Preparation and Performance Evaluation of Amphiphilic Polymers for Enhanced Heavy Oil Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
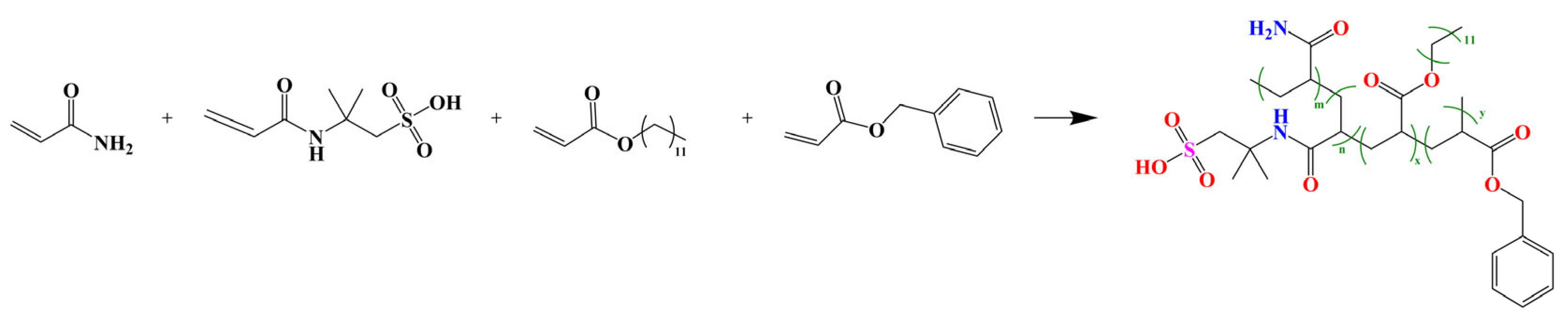
2.2. Synthesis of PAALB
2.3. Polymer Characterization
2.4. Apparent Viscosity Measurement
2.5. Critical Association Concentration Measurement
2.6. Polymer Self-Emulsification Ability Evaluation
2.7. Viscosity Reduction Performance Measurement
2.8. Emulsion Structure Testing
2.9. Interfacial Tension Measurement
2.10. Contact Angle Measurement
2.11. Polymer Flooding Performance
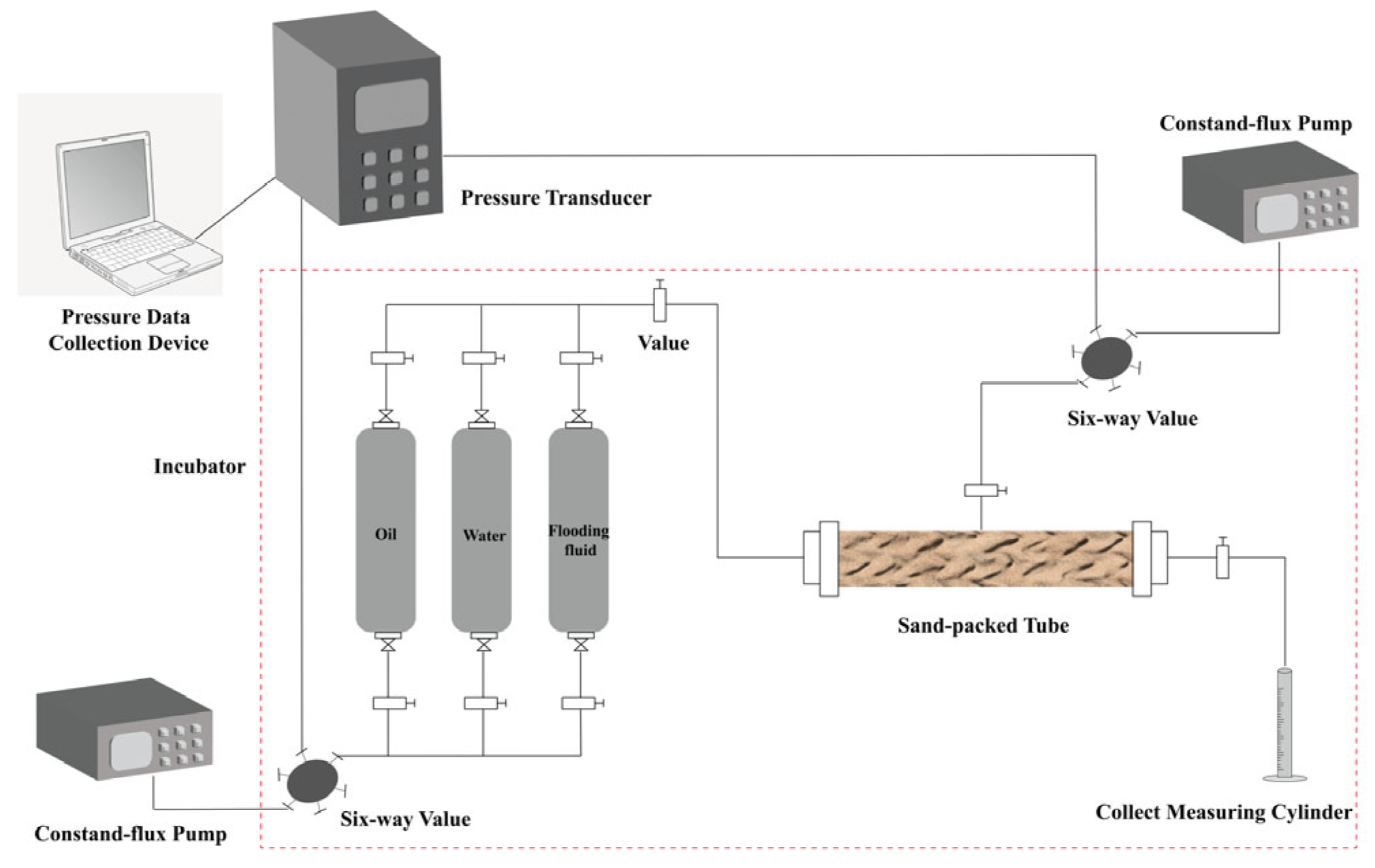
2.11.1. Sand-Packed Tube Flooding Experiments
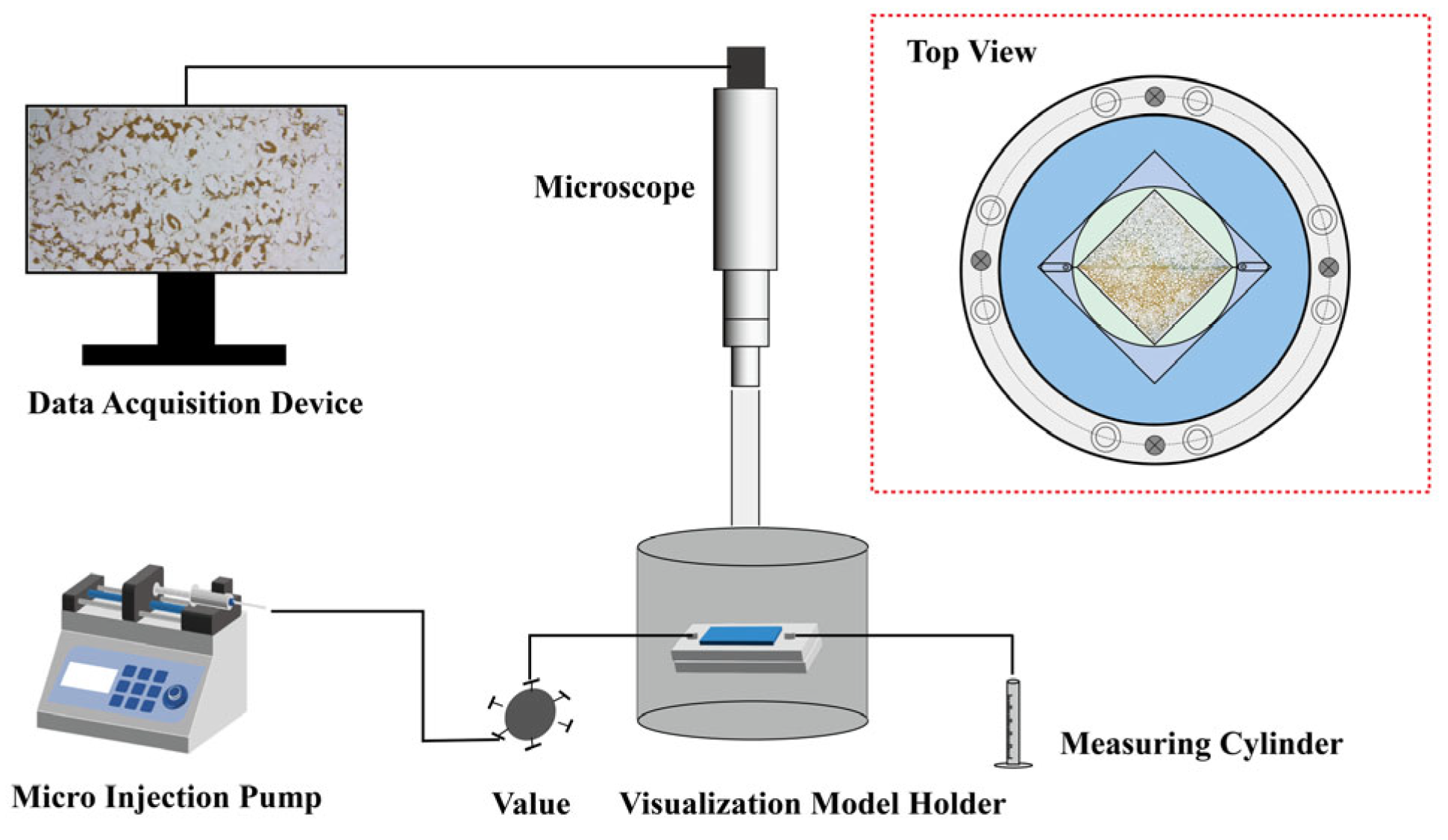
2.11.2. Microscopic Model Flooding Experiments
3. Results and Discussion
3.1. Characterization of PAALB
3.1.1. FT-IR and 1H-NMR
3.1.2. Elemental Analysis
3.1.3. Relative Molecular Mass of PAALB and Its Distribution
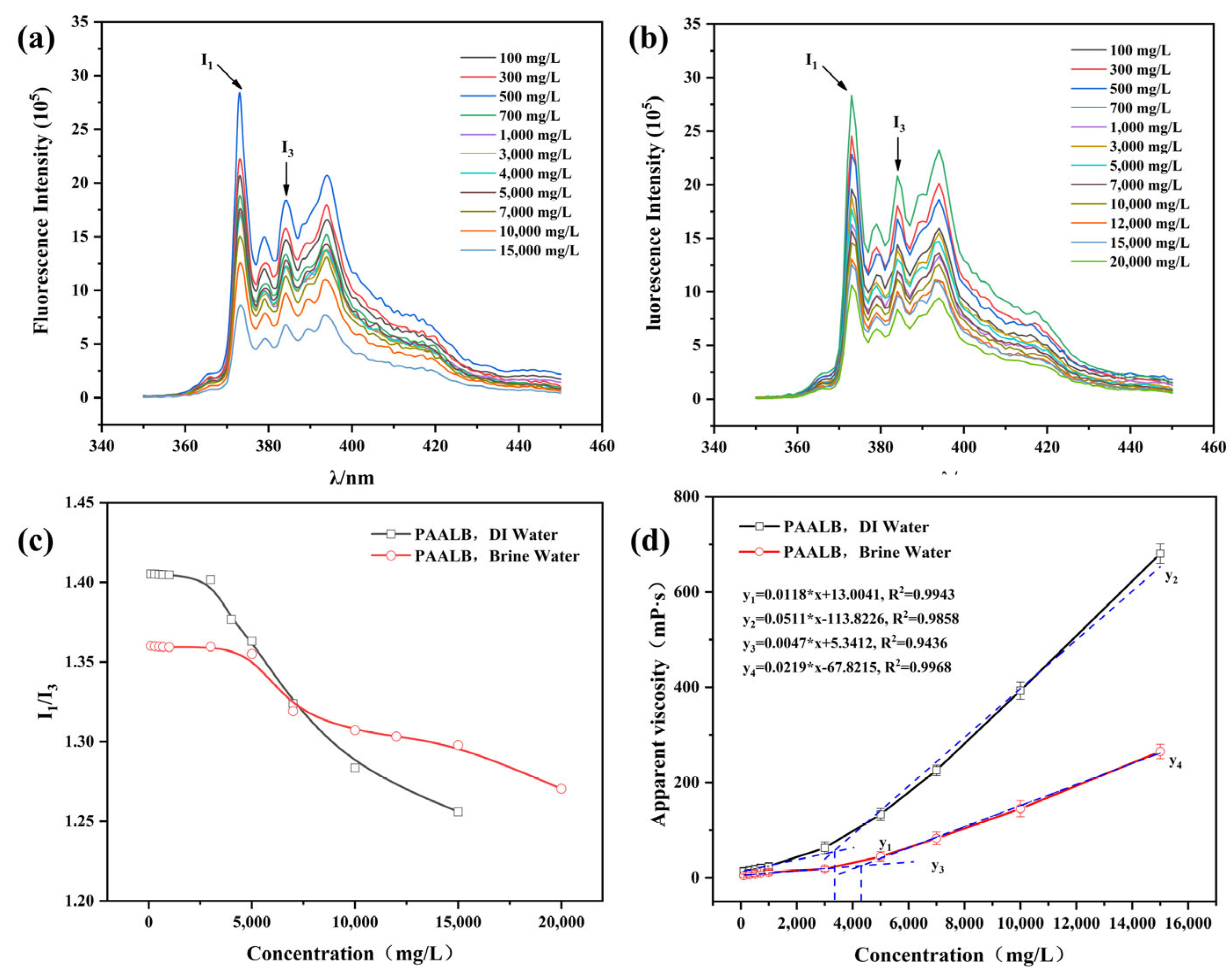
3.2. Critical Association Concentration of Polymer
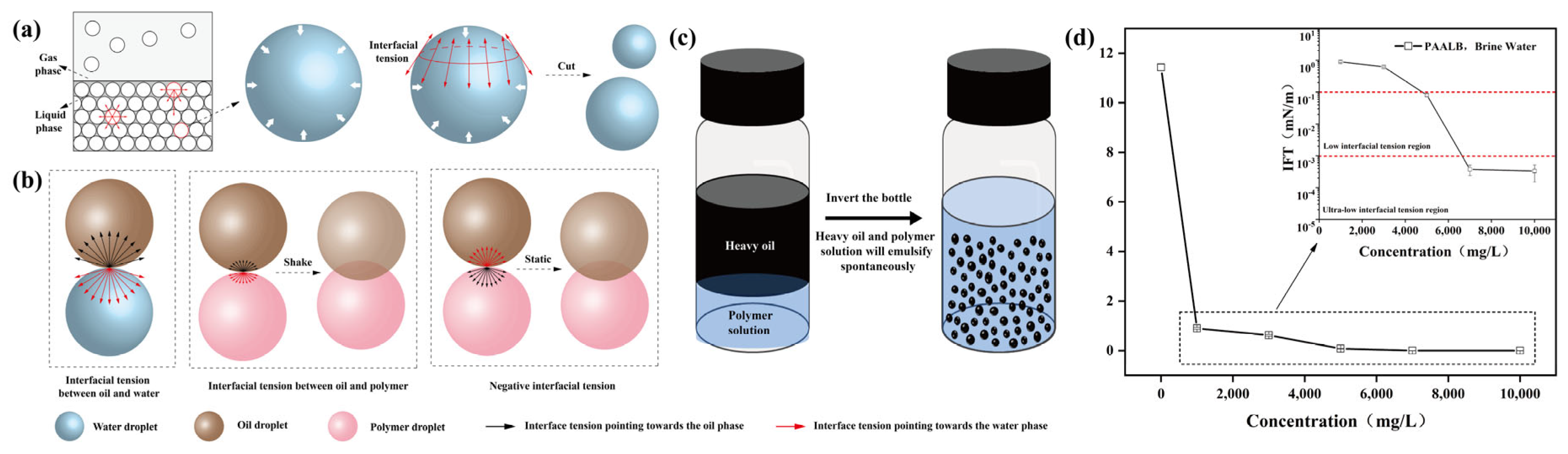
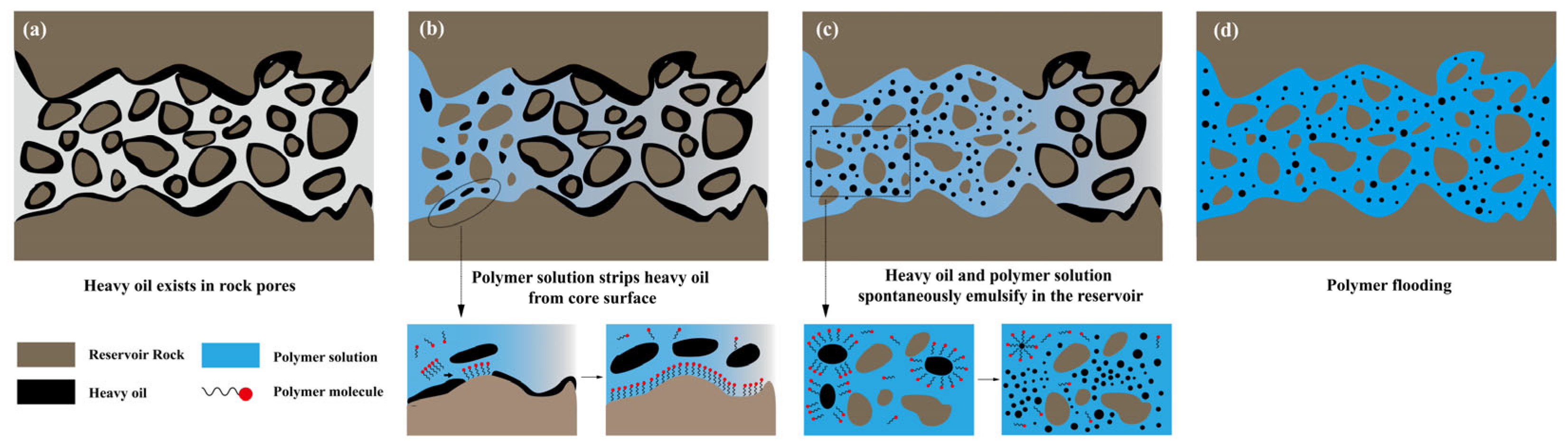
3.3. Polymer Self-Emulsification Performance and Mechanism
3.3.1. Polymer Self-Emulsification Performance
3.3.2. Self-Emulsification Mechanism
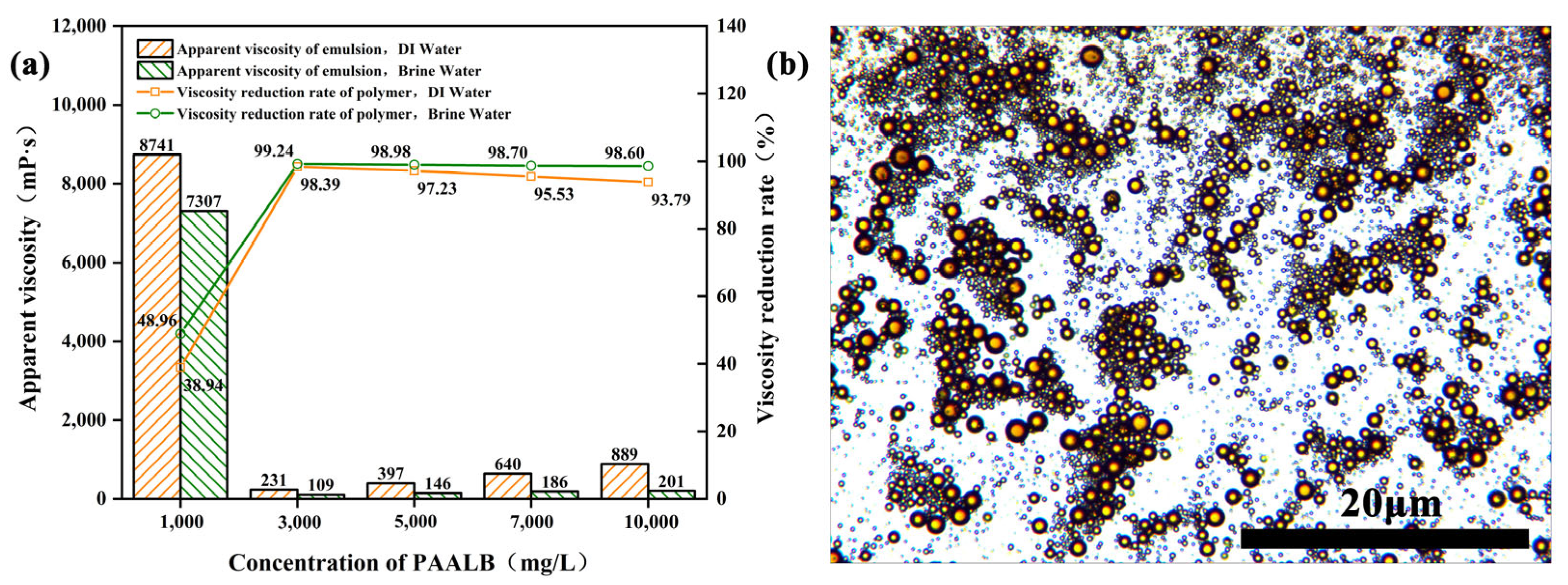
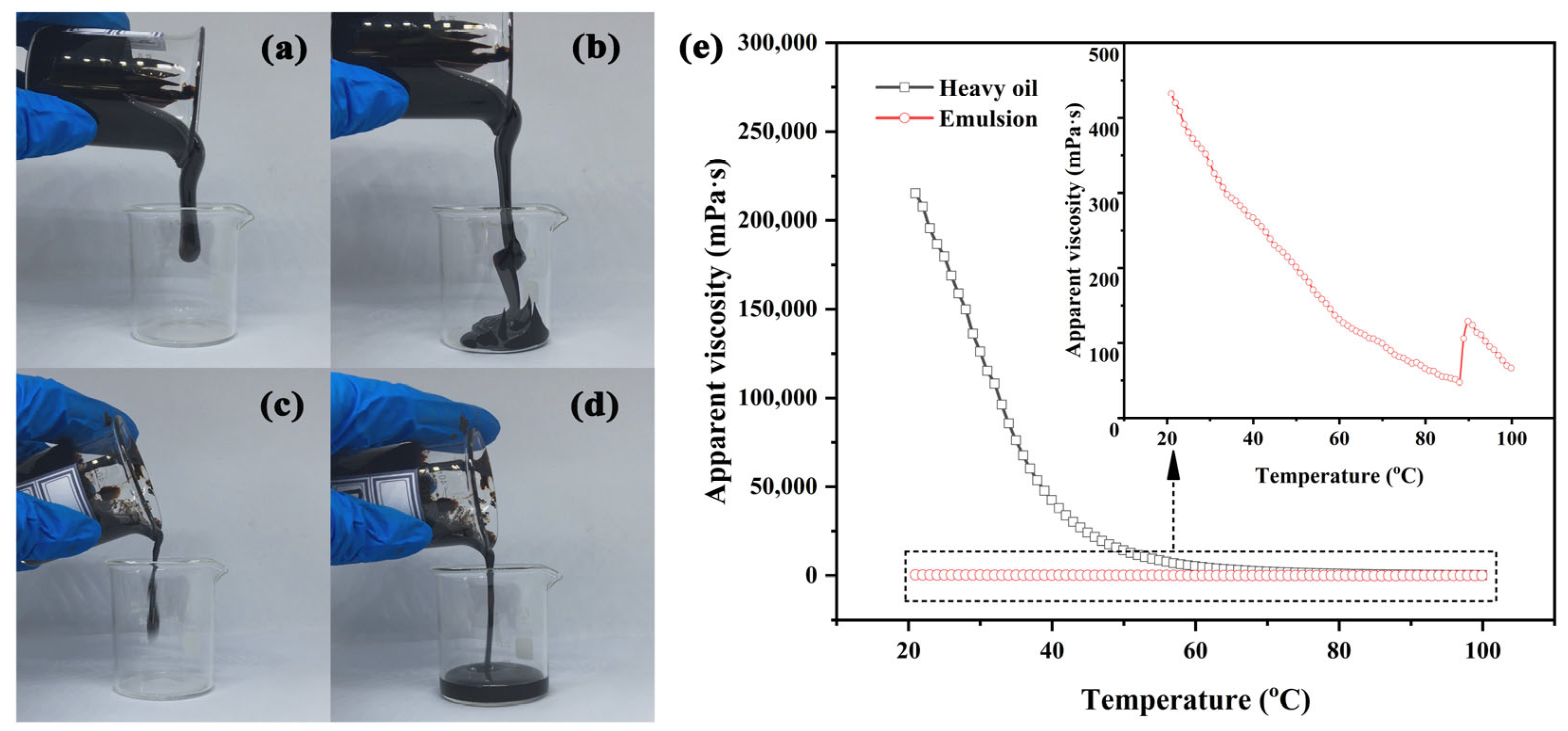
3.4. Heavy Oil Viscosity Reduction Performance of Polymer
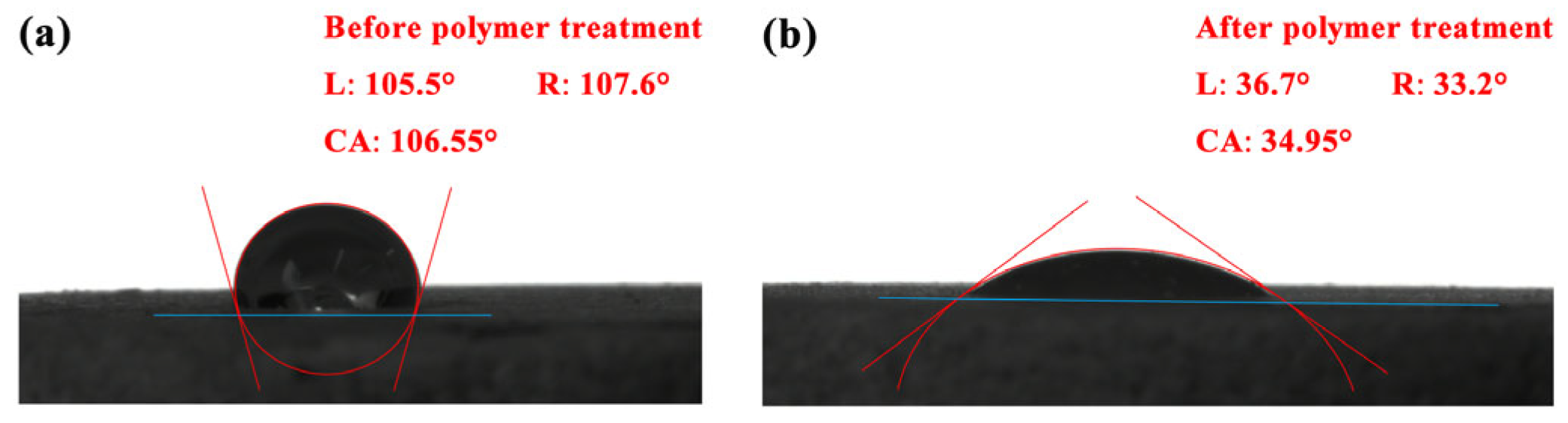
3.5. Wettability Change
3.6. Polymer Oil Displacement Performance
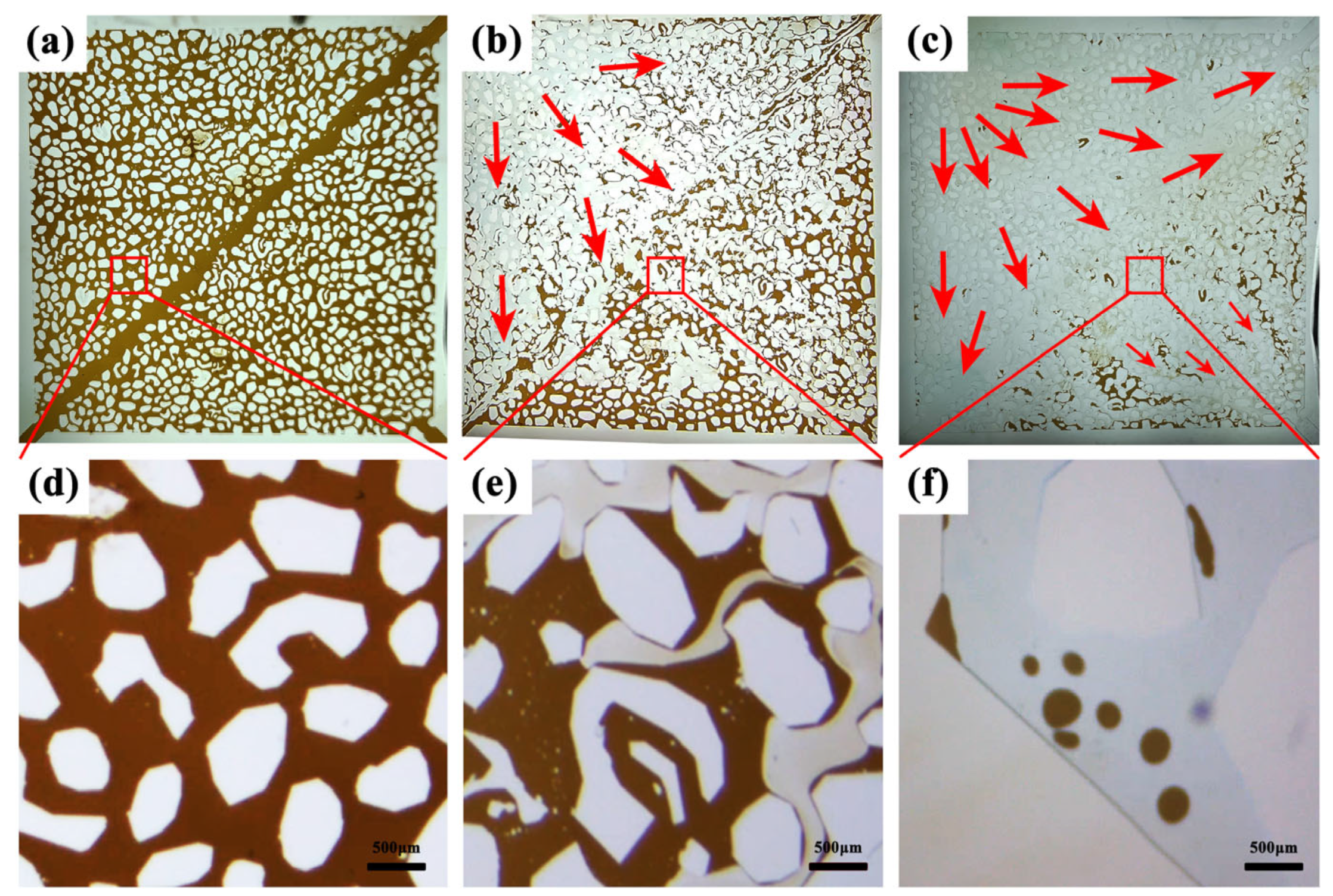
3.6.1. Sand-Packed Tube Flooding Experiments
3.6.2. Microscopic Model Flooding Experiments
3.7. Amphiphilic Polymer Flooding Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Wu, Z.; Huiqing, L.; Cao, P.; Yang, R. Experimental investigation on improved vertical sweep efficiency by steam-air injection for heavy oil reservoirs. Fuel 2021, 285, 119138. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Sun, J.H.; Zhang, F.S.; Wu, Y.W.; Liu, G.L.; Li, X.N.; Su, H.M.; Zhu, Z.Y. Overview of emulsified viscosity reducer for enhancing heavy oil recovery. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012009. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Taleb, M.A.M.; Wen, Z.; Chen, X. A Review of the Seepage Mechanisms of Heavy Oil Emulsions during Chemical Flooding. Energies 2022, 15, 8397. [Google Scholar] [CrossRef]
- Kumar Sharma, V.; Singh, A.; Tiwari, P. An experimental study of pore-scale flow dynamics and heavy oil recovery using low saline water and chemical flooding. Fuel 2023, 334, 126756. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.; Gao, W.; Cao, J.; Sun, B.; Zhang, J. Experimental investigation on the effect of interfacial properties of chemical flooding for enhanced heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132335. [Google Scholar] [CrossRef]
- Wang, H.; Wei, B.; Hou, J.; Liu, Y.; Du, Q. Heavy oil recovery in blind-ends during chemical flooding: Pore scale study using microfluidic experiments. J. Mol. Liq. 2022, 368, 120724. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhong, L.; Yuan, S.; Wang, Q.; Wei, C. Study on the emulsification characteristics of heavy oil during chemical flooding. Phys. Fluids 2023, 35, 53330. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, H.; Li, Y.; Li, Y.; Chen, X.; Zhuang, Y. Experimental Investigation of Synergy of Components in Surfactant/Polymer Flooding Using Three-Dimensional Core Model. Transp. Porous Media 2018, 126, 317–335. [Google Scholar] [CrossRef]
- Li, W.; Dai, C.; Ouyang, J.; Aziz, H.; Tao, J.; He, X.; Zhao, G. Adsorption and retention behaviors of heterogeneous combination flooding system composed of dispersed particle gel and surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 250–261. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, T.; Lin, X.; Wang, X.; Su, L. Chemicals loss and the effect on formation damage in reservoirs with ASP flooding enhanced oil recovery. J. Nat. Gas Sci. Eng. 2016, 33, 1381–1389. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, S.; Shi, F.; Jia, X. Experimental study of chemical concentration variation of ASP flooding. Sci. China Ser. E Technol. Sci. 2009, 52, 1882–1890. [Google Scholar] [CrossRef]
- Xiao, H.M.; Sun, L.H.; Kou, H.H. Mass Transfer Mechanisms of ASP Flooding in Porous Media. Adv. Mater. Res. 2012, 550–553, 2738–2744. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Liu, Y.; Wang, M.; Tan, Y. Long Branched-Chain Amphiphilic Copolymers: Synthesis, Properties, and Application in Heavy Oil Recovery. Energy Fuels 2018, 32, 7002–7010. [Google Scholar] [CrossRef]
- Qin, X.; Zhu, S.; Shi, Q.; Li, C.; Baykara, H. Synthesis and Properties of a Dendrimer Amphiphilic Polymer as Enhanced Oil Recovery Chemical. J. Chem. 2023, 2023, 4271446. [Google Scholar] [CrossRef]
- Chen, M.; Chen, W.; Wang, Y.; Ding, M.; Zhang, Z.; Liu, D.; Mao, D. Hydrogen-Bonded amphiphilic polymer viscosity reducer for enhancing heavy oil Recovery: Synthesis, characterization and mechanism. Eur. Polym. J. 2022, 180, 111589. [Google Scholar] [CrossRef]
- Liu, Z.; Mendiratta, S.; Chen, X.; Zhang, J.; Li, Y. Amphiphilic-Polymer-Assisted Hot Water Flooding toward Viscous Oil Mobilization. Ind. Eng. Chem. Res. 2019, 58, 16552–16564. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Zheng, W.; Zhou, B.; Zhao, H.; Li, X.; Zhang, L.; Zhu, Z.; Kang, W.; Ketova, Y.A.; et al. Effect of hydrophobic group content on the properties of betaine-type binary amphiphilic polymer. J. Mol. Liq. 2020, 311, 113358. [Google Scholar] [CrossRef]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W. Study on the stability of heavy crude oil-in-water emulsions stabilized by two different hydrophobic amphiphilic polymers. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 299–306. [Google Scholar] [CrossRef]
- Zhu, Z.; Kang, W.; Sarsenbekuly, B.; Yang, H.; Dai, C.; Yang, R.; Fan, H. Preparation and solution performance for the amphiphilic polymers with different hydrophobic groups. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Zhi, J.; Liu, Y.; Chen, J.; Jiang, N.; Xu, D.; Bo, L.; Qu, G. Performance Evaluation and Oil Displacement Effect of Amphiphilic Polymer Heavy Oil Activator. Molecules 2023, 28, 5257. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, X.; Xie, L.; Chen, Y.; Ren, W.; Gu, W.; Zhang, M.; Zhou, H. Synthesis and Molecular Dynamics Simulation of Amphiphilic Low Molecular Weight Polymer Viscosity Reducer for Heavy Oil Cold Recovery. Energies 2021, 14, 6856. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Yang, M.; Jiang, J.; Xu, D.; Feng, H.; Lu, Y.; Kang, W.; Bai, B.; Hou, J. Spontaneous Emulsification via Once Bottom-Up Cycle for the Crude Oil in Low-Permeability Reservoirs. Energy Fuels 2018, 32, 3119–3126. [Google Scholar] [CrossRef]
- Li, Z.; Kang, W.; Bai, B.; Wu, H.; Gou, C.; Yuan, Y.; Xu, D.; Lu, Y.; Hou, J. Fabrication and Mechanism Study of the Fast Spontaneous Emulsification of Crude Oil with Anionic/Cationic Surfactants as an Enhanced Oil Recovery (EOR) Method for Low-Permeability Reservoirs. Energy Fuels 2019, 33, 8279–8288. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, M.; Yue, X.; Hou, J. Synergy of alkali and surfactant in emulsification of heavy oil in brine. Colloids Surf. A Physicochem. Eng. Asp. 2006, 273, 219–228. [Google Scholar] [CrossRef]
- Wu, T.; Firoozabadi, A. Surfactant-Enhanced Spontaneous Emulsification Near the Crude Oil–Water Interface. Langmuir 2021, 37, 4736–4743. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Wu, H.; Yang, M.; Feng, H.; Xu, D.; Hou, J.; Kang, W.; Yang, H.; Zhao, Y.; et al. A Novel Ultra-Low IFT Spontaneous Emulsification System for Enhanced Oil Recovery in Low Permeability Reservoirs. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar]
- Wang, Y.; Li, M.; Hou, J.; Zhang, L.; Jiang, C. Design, synthesis and properties evaluation of emulsified viscosity reducers with temperature tolerance and salt resistance for heavy oil. J. Mol. Liq. 2022, 356, 118977. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.-D.; Li, Q.-Y.; Yang, J.-J.; Zhang, Z.-J. New Amphiphilic Polymer with Emulsifying Capability for Extra Heavy Crude Oil. Ind. Eng. Chem. Res. 2018, 57, 17013–17023. [Google Scholar] [CrossRef]
- Wu, R.; Yan, Y.; Li, X.; Tan, Y. Preparation and evaluation of double-hydrophilic diblock copolymer as viscosity reducers for heavy oil. J. Appl. Polym. Sci. 2022, 140, e53278. [Google Scholar] [CrossRef]
- Zheng, C.; Fu, H.; Wang, Y.; Zhang, T.; Duan, W. Preparation and mechanism of hyperbranched heavy oil viscosity reducer. J. Pet. Sci. Eng. 2021, 196, 107941. [Google Scholar] [CrossRef]
- Mao, J.; Cao, H.; Zhang, H.; Du, A.; Xue, J.; Lin, C.; Yang, X.; Wang, Q.; Mao, J.; Chen, A. Design of salt-responsive low-viscosity and high-elasticity hydrophobic association polymers and study of association structure changes under high-salt conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129512. [Google Scholar] [CrossRef]
- Pu, W.; Jiang, F.; Wei, B.; Tang, Y.; He, Y. Influences of structure and multi-intermolecular forces on rheological and oil displacement properties of polymer solutions in the presence of Ca2+/Mg2+. RSC Adv. 2017, 7, 4430–4436. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- López-Montilla, J.C.; Herrera-Morales, P.E.; Pandey, S.; Shah, D.O. Spontaneous Emulsification: Mechanisms, Physicochemical Aspects, Modeling, and Applications. J. Dispers. Sci. Technol. 2002, 23, 219–268. [Google Scholar] [CrossRef]
- Li, Z.; Xu, D.; Yuan, Y.; Wu, H.; Hou, J.; Kang, W.; Bai, B. Advances of spontaneous emulsification and its important applications in enhanced oil recovery process. Adv. Colloid Interface Sci. 2020, 277, 102119. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.-Y. Apparent viscosity characteristics and prediction model of an unstable oil-in-water or water-in-oil dispersion system. J. Dispers. Sci. Technol. 2018, 40, 1645–1656. [Google Scholar] [CrossRef]
