Exploring Schwann Cell Behavior on Electrospun Polyhydroxybutyrate Scaffolds with Varied Pore Sizes and Fiber Thicknesses: Implications for Neural Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Fabrication
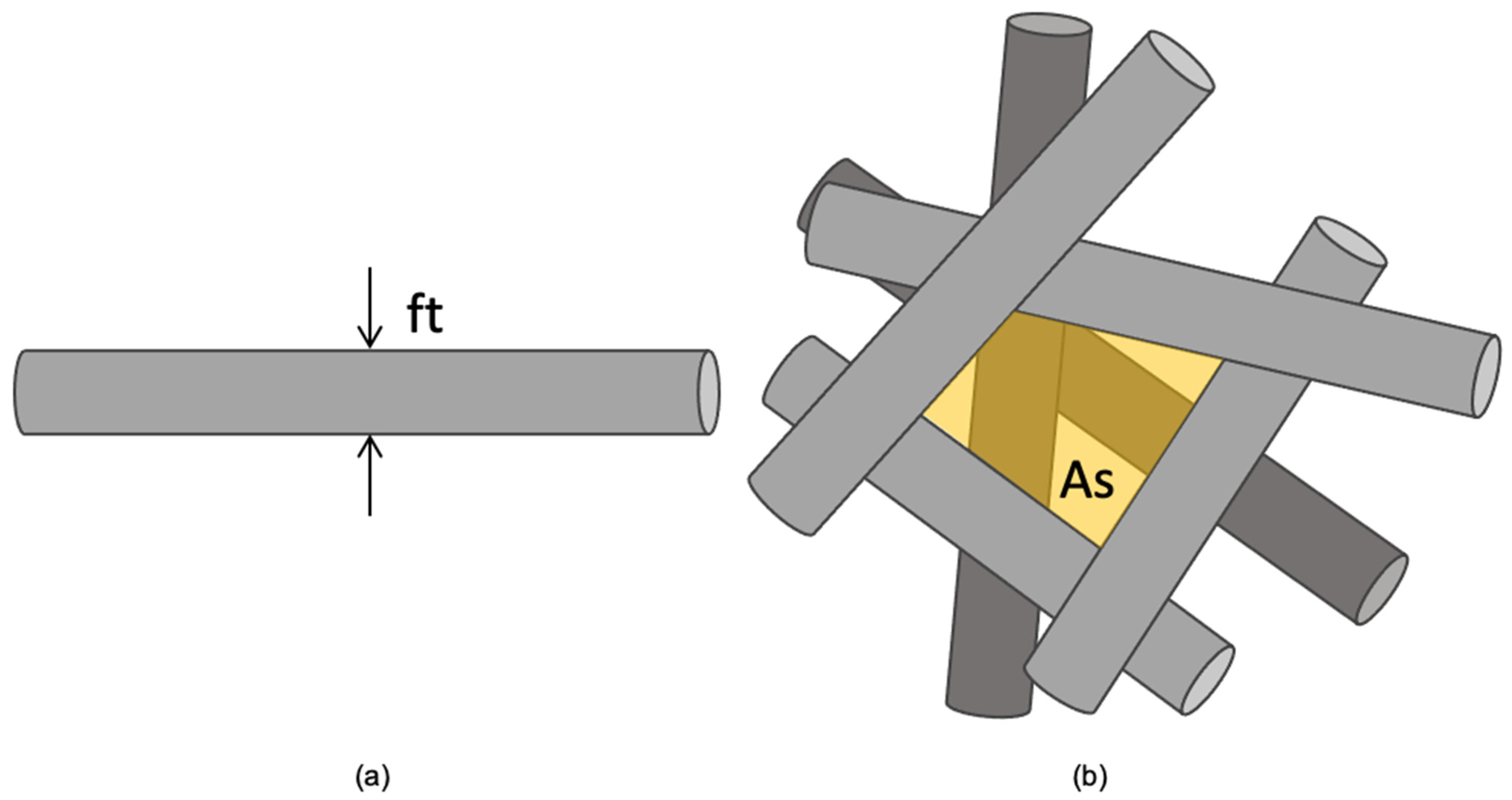
2.2. Scaffold Morphological Characterization
2.3. Cell-Scaffold Imaging
2.4. Cell Viability Assay
2.5. Statistical Analysis
3. Results
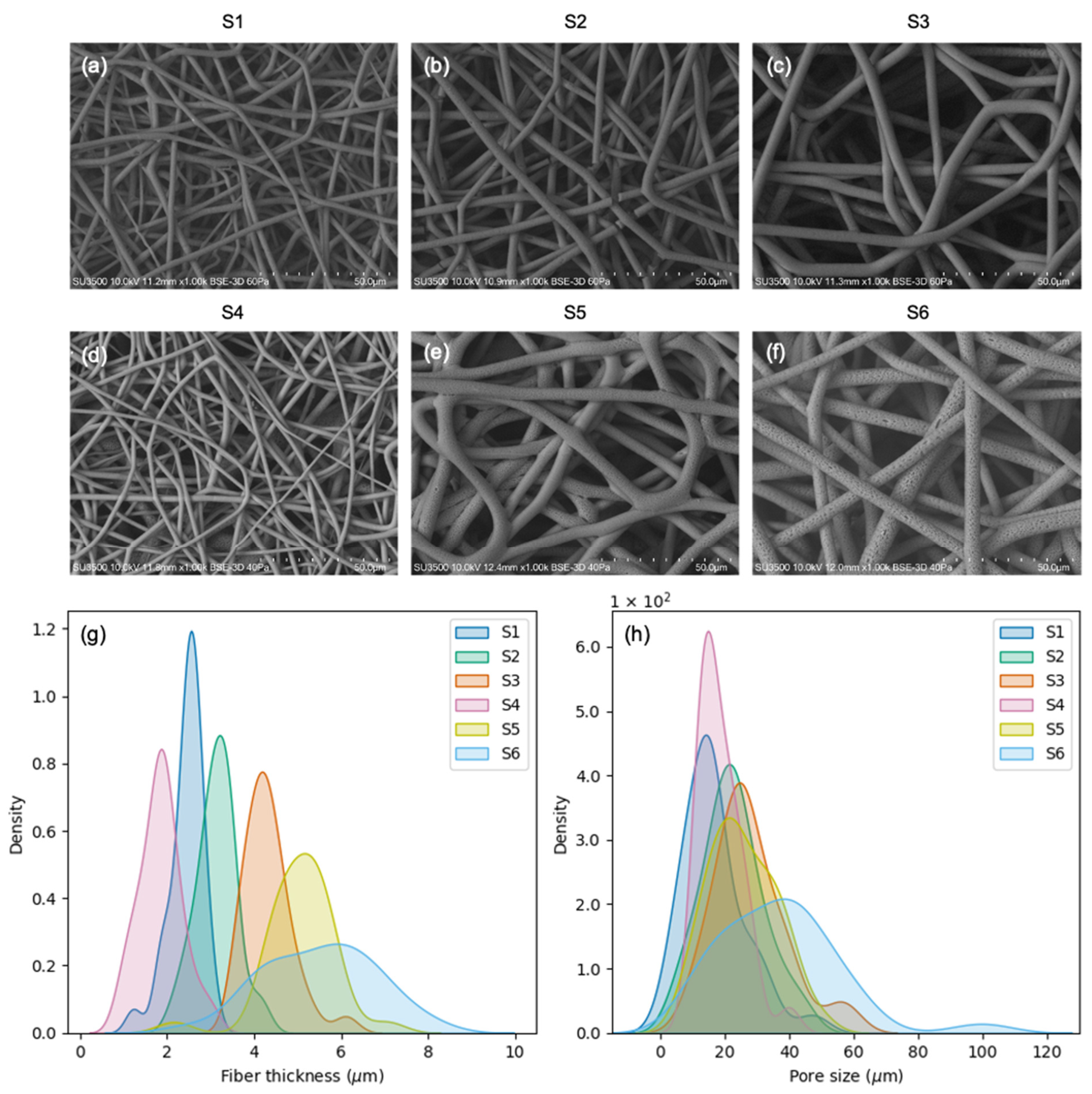
