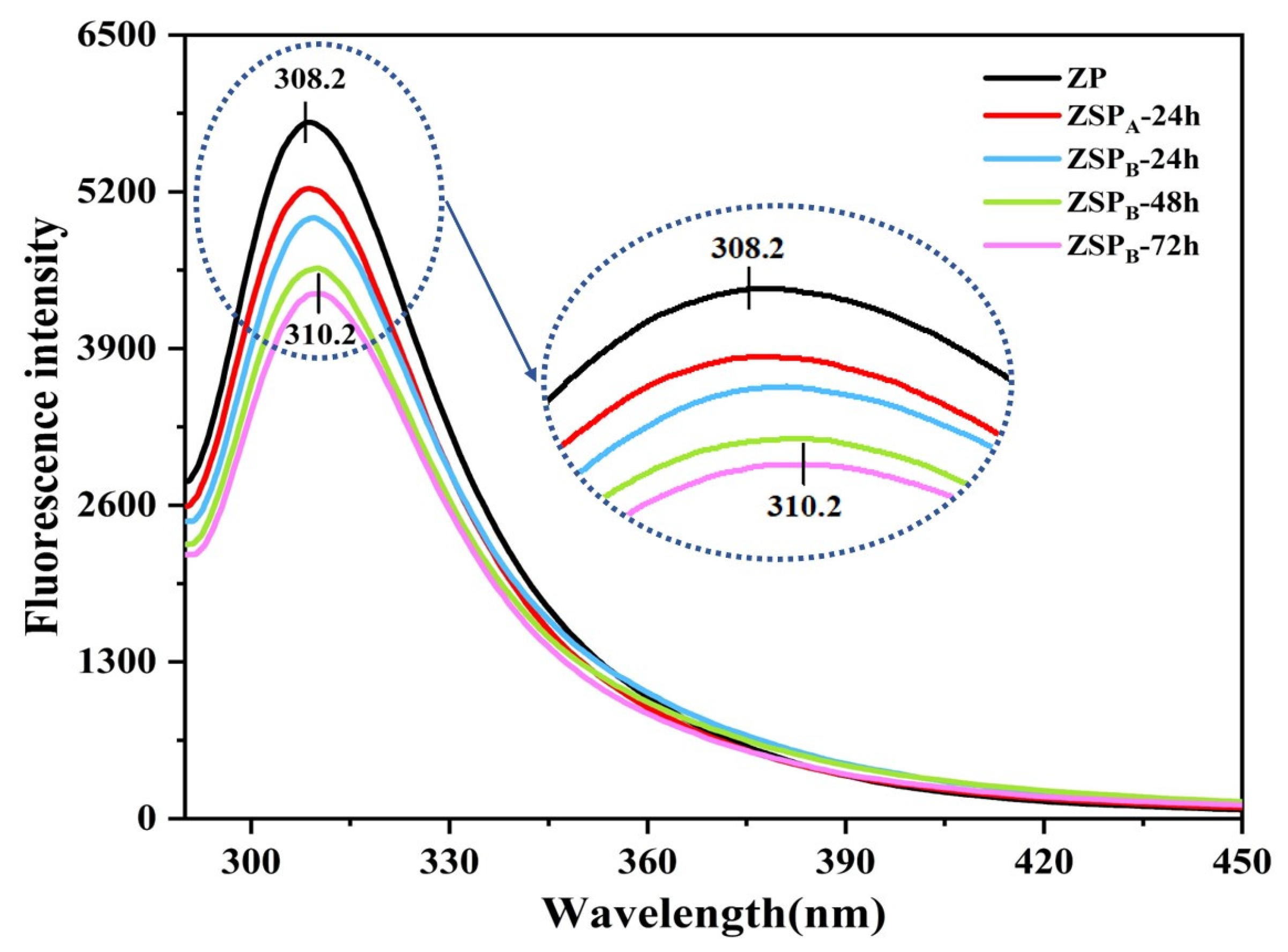
2.4.4. Fluorescence Spectroscopy
2.4.5. Evaluation of Nanoparticle Stability
2.4.6. Wettability Measurements
2.5. Preparation of ZSP-Stabilized Pickering Emulsions
2.6. Emulsions Stability
2.7. Microstructure of Emulsions
2.8. Oxidation Stability of Pickering Emulsions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Zein/SSPS Conjugated Nanoparticles (ZSP)
3.1.1. Measurements of Free Amine Content and Degree of Grafting (DG)
3.1.2. SDS-PAGE
3.2. Structural Properties of Zein/SSPS Conjugated Nanoparticles
3.2.1. FTIR
3.2.2. Fluorescence Spectrum
3.3. Physical Stability of Native ZP and ZSP
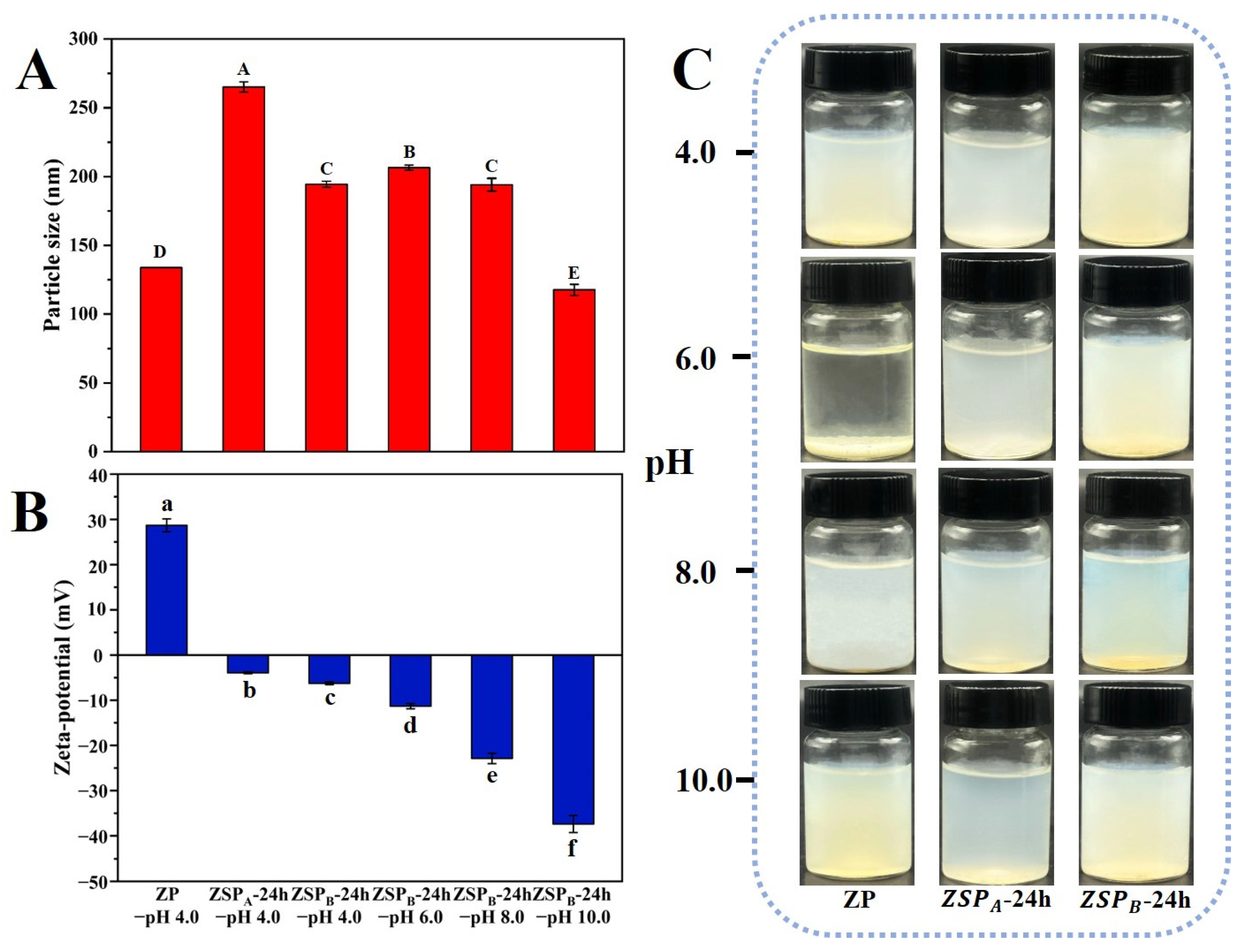
3.3.1. Effect of pH on the Stability of Native ZP and ZSP
3.3.2. Nanoparticle Stability at pH 6.0
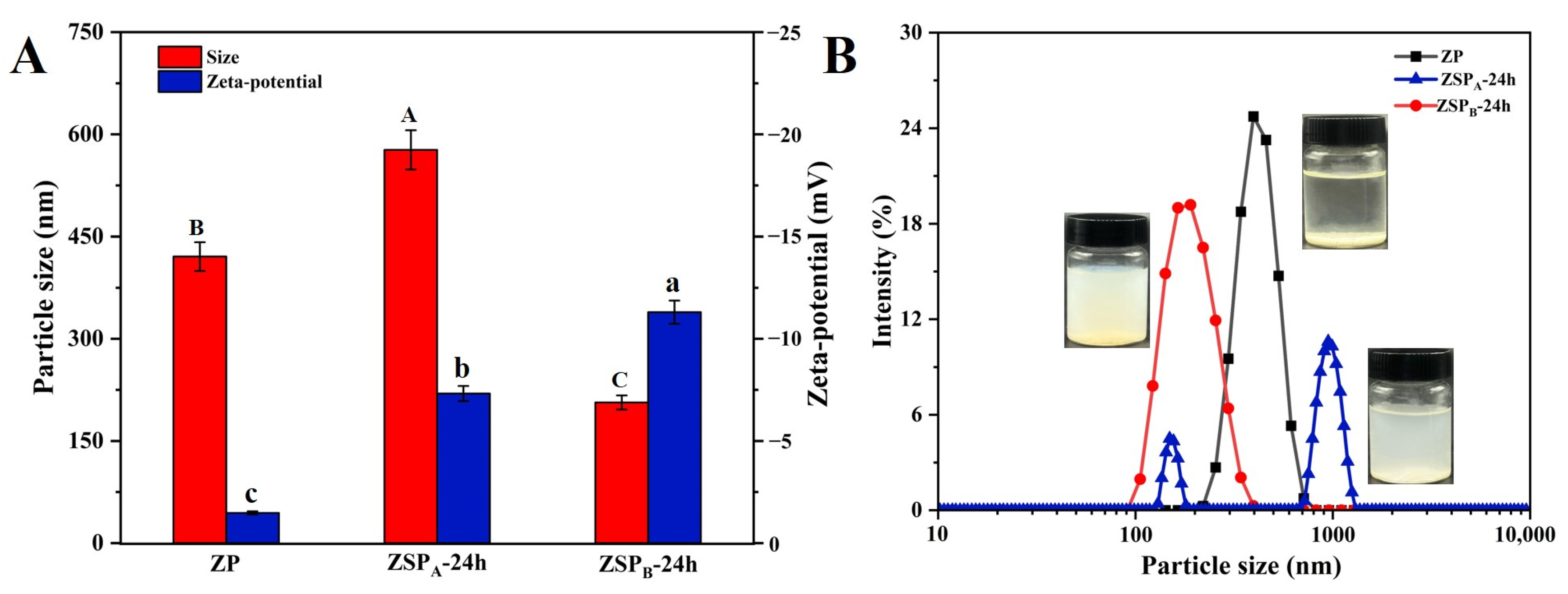
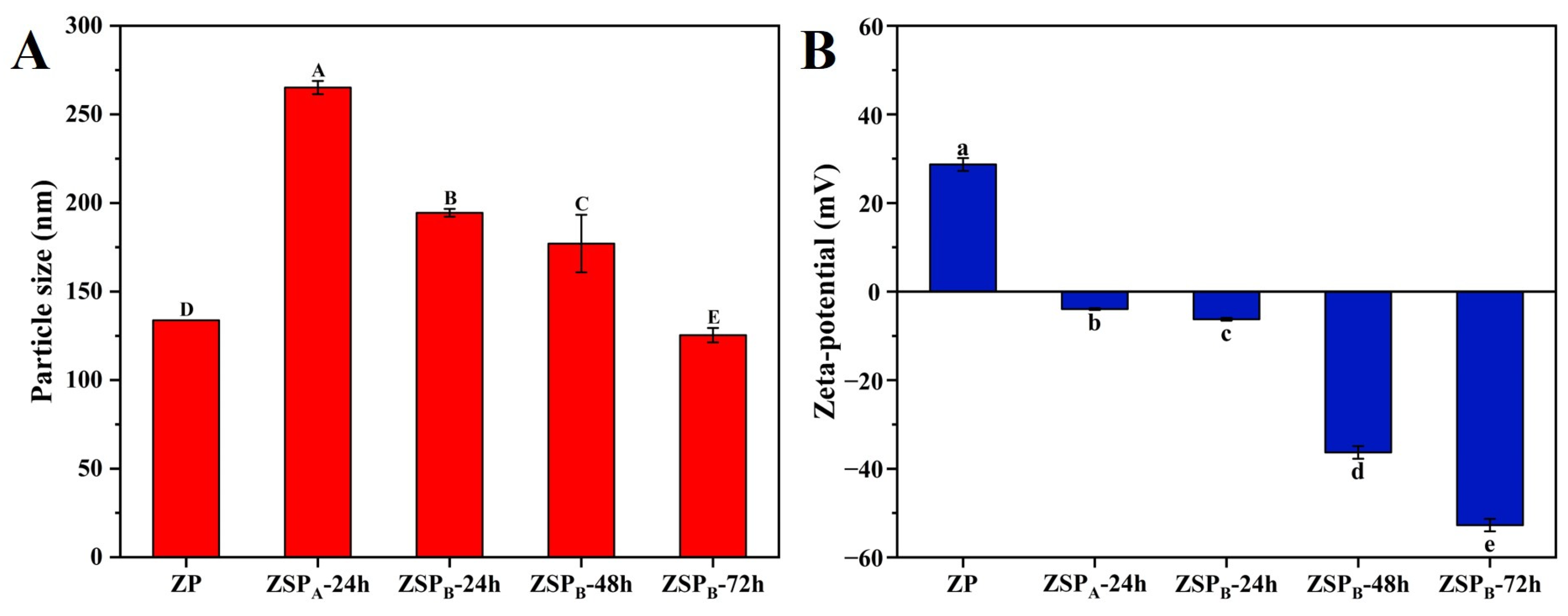
3.3.3. Analysis of Nanoparticle Size and Potential under Different Reaction Conditions
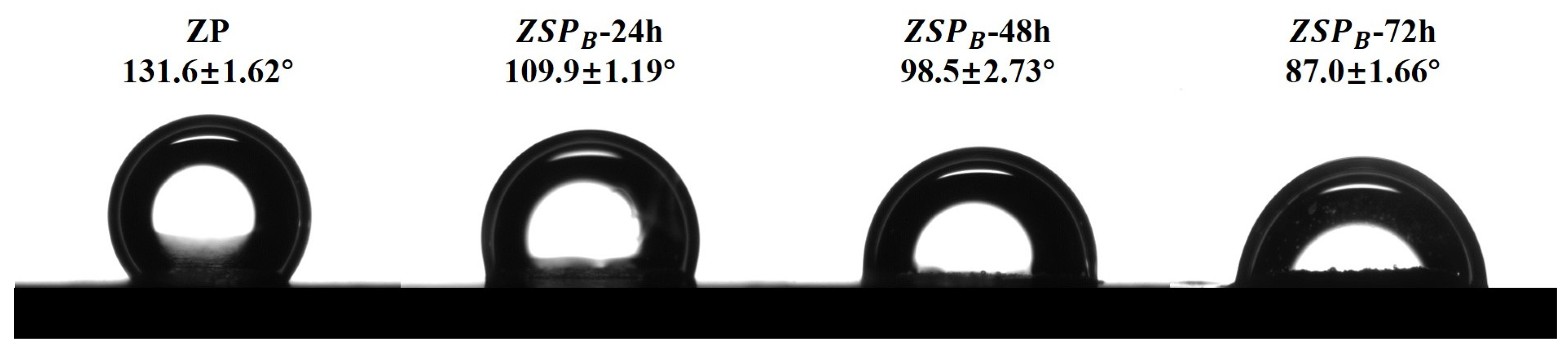
3.4. Wettability of Native ZP and ZSP
3.5. Properties of Pickering Emulsions Stabilized by Native ZP and ZSP
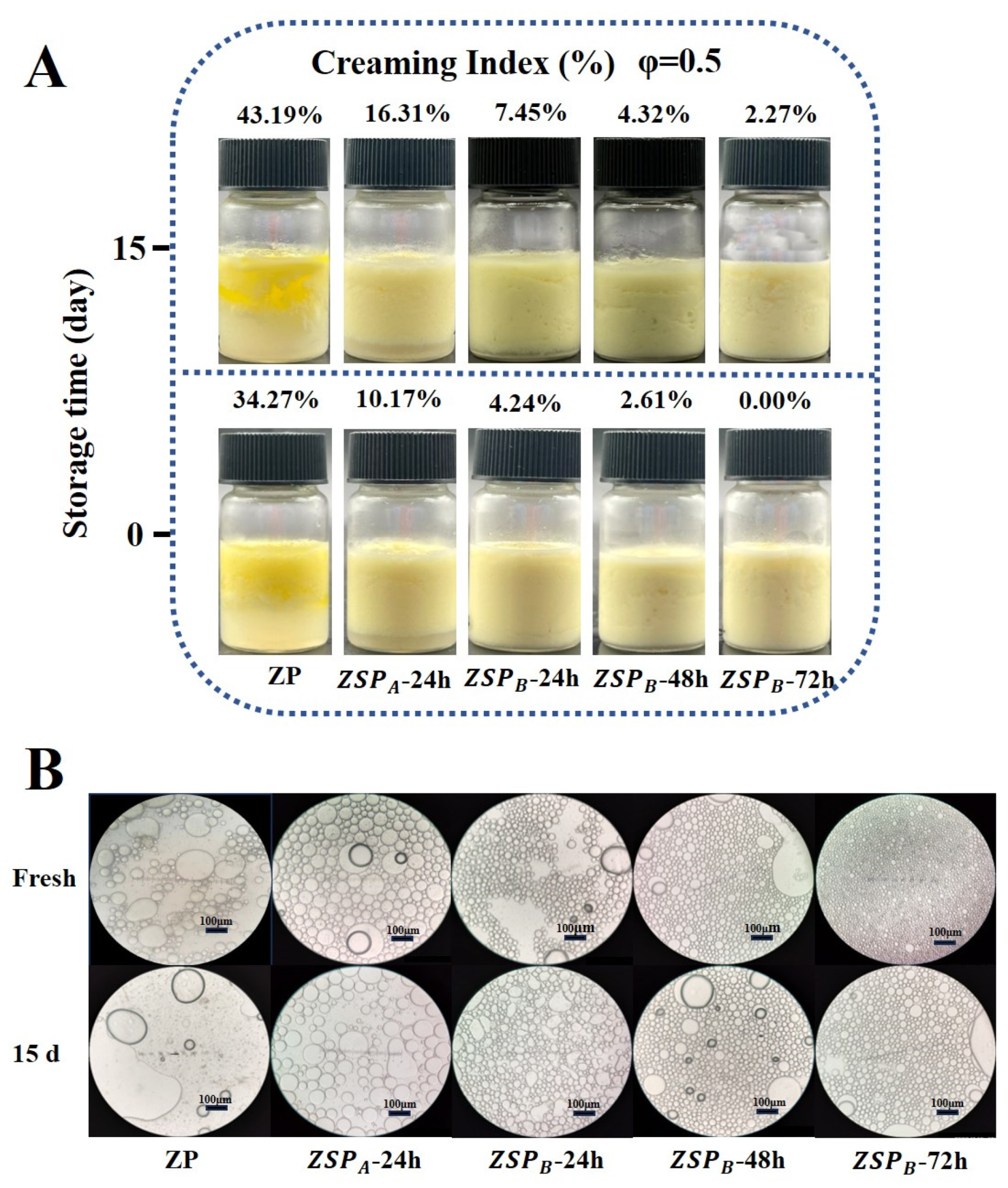
3.5.1. Visual Appearance, Optical Micrographs and Creaming Index (CI)
3.5.2. Confocal Laser Scanning Microscopy (CLSM)
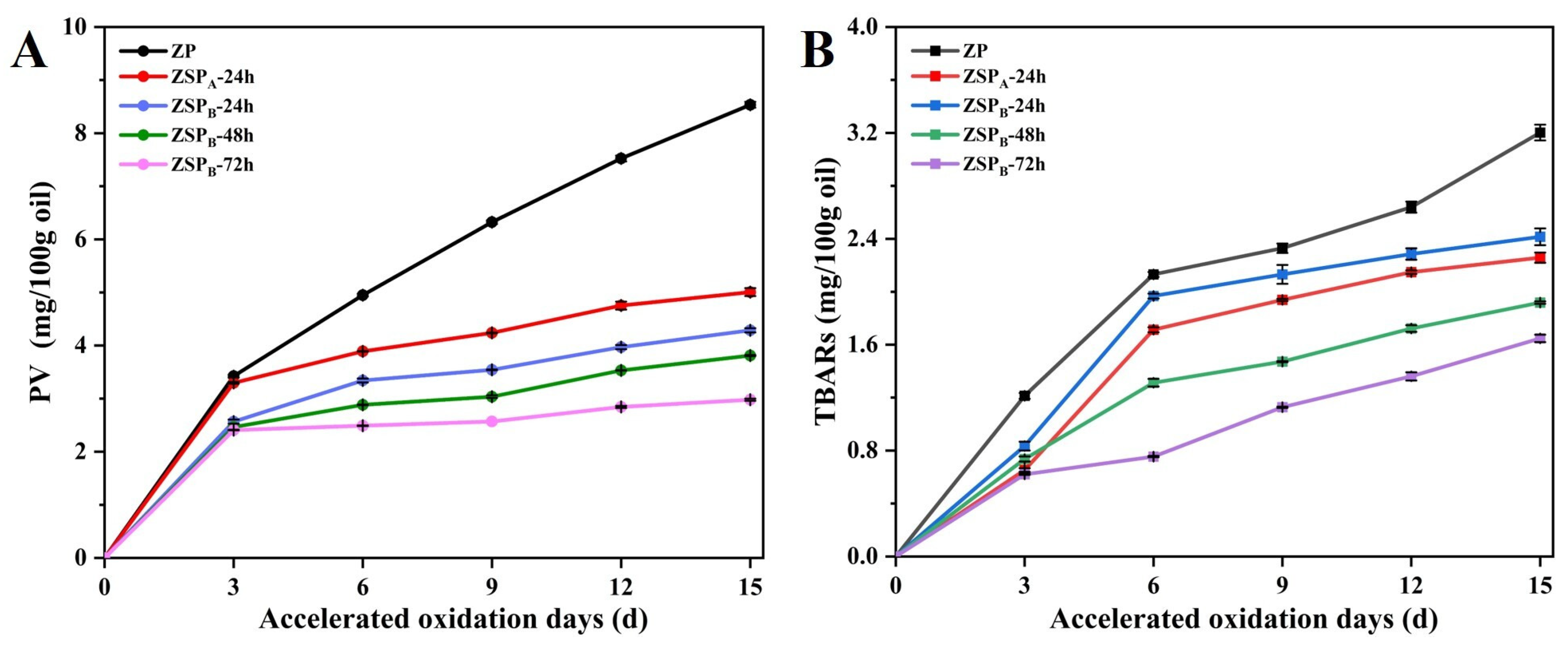
3.5.3. Oxidative Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, D.; Deng, S.; Mcclements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J. Colloid Interface Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, J. Waxy maize starch nanoparticles incorporated tea polyphenols to stabilize Pickering emulsion and inhibit oil oxidation. Carbohyd. Polym. 2022, 296, 119991. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wu, Y.; He, K.; Li, Y.; Luo, X.; Li, B.; Wang, C.; Liu, S. Flexible cellulose nanofibrils as novel Pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Rousseau, D. Trends in structuring edible emulsions with Pickering fat crystals. Curr. Opin. Colloid Interface Sci. 2013, 18, 283–291. [Google Scholar] [CrossRef]
- Xu, T.; Yang, J.; Hua, S.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. Characteristics of starch-based Pickering emulsions from the interface perspective. Trends Food Sci. Technol. 2020, 105, 334–346. [Google Scholar] [CrossRef]
- De Folter, J.W.J.; Van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807–6815. [Google Scholar] [CrossRef]
- Kim, D.Y.; Shin, W.S. Functional improvements in bovine serum albumin-fucoidan conjugate through the Maillard reaction. Food Chem. 2016, 190, 974–981. [Google Scholar] [CrossRef]
- Affes, S.; Nasri, R.; Li, S.; Thami, T.; Van Der Lee, A.; Nasri, M.; Maalej, H. Effect of glucose-induced Maillard reaction on physical, structural and antioxidant properties of chitosan derivatives-based films. Carbohyd. Polym. 2021, 255, 117341. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Sun, Q.; Wang, J.; Zheng, B.; Guo, Z. Structural characteristics and emulsifying properties of myofibrillar protein-dextran conjugates induced by ultrasound Maillard reaction. Ultrason. Sonochem. 2021, 72, 105458. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Muhoza, B.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C. Whey protein isolate-dextran conjugates: Decisive role of glycation time dependent conjugation degree in size control and stability improvement of colloidal nanoparticles. LWT 2021, 148, 111766. [Google Scholar] [CrossRef]
- Masoumi, B.; Tabibiazar, M.; Fazelioskouei, T.; Mohammadifar, M.; Hamishehkar, H. Pickering emulsion stabilized by conjugated sodium caseinate-ascorbic acid nanoparticles: Synthesis and physicochemical characterization. Food Hydrocoll. 2023, 145, 109168. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, L.; Li, D.; Chang, Y. Lutein-loaded glycosylated zein nanoparticles-preparation, characterization, and stability in functional drink. LWT 2023, 187, 115308. [Google Scholar] [CrossRef]
- Nakamura, A.; Furuta, H.; Maeda, H.; Takao, T.; Nagamatsu, Y. Structural studies by stepwise enzymatic degradation of the main backbone of soybean soluble polysaccharides consisting of galacturonan and rhamnogalacturonan. Biosci. Biotech. Biochem. 2002, 66, 1301–1313. [Google Scholar] [CrossRef]
- Tavasoli, S.; Maghsoudlou, Y.; Jafari, S.M.; Tabarestani, H.S. Improving the emulsifying properties of sodium caseinate through conjugation with soybean soluble polysaccharides. Food Chem. 2022, 377, 131987. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.; Gong, J.; Miller, S.S.; Wang, Q.; Hua, Y. Stability of citral in oil-in-water emulsions protected by a soy protein–polysaccharide Maillard reaction product. Food Res. Int. 2015, 69, 357–363. [Google Scholar] [CrossRef]
- Peng, X.; Xu, Y.; Liu, T.; Tang, C. Molecular mechanism for improving emulsification efficiency of soy glycinin by glycation with soy soluble polysaccharide. J. Agric. Food Chem. 2018, 66, 12316–12326. [Google Scholar] [CrossRef]
- Yin, B.; Wang, C.; Liu, Z.; Yao, P. Peptide-polysaccharide conjugates with adjustable hydrophilicity/hydrophobicity as green and pH sensitive emulsifiers. Food Hydrocoll. 2017, 63, 120–129. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lazzaro, F.; Saint-Jalmes, A.; Violleau, F.; Lopez, C.; Gaucher-Delmas, M.; Madec, M.; Beaucher, E.; Gaucheron, F. Gradual disaggregation of the casein micelle improves its emulsifying capacity and decreases the stability of dairy emulsions. Food Hydrocoll. 2017, 63, 189–200. [Google Scholar] [CrossRef]
- Kargar, M.; Spyropoulos, F.; Norton, I.T. The effect of interfacial microstructure on the lipid oxidation stability of oil-in-water emulsions. J. Colloid Interface Sci. 2011, 357, 527–533. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; Da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Moreno, F.J.; Olano, A.; Villamiel, M. Structural characterization of bovine β-lactoglobulin−galactose/tagatose Maillard complexes by electrophoretic, chromatographic, and spectroscopic methods. J. Agric. Food Chem. 2008, 56, 4244–4252. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Wu, J.; A’Yun, Q.; Van der Meeren, P. Maillard conjugation as an approach to improve whey proteins functionality: A review of conventional and novel preparation techniques. Trends Food Sci. Technol. 2019, 91, 1–11. [Google Scholar] [CrossRef]
- Cermeño, M.; Felix, M.; Connolly, A.; Brennan, E.; Coffey, B.; Ryan, E.; Fitzgerald, R.J. Role of carbohydrate conjugation on the emulsification and antioxidant properties of intact and hydrolysed whey protein concentrate. Food Hydrocoll. 2019, 88, 170–179. [Google Scholar] [CrossRef]
- Mattice, K.D.; Marangoni, A.G. Physical properties of zein networks treated with microbial transglutaminase. Food Chem. 2021, 338, 128010. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Tabatabaee Amid, B.; Mirhosseini, H. Stabilization of water in oil in water (w/o/w) emulsion using whey protein isolate-conjugated durian seed gum: Enhancement of interfacial activity through conjugation process. Colloids Surf. B Biointerfaces 2014, 113, 107–114. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, H.; Hao, L.; Li, Z.; Xu, H.; Chen, H.; Zhou, X. Dialdehyde carboxymethyl cellulose-zein conjugate as water-based nanocarrier for improving the efficacy of pesticides. Ind. Crop. Prod. 2020, 150, 112358. [Google Scholar] [CrossRef]
- Salarbashi, D.; Noghabi, M.S.; Bazzaz, B.S.F.; Shahabi-Ghahfarrokhi, I.; Jafari, B.; Ahmadi, R. Eco-friendly soluble soybean polysaccharide/nanoclayna + bionanocomposite: Properties and characterization. Carbohyd. Polym. 2017, 169, 524–532. [Google Scholar] [CrossRef]
- Song, C.L.; Zhao, X.H. The preparation of an oligochitosan-glycosylated and cross-linked caseinate obtained by a microbial transglutaminase and its functional properties. Int. J. Dairy Technol. 2014, 67, 110–116. [Google Scholar] [CrossRef]
- Farhat, I.A.; Orset, S.; Moreau, P.; Blanshard, J.M.V. FTIR study of hydration phenomena in protein–sugar systems. J. Colloid Interface Sci. 1998, 207, 200–208. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crop. Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Huang, S.; He, J.; Han, L.; Lin, H.; Liu, G.; Zhang, W. Zein-polyglycerol conjugates with enhanced water solubility and stabilization of high oil loading emulsion. J. Agric. Food Chem. 2020, 68, 11810–11816. [Google Scholar] [CrossRef]
- Ke, C.; Li, L. Influence mechanism of polysaccharides induced Maillard reaction on plant proteins structure and functional properties: A review. Carbohyd. Polym. 2023, 302, 120430. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, J.; Wu, Z.; Wang, H. Enhanced physicochemical stability of lutein-enriched emulsions by polyphenol-protein-polysaccharide conjugates and fat-soluble antioxidant. Food Hydrocoll. 2020, 101, 105447. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Lin, Y.; Ma, M. Synthesis and structural characterization of lysozyme-pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Zhang, Y.; Wen, Z.; Zhao, L.; Lu, Y. Glycosylated α-lactalbumin-based nanocomplex for curcumin: Physicochemical stability and DPPH-scavenging activity. Food Hydrocoll. 2016, 61, 369–377. [Google Scholar] [CrossRef]
- Cui, S.; Mcclements, D.J.; Shi, J.; Xu, X.; Ning, F.; Liu, C.; Zhou, L.; Sun, Q.; Dai, L. Fabrication and characterization of low-fat Pickering emulsion gels stabilized by zein/phytic acid complex nanoparticles. Food Chem. 2023, 402, 134179. [Google Scholar] [CrossRef]
- Lin, J.; Meng, H.; Yu, S.; Wang, Z.; Ai, C.; Zhang, T.; Guo, X. Genipin-crosslinked sugar beet pectin-bovine serum albumin nanoparticles as novel Pickering stabilizer. Food Hydrocoll. 2021, 112, 106306. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Li, T.; Wang, Y.; Jiang, J.; Zhang, X.; Huang, J.; Xia, B.; Shum, H.C.; Yang, Z.; et al. Ultra-stable Pickering emulsions stabilized by zein-cellulose conjugate particles with tunable interfacial affinity. Food Hydrocoll. 2023, 134, 108055. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, S.; Zhu, J.; Qi, J.; Guo, J.; Wu, L.; Tang, C.; Yang, X. Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles. Food Hydrocoll. 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium caseinate stabilized zein colloidal particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Pérez-Ciordia, S.; Marı́n-Arroyo, M.R.; Mcclements, D.J. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: Impact of protein–carbohydrate Maillard conjugation. J. Agric. Food Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; Espinal-Ruiz, M.; Mcclements, D.J. Effect of Maillard conjugates on the physical stability of zein nanoparticles prepared by liquid antisolvent coprecipitation. J. Agric. Food Chem. 2015, 63, 8510–8518. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Q.; Zhang, S.; Huang, Y.; Zhu, H.; Qi, B.; Li, Y. Oxidized dextran improves the stability and effectively controls the release of curcumin loaded in soybean protein nanocomplexes. Food Chem. 2024, 431, 137089. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, X.; Wu, Z.; Yin, S.; Zhu, J.; Tang, C.; Yang, X. Fabrication of zein/pectin hybrid particle-stabilized Pickering high internal phase emulsions with robust and ordered interface architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.; Li, X.; Tang, C. Novel nanoparticles from insoluble soybean polysaccharides of okara as unique Pickering stabilizers for oil-in-water emulsions. Food Hydrocoll. 2019, 94, 255–267. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Tan, Z.; Zhang, G.; Xu, X.; Song, L. Improved thermal and oxidation stabilities of Pickering high internal phase emulsions stabilized using glycated pea protein isolate with glycation extent. LWT 2022, 162, 113465. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Singh, A.K.; Arora, S.; Lal, D.; Sabikhi, L. Development of stable flaxseed oil emulsions as a potential delivery system ω-3 fatty acids. J. Food Sci. Technol. 2015, 52, 4256–4265. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E. Interfacial antioxidants: A review of natural and synthetic emulsifiers and coemulsifiers that can inhibit lipid oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef]


| Sample Nomenclature | Ratio of Zein to SSPS (w/w) | Maillard Reaction pH | Maillard Reaction Time |
|---|---|---|---|
| ZP | 1:0 | -- | -- |
| ZSPA-24 h | 1:1 | pH 7.0 | 24 h |
| ZSPB-24 h | 1:1 | pH 10.0 | 24 h |
| ZSPB-48 h | 1:1 | pH 10.0 | 48 h |
| ZSPB-72 h | 1:1 | pH 10.0 | 72 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Wang, Y.; He, Y.; Wei, P.; Li, C.; Xiong, X. Pickering Emulsions Stabilized by Conjugated Zein-Soybean Polysaccharides Nanoparticles: Fabrication, Characterization and Functional Performance. Polymers 2023, 15, 4474. https://doi.org/10.3390/polym15234474
Yao L, Wang Y, He Y, Wei P, Li C, Xiong X. Pickering Emulsions Stabilized by Conjugated Zein-Soybean Polysaccharides Nanoparticles: Fabrication, Characterization and Functional Performance. Polymers. 2023; 15(23):4474. https://doi.org/10.3390/polym15234474
Chicago/Turabian StyleYao, Lili, Ying Wang, Yangyang He, Ping Wei, Chen Li, and Xiong Xiong. 2023. "Pickering Emulsions Stabilized by Conjugated Zein-Soybean Polysaccharides Nanoparticles: Fabrication, Characterization and Functional Performance" Polymers 15, no. 23: 4474. https://doi.org/10.3390/polym15234474
APA StyleYao, L., Wang, Y., He, Y., Wei, P., Li, C., & Xiong, X. (2023). Pickering Emulsions Stabilized by Conjugated Zein-Soybean Polysaccharides Nanoparticles: Fabrication, Characterization and Functional Performance. Polymers, 15(23), 4474. https://doi.org/10.3390/polym15234474