- Luo, H.; Wen, J.; Lv, C.; Wang, Z. Modeling of viscosity of unstable crude oil–water mixture by characterization of energy consumption and crude oil physical properties. J. Pet. Sci. Eng. 2022, 212, 110222. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Zhao, J.; Mei, N. Viscosity estimation and component identification for an oil-water emulsion with the inversion method. Appl. Therm. Eng. 2017, 111, 759–767. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Liao, Y.; Mao, J.; Yang, X.; Lin, C.; Wang, Q.; Huang, Z.; Xu, T.; Liu, B.; et al. Salt resistance study and molecular dynamics simulation of hydrophobic-association polymer with internal salt structure. J. Mol. Liq. 2022, 367, 120520. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Zewen, Y.; Liu, Y.; Meng, X.; Zhang, W.; Zhang, H.; Yang, G.; Shaojie, W. High-efficiency emulsification anionic surfactant for enhancing heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128654. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, L.; Lyu, C.; Liu, Y.; Zhang, S. W/O emulsions generated by heavy oil in porous medium and its effect on re-emulsification. Fuel 2022, 310, 122344. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Liu, Z.; Trivedi, J.; Tang, Y.; Sui, M. Visualized investigation of the immiscible displacement: Influencing factors, improved method, and EOR effect. Fuel 2023, 331, 125841. [Google Scholar] [CrossRef]



| Relative Density | Viscosity (50 °C) | Saturates | Aromatics | Resin | Asphaltene | Wax |
|---|---|---|---|---|---|---|
| 0.983 g/cm3 | 14,315 mPa·s | 46.17% | 17.64% | 10.11% | 10.69% | 2.62% |
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | Total Dissolved Solids |
|---|---|---|---|---|---|---|---|
| 11,500 | 126 | 6600 | 62 | 29,200 | 260 | 652 | 48,400 |
| Polymer | Length (cm) | Diameter (cm) | Porosity (%) | Permeability (μm2) | Oil Saturation (%) |
|---|---|---|---|---|---|
| HPAM | 30.00 | 2.50 | 36.06 | 5.13 | 89.17 |
| PAALB | 30.00 | 2.50 | 35.71 | 5.07 | 92.28 |
| Element | C | H | O | N | S |
|---|---|---|---|---|---|
| Theoretical content (wt %) | 55.07 | 7.57 | 23.19 | 9.02 | 5.15 |
| Measurement content (wt %) | 50.77 | 7.41 | 28.54 | 8.35 | 5.02 |
| Mn | Mw | Mz | Mz+1 | Mv | PDI |
|---|---|---|---|---|---|
| 307,883 | 679,306 | 1,048,818 | 1,333,611 | 623,887 | 2.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, D.; Guo, J.; Xiong, R.; Zhang, X.; Kang, C.; Kiyingi, W. Preparation and Performance Evaluation of Amphiphilic Polymers for Enhanced Heavy Oil Recovery. Polymers 2023, 15, 4606. https://doi.org/10.3390/polym15234606
Fei D, Guo J, Xiong R, Zhang X, Kang C, Kiyingi W. Preparation and Performance Evaluation of Amphiphilic Polymers for Enhanced Heavy Oil Recovery. Polymers. 2023; 15(23):4606. https://doi.org/10.3390/polym15234606
Chicago/Turabian StyleFei, Dongtao, Jixiang Guo, Ruiying Xiong, Xiaojun Zhang, Chuanhong Kang, and Wyclif Kiyingi. 2023. "Preparation and Performance Evaluation of Amphiphilic Polymers for Enhanced Heavy Oil Recovery" Polymers 15, no. 23: 4606. https://doi.org/10.3390/polym15234606