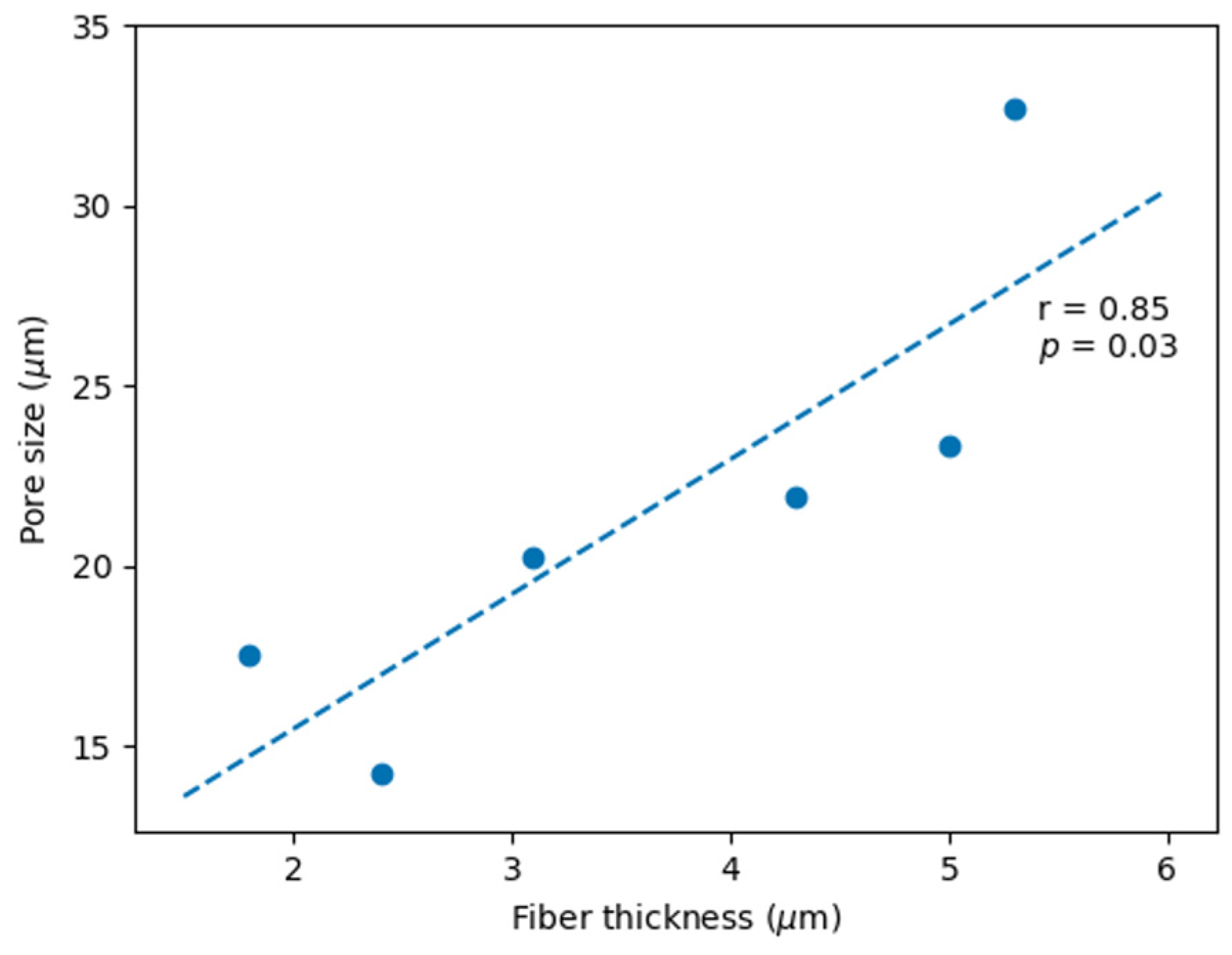
3.1. PHB Scaffold Morphology
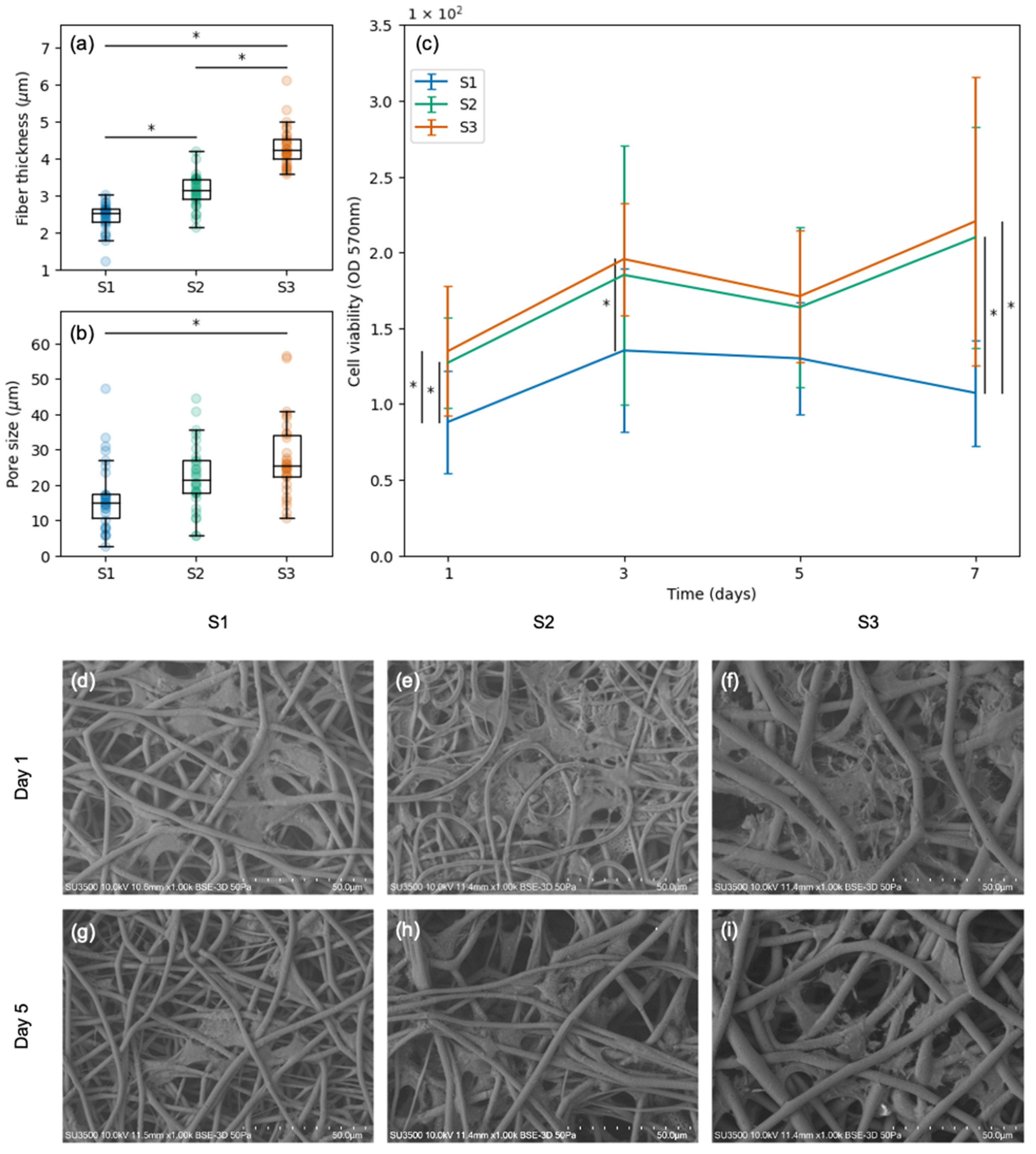
3.2. Viability and Morphology of SCs Attached to Scaffolds with Varied Pore Sizes and Fiber Thicknesses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, W.W. Evaluation and Management of Peripheral Nerve Injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral Nerve Injury and Myelination: Potential Therapeutic Strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Seddighi, A.; Nikouei, A.; Seddighi, A.S.; Zali, A.R.; Tabatabaei, S.M.; Sheykhi, A.R.; Yourdkhani, F.; Naeimian, S. Peripheral Nerve Injury: A Review Article. Int. Clin. Neurosci. J. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of Biomaterial Scaffold for Nerve Tissue Engineering: Biomaterial Mediated Neural Regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Tonda-Turo, C.; Ciardelli, G. Chapter 9 Artificial Scaffolds for Peripheral Nerve Reconstruction. In International Review of Neurobiology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 87, pp. 173–198. [Google Scholar]
- Obruča, S.; Dvořák, P.; Sedláček, P.; Koller, M.; Sedlář, K.; Pernicová, I.; Šafránek, D. Polyhydroxyalkanoates Synthesis by Halophiles and Thermophiles: Towards Sustainable Production of Microbