Facile Preparation of PVDF/CoFe2O4-ZnO Hybrid Membranes for Water Depollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
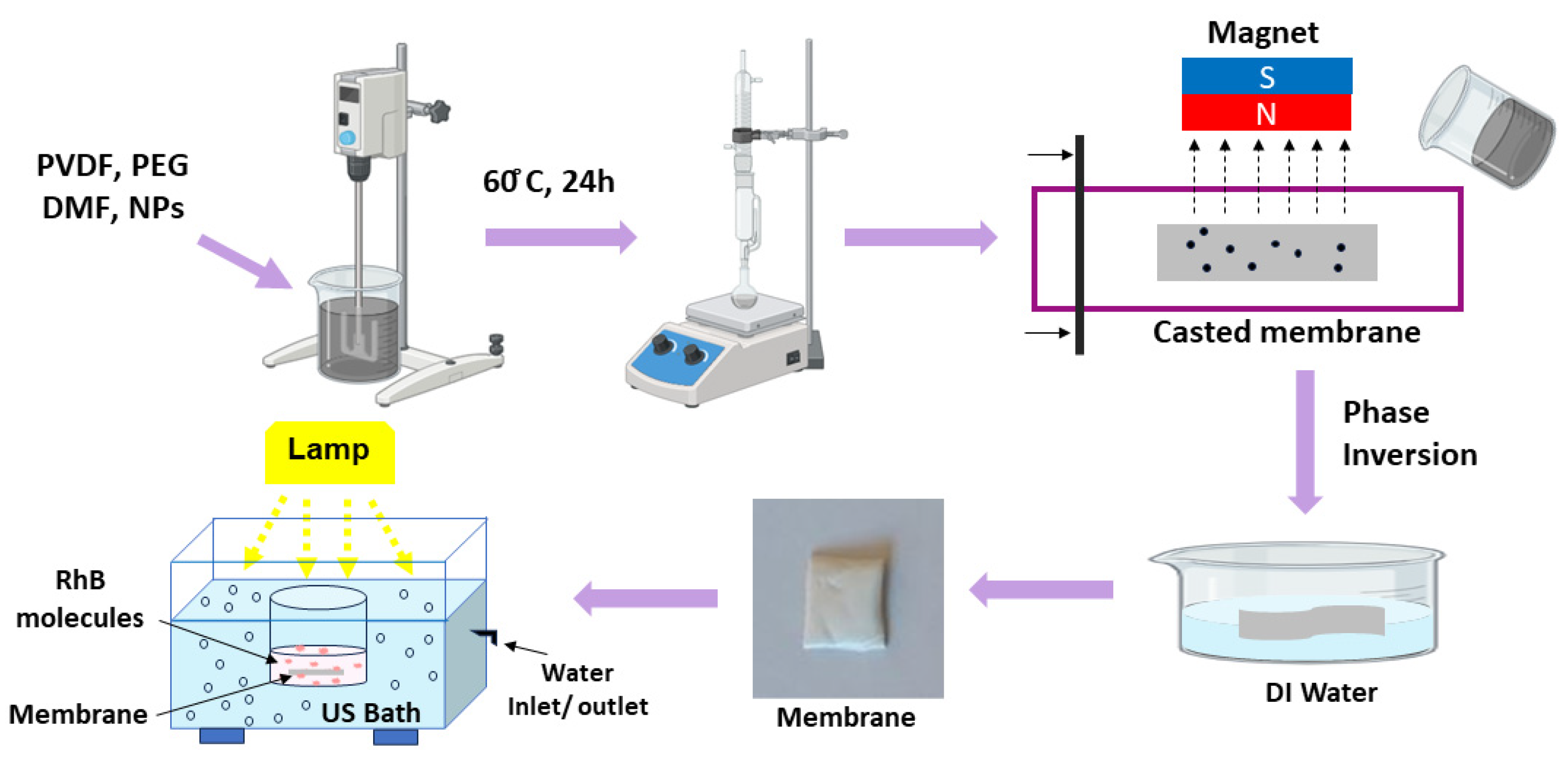
2.2. Sample Preparation
2.2.1. Synthesis of CoFe2O4 and CoFe2O4-ZnO Nanoparticles
2.2.2. Membrane Preparation
2.3. Methods
2.4. Photocatalytic Activity Evaluation
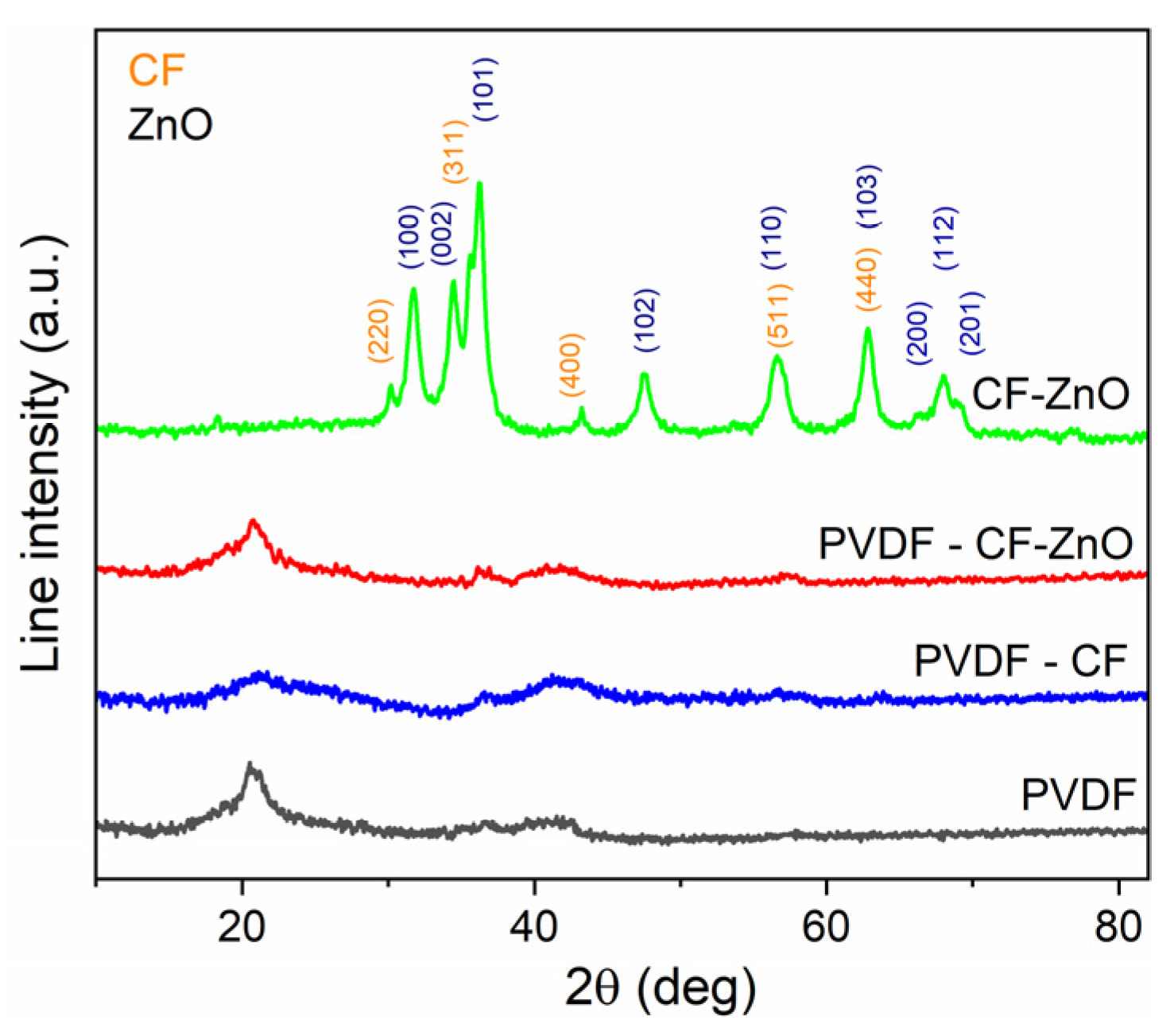
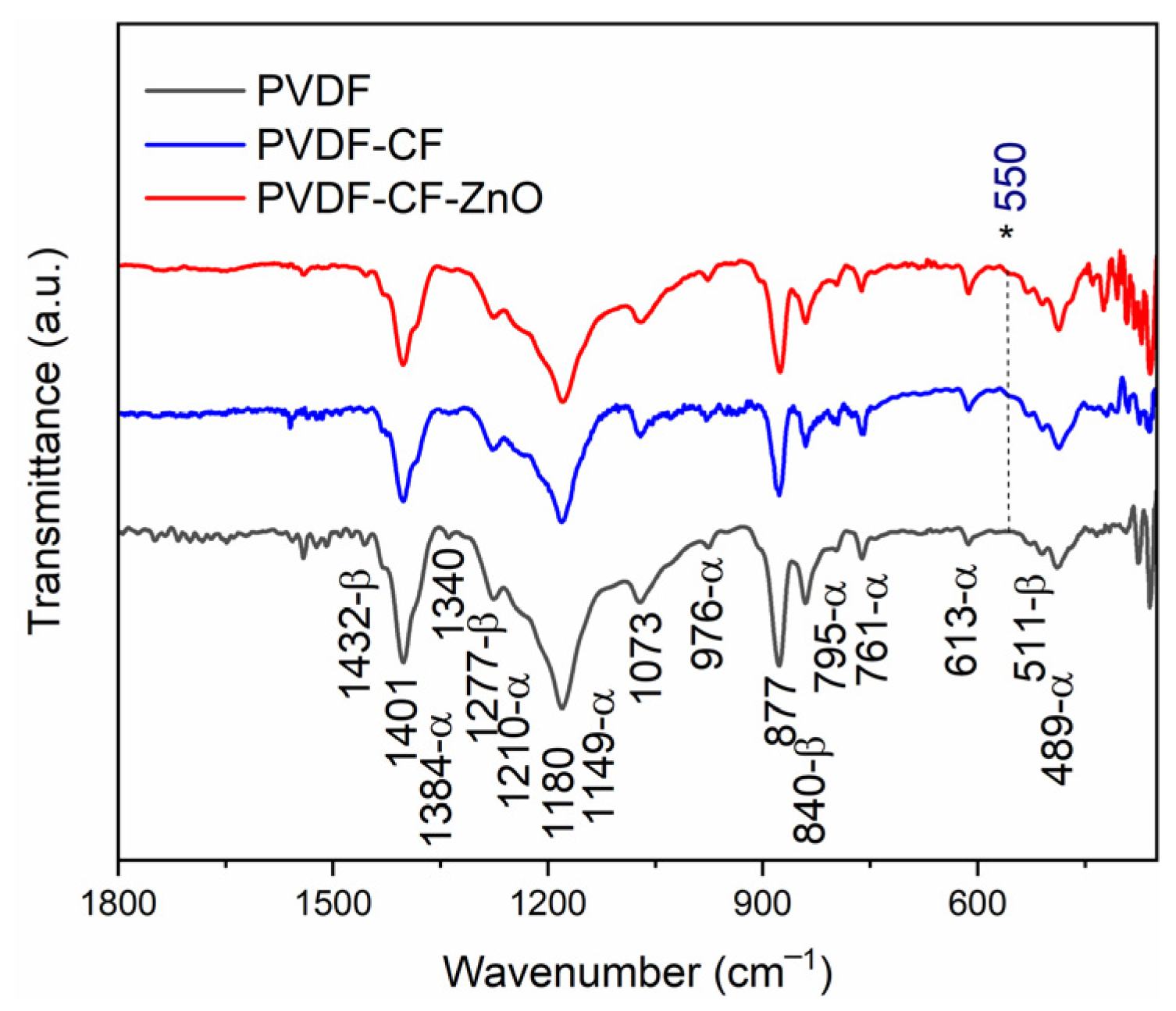
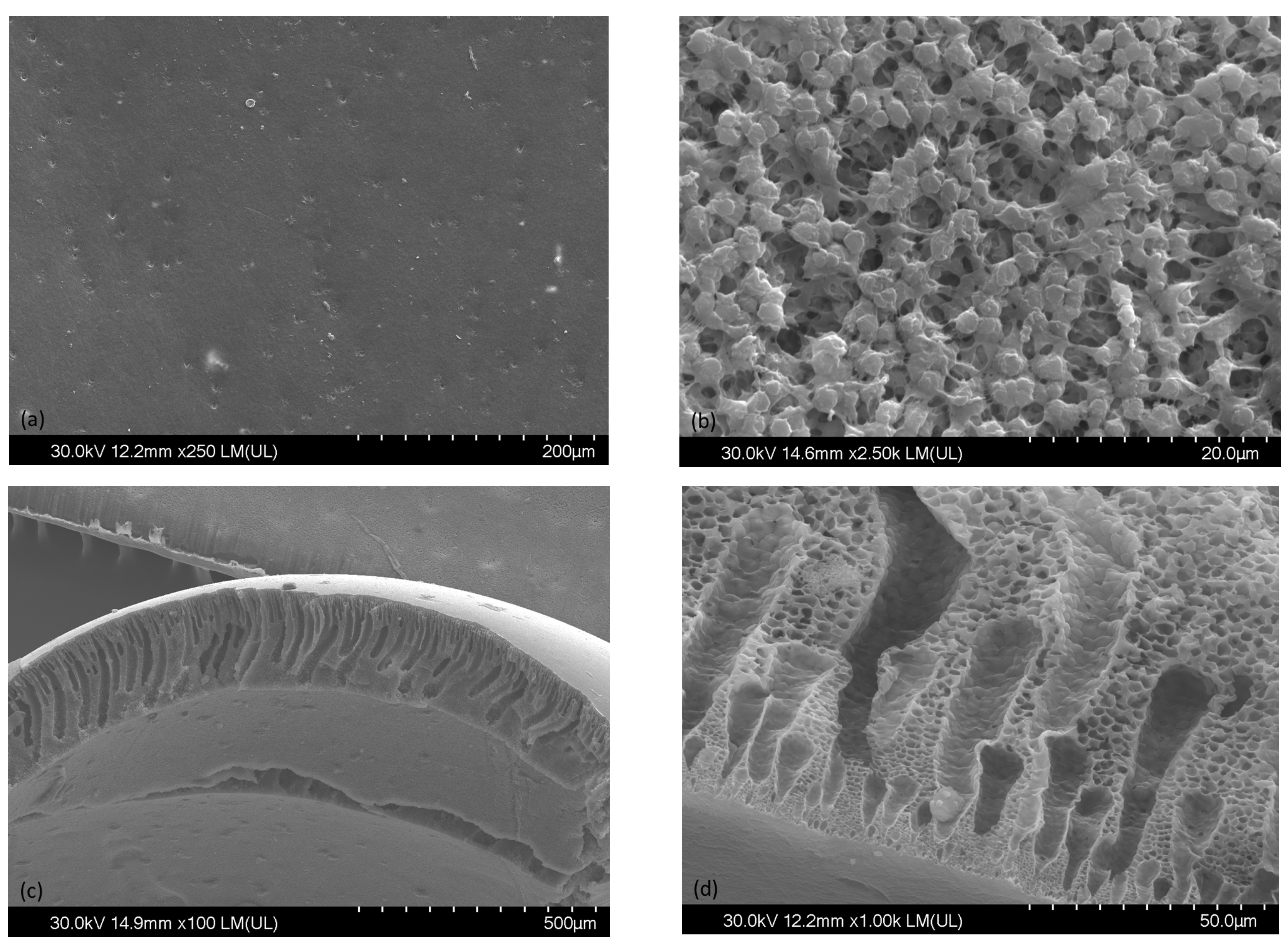
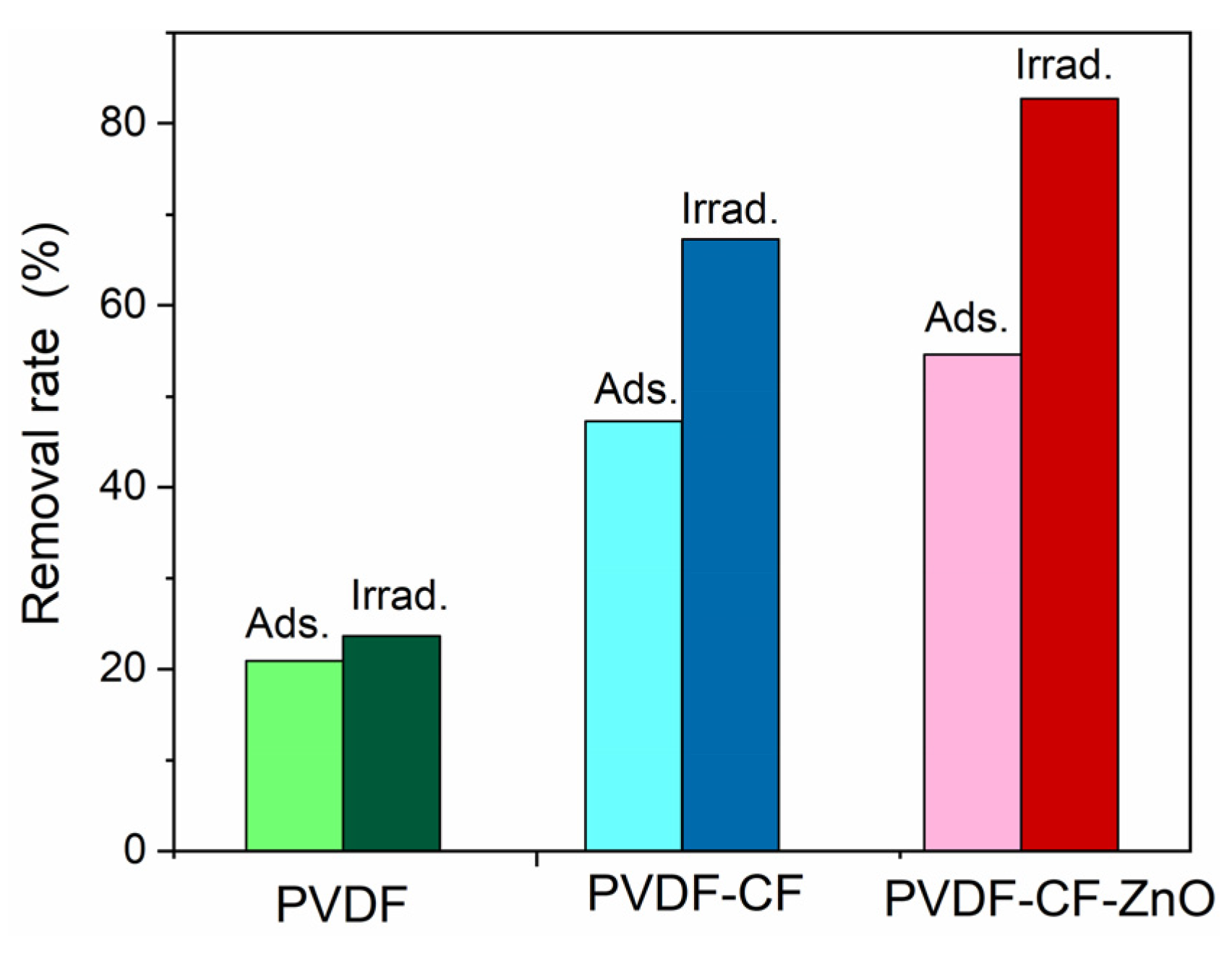
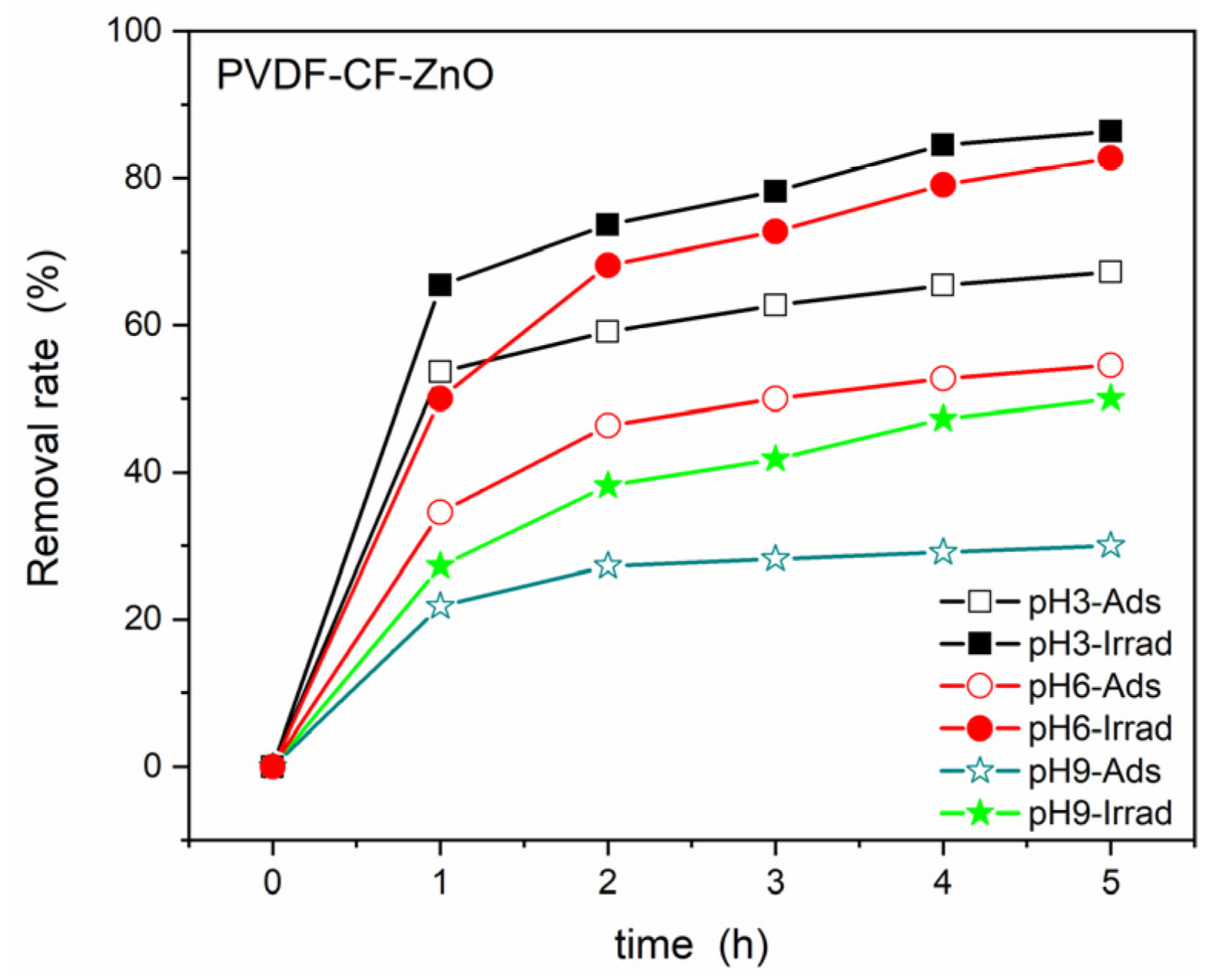
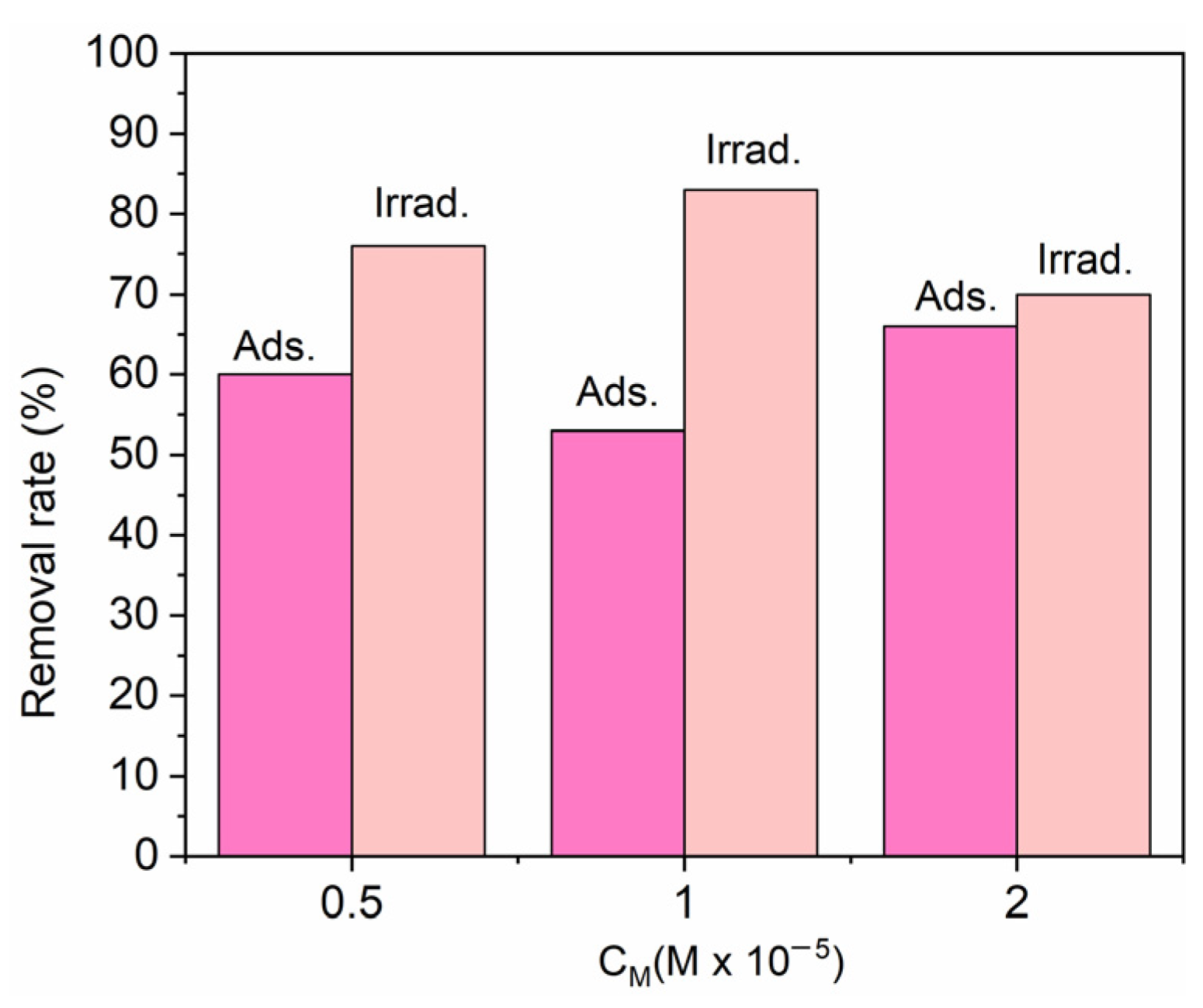
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altaf, C.T.; Colak, T.O.; Erdem, E.; Unal, U.; Misirlioglu, F.B.; Condorelli, G.G.; Demirci Sankir, N.; Sankir, M. Disulfonated polyarylene ether sulfone membrane for graphitic carbon nitride/zinc oxide-based photo-supercapacitors. Electrochim. Acta 2023, 456, 142415. [Google Scholar] [CrossRef]
- Li, Z.M.; Wei, J.G.; Shan, F.; Yang, J.; Wang, X.L. PVDF/PMMA brushes membrane for lithium-ion rechargeable batteries prepared via preirradiation grafting technique. J. Polym. Sci. Polym. Phys. 2008, 46, 751–758. [Google Scholar] [CrossRef]
- Han, Y.; Winston Ho, W.S. Polymeric membranes for CO2 separation and capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Shokri, A. Synthesize and characterization of polysulfone membrane for the separation of hydrogen sulfide from natural gas. Surf. Interfaces 2021, 25, 101233. [Google Scholar] [CrossRef]
- Popa, A.; Toloman, D.; Stefan, M.; Petran, A.; Macavei, S.; Ulinici, S.; Stan, M.; Barbu-Tudoran, L.; Vlassa, M.; Pana, O. Hybrid PVDF-P(L-DOPA)-ZnO membranes for dyes and antibiotics removal through simultaneous action of adsorption and photocatalysis processes. J. Environ. Chem. Eng. 2021, 9, 106812. [Google Scholar] [CrossRef]
- Popa, A.; Toloman, D.; Stan, M.; Stefan, M.; Radu, T.; Vlad, G.; Ulinici, S.; Baisan, G.; Macavei, S.; Barbu-Tudoran, L.; et al. Tailoring the RhB removal rate by modifying the PVDF membrane surface through ZnO particles deposition. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1642–1652. [Google Scholar] [CrossRef]
- Du, J.; Wu, C.; Wang, B.; Hao, J.; Qin, W.; Zhang, Y.; Wu, X.; Wang, X. Electrochemical performance of acetalized polyvinyl alcohol membrane for quasi-solid-state supercapacitors. J. Energy Storage 2023, 72, 108317. [Google Scholar] [CrossRef]
- Seshasayee, M.S.; Yu, Z.; Arthanareeswaran, G.; Das, D.B. Preparation of nanoclay embedded polymeric membranes for the filtration of natural organic matter (NOM) in a circular crossflow filtration system. J. Water Process. Eng. 2020, 37, 101408. [Google Scholar] [CrossRef]
- Kerr-Phillips, T.; Schon, B.; Barke, D. Polymeric Materials and Microfabrication Techniques for Liquid Filtration Membranes. Polymers 2022, 14, 4059. [Google Scholar] [CrossRef]
- Jain, H.; Garg, M.C. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination—A review. Environ. Technol. Innov. 2021, 23, 101561. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, Y.; Luo, J.; Darling, S.B. Drawing on Membrane Photocatalysis for Fouling Mitigation. ACS Appl. Mater. Interfaces 2021, 13, 14844–14865. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, C.F.; Huang, Q.L.; Liu, H.L.; Hao, J.Q.; Song, L. Magnetic field induced orderly arrangement of Fe3O4/GO composite particles for preparation of Fe3O4/GO/PVDF membrane. J. Membr. Sci. 2018, 548, 184–193. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, H.; Pei, W.; Liu, W.; Shi, F.; Li, Y.; Huo, Y. Integrated photocatalysis-adsorption-membrane separation in rotating reactor for synergistic removal of RhB. Chemosphere 2021, 270, 129424. [Google Scholar] [CrossRef] [PubMed]
- Toloman, D.; Popa, A.; Stefan, M.; Pana, O.; Silipas, T.D.; Macavei, S.; Barbu-Tudoran, L. Impact of Gd ions from the lattice of TiO2 nanoparticles on the formation of reactive oxygen species during the degradation of RhB under visible light irradiation. Mater. Sci. Semicond. Process. 2017, 71, 61–68. [Google Scholar] [CrossRef]
- Wu, S.L.; Liu, F.; Yang, H.C.; Darling, S.B. Recent progress in molecular engineering to tailor organic–inorganic interfaces in composite membranes. Mol. Syst. Des. Eng. 2020, 5, 433–444. [Google Scholar] [CrossRef]
- Luo, J.; Chen, W.; Song, H.; Liu, J. Fabrication of hierarchical layer-by-layer membrane as the photocatalytic degradation of foulants and effective mitigation of membrane fouling for wastewater treatment. Sci. Total Environ. 2020, 699, 134398. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhang, J.; Sun, Z.; Zhao, J.; Zhang, J.; Zuo, W. Precisely-controlled modification of PVDF membranes with 3D TiO2/ZnO nanolayer: Enhanced anti-fouling performance by changing hydrophilicity and photocatalysis under visible light irradiation. J. Membr. Sci. 2017, 528, 359–368. [Google Scholar] [CrossRef]
- Hanh Le, T.M.; Wang, R.; Sairiam, S. Self-protecting PVDF-PDA-TiO2 membranes towards highly efficient and prolonged dye wastewater treatment by photocatalytic membranes. J. Membr. Sci. 2023, 683, 121789. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontàs, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In situ growth and crystallization of TiO2 on polymeric membranes for the photocatalytic degradation of diclofenac and 17α-ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; An, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Toloman, D.; Stefan, M.; Macavei, S.; Barbu-Tudoran, L.; Popa, A. Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal. Coatings 2023, 13, 594. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Z.; Pan, Y.; Zeng, G. Antibacterial photocatalytic self-cleaning poly(vinylidene fluoride) membrane for dye wastewater treatment. Polym. Adv. Technol. 2018, 29, 254–262. [Google Scholar] [CrossRef]
- Du, J.; Tian, Y.; Li, N.; Zhang, J.; Zuo, W. Enhanced antifouling performance of ZnS/GO/PVDF hybrid membrane by improving hydrophilicity and photocatalysis. Polym. Adv. Technol. 2019, 30, 351–359. [Google Scholar] [CrossRef]
- Yang, C.; Wang, P.; Li, J.; Wang, Q.; Xu, P.; You, S.; Zheng, Q.; Zhang, G. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe(III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417, 129340. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Sun, T.; He, Y. A novel strategy based on magnetic field assisted preparation of magnetic and photocatalytic membranes with improved performance. J. Membr. Sci. 2020, 612, 118378. [Google Scholar] [CrossRef]
- Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Zhu, Z.; Feng, Y.; Wu, C. Changing conventional blending photocatalytic membranes (BPMs): Focus on improving photocatalytic performance of Fe3O4/g-C3N4/PVDF membranes through magnetically induced freezing casting method. Chem. Eng. J. 2019, 365, 405–414. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Yuan, H.; Ren, J. Preparation of poly(vinylidene fluoride) (PVDF)/acetalyzed poly (vinyl alcohol) ultrafiltration membrane with the enhanced hydrophilicity and the anti-fouling property. Chem. Eng. Res. Des. 2017, 121, 348–359. [Google Scholar] [CrossRef]
- Wu, J.; Shen, X.; Yang, L.; Wang, K.; Chen, K. Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites. Appl. Surf. Sci. 2010, 256, 2826–2830. [Google Scholar] [CrossRef]
- Topkaya, R.; Akman, O.; Kazan, S.; Aktas, B.; Durmus, Z.; Baykal, A. Surface spin disorder and spin-glass-like behavior in manganese-substituted cobalt ferrite nanoparticles. J. Nanopart. Res. 2012, 14, 1156. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Pankratov, D.A.; Gubin, S.P.; Kosobudsky, I.D.; Beltran, M.; Khodorkovsky, Y.; Tishin, A.M. Electron paramagnetic resonance of ferrite nanoparticles. J. Appl. Phys. 2001, 89, 2293–2298. [Google Scholar] [CrossRef]
- Buryi, M.; Babin, V.; Chang, Y.-Y.; Remeš, Z.; Mičová, J.; Šimek, D. Influence of precursor age on defect states in ZnO nanorods. Appl. Surf. Sci. 2020, 525, 146448. [Google Scholar] [CrossRef]
- Safari, M.; Mazloom, J. Electrochemical performance of spindle-like Fe2Co-MOF and derived magnetic yolk-shell CoFe2O4 microspheres for supercapacitor applications. J. Solid State Electrochem. 2021, 25, 2189–2200. [Google Scholar] [CrossRef]
- Stefan, M.; Toloman, D.; Popa, A.; Mesaros, A.; Vasile, O.R.; Leostean, C.; Pana, O. Interface charge transfer process in ZnO:Mn/ZnS nanocomposites. J. Nanopart. Res. 2016, 18, 59. [Google Scholar] [CrossRef]
- Ferdosi, E.; Ghanbari, B.D. Investigation the photocatalytic activity of CoFe2O4/ZnO and CoFe2O4/ZnO/Ag nanocomposites for purification of dye pollutants. Sep. Purif. Technol. 2019, 211, 35–39. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 12650. [Google Scholar] [CrossRef]
- Shen, T.; Zhong, L.; Liu, X.; Zhang, J.; Zhang, D.; He, K.; Yuan, C.; Xu, Y.; Dai, L. Decorating Au nanoparticles onto optimized P(tBA-co-DMAEMA) carriers for ameliorative catalytic capability. J. Appl. Polym. Sci. 2020, 137, 48920. [Google Scholar] [CrossRef]
- Zioui, D.; Martins, P.M.; Aoudjit, L.; Salazar, H.; Lanceros-Méndez, S. Wastewater Treatment of Real Effluents by Microfiltration Using Poly(vinylidene fluoride–hexafluoropropylene) Membranes. Polymers 2023, 15, 1143. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wei, X.; Liu, G.; Li, J. ZnO nanoparticles implanted in TiO2 macrochannels as an effective direct Z scheme heterojunction photocatalyst for degradation of RhB. Appl. Surf. Sci. 2018, 456, 666–675. [Google Scholar] [CrossRef]
- Ahmadi, M.; Motlagh, H.R.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorf, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.; Tang, Y.; Zhang, X.; Ji, S.; Luo, L.; Jiang, F. Photoinduced RhB-sensitized effect on a novel AgI/BiOCl/biochar photocatalyst to boost its photocatalytic performance for 17α-ethinyl estradiol degradation. Sep. Purif. Technol. 2024, 332, 125774. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, T.; Wang, M.; Yun, R.; Xiang, X. Recent Advances in Heterogeneous Photo-Driven Oxidation of Organic Molecules by Reactive Oxygen Species. ChemSusChem 2020, 13, 5173–5184. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.M.; Cohen, G.; Cederbaum, A.I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 1981, 20, 6006–6012. [Google Scholar] [CrossRef]
- Alyani, S.J.; Pirbazari, A.E.; Khalilsaraei, F.E.; Kolur, N.A.; Gilani, N. Growing Co-doped TiO2 nanosheets on reduced graphene oxide for efficient photocatalytic removal of tetracycline antibiotic from aqueous solution and modeling the process by artificial neural network. J. Alloys Compd. 2019, 799, 169–182. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Toloman, D.; Popa, A.; Macavei, S.; Falamas, A.; Suciu, R.; Barbu-Tudoran, L.; Marincas, O.; Pana, O. New emerging magnetic, optical and photocatalytic properties of Tb doped TiO2 interfaced with CoFe2O4 nanoparticles. Appl. Surf. Sci. 2021, 570, 151172. [Google Scholar] [CrossRef]
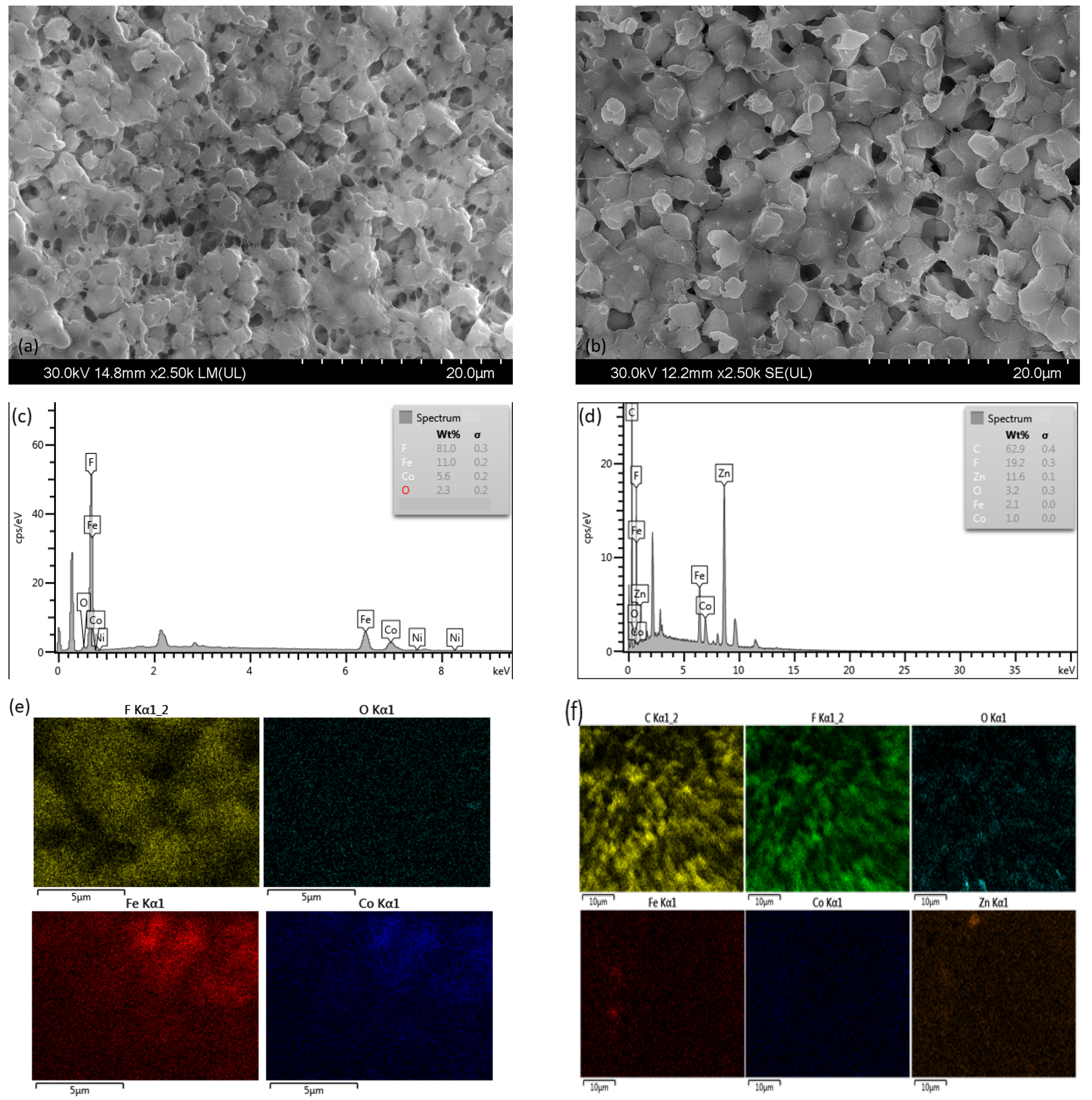
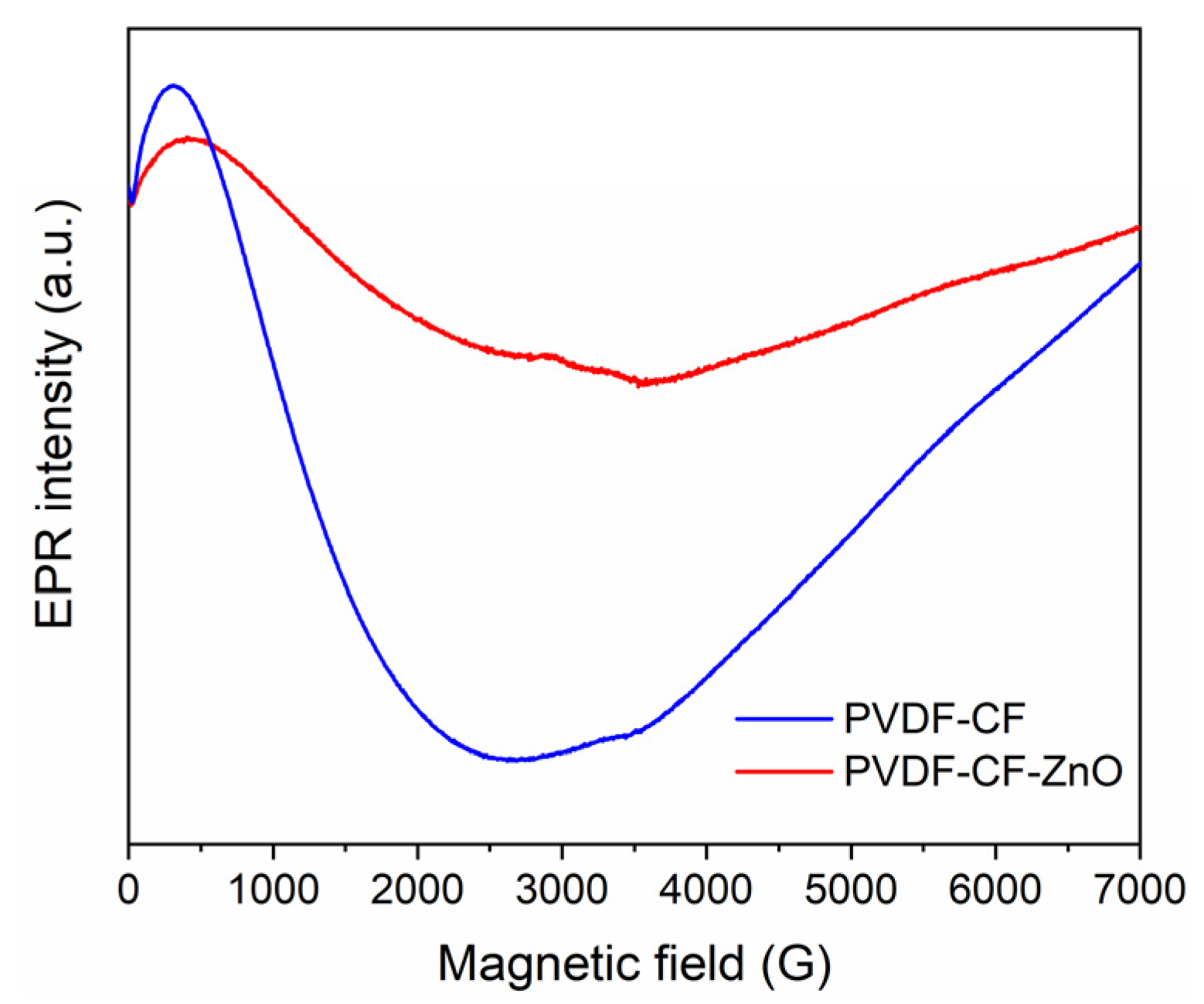
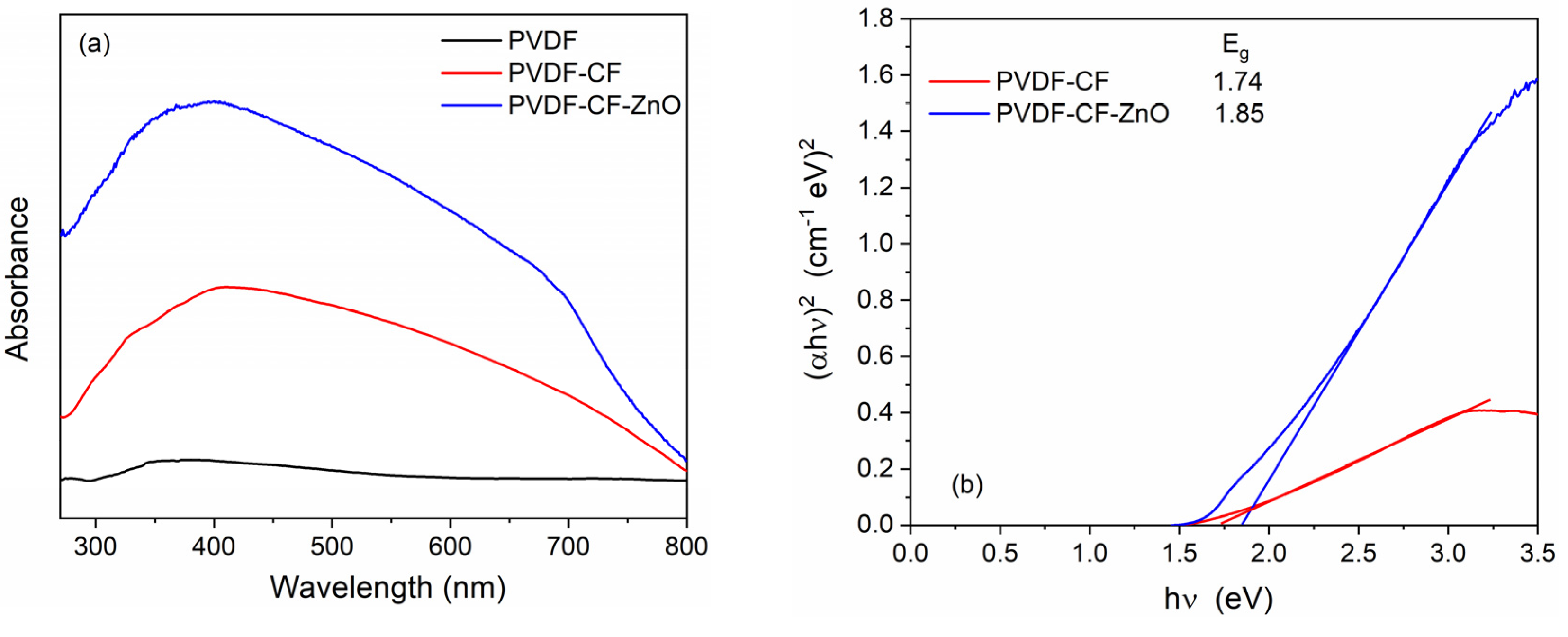
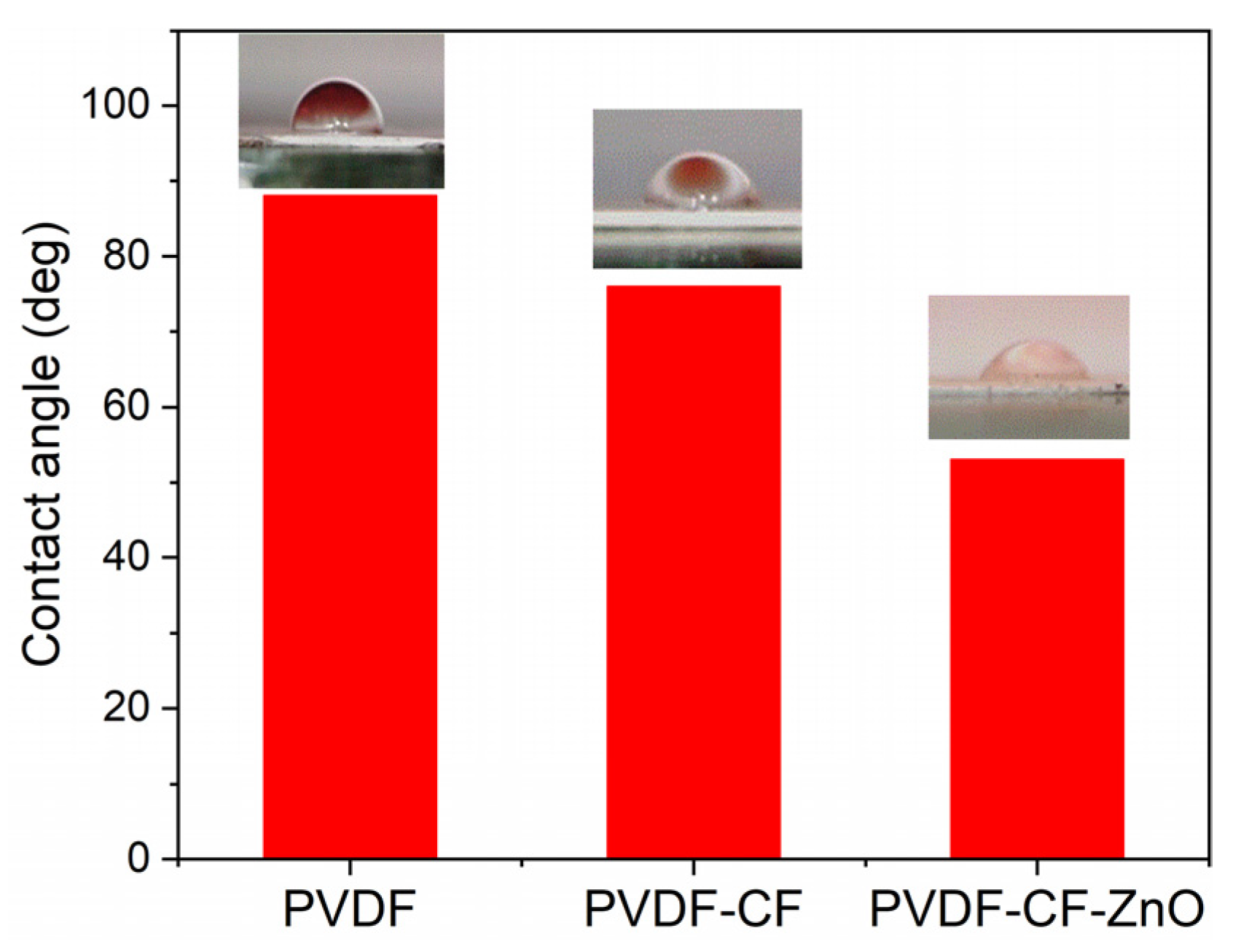
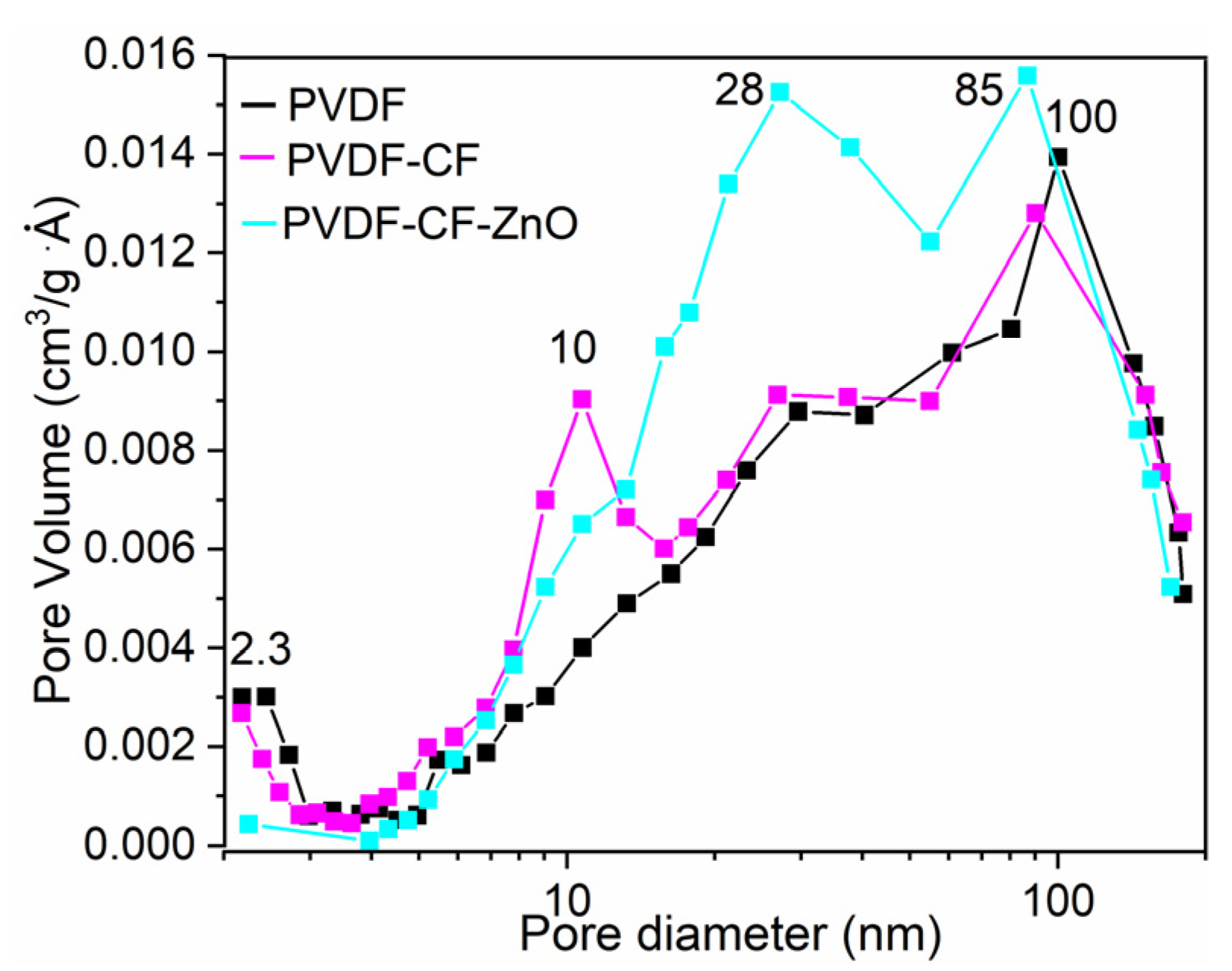
| Sample | SBET (m2/g) | Pores Volume (cm3/g) | D (nm) |
|---|---|---|---|
| PVDF | 3.27 | 0.0079 | 15.63 |
| PVDF-CF | 4.01 | 0.0173 | 24.74 |
| PVDF-CF-ZnO | 3.94 | 0.0177 | 28.56 |
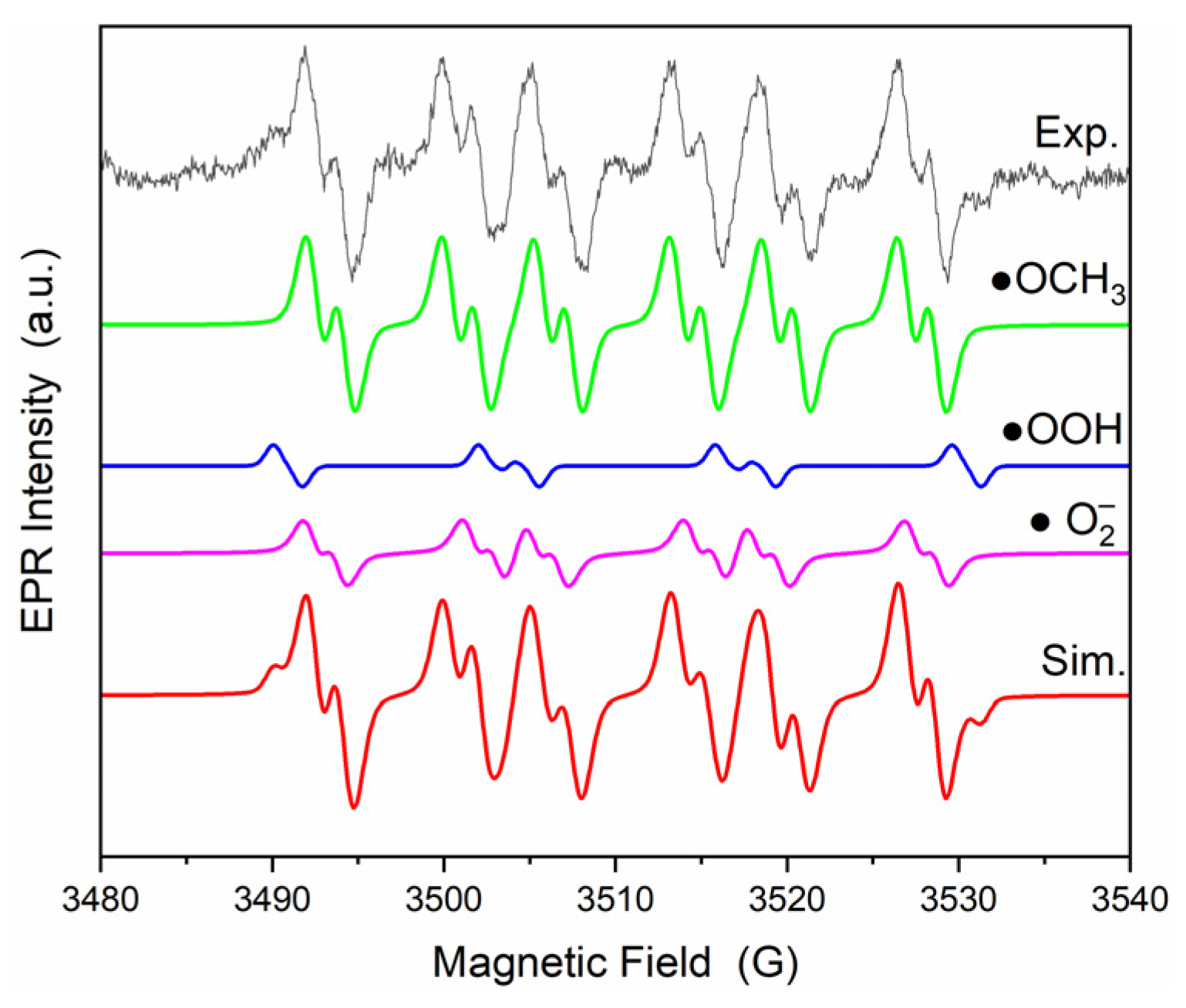
| Sample | •OCH3 (%) | •OOH (%) | (%) |
|---|---|---|---|
| PVDF-CF | 0 | 23 | 77 |
| PVDF-CF-ZnO | 30 | 6 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.; Stefan, M.; Macavei, S.; Perhaita, I.; Tudoran, L.B.; Toloman, D. Facile Preparation of PVDF/CoFe2O4-ZnO Hybrid Membranes for Water Depollution. Polymers 2023, 15, 4547. https://doi.org/10.3390/polym15234547
Popa A, Stefan M, Macavei S, Perhaita I, Tudoran LB, Toloman D. Facile Preparation of PVDF/CoFe2O4-ZnO Hybrid Membranes for Water Depollution. Polymers. 2023; 15(23):4547. https://doi.org/10.3390/polym15234547
Chicago/Turabian StylePopa, Adriana, Maria Stefan, Sergiu Macavei, Ioana Perhaita, Lucian Barbu Tudoran, and Dana Toloman. 2023. "Facile Preparation of PVDF/CoFe2O4-ZnO Hybrid Membranes for Water Depollution" Polymers 15, no. 23: 4547. https://doi.org/10.3390/polym15234547
APA StylePopa, A., Stefan, M., Macavei, S., Perhaita, I., Tudoran, L. B., & Toloman, D. (2023). Facile Preparation of PVDF/CoFe2O4-ZnO Hybrid Membranes for Water Depollution. Polymers, 15(23), 4547. https://doi.org/10.3390/polym15234547







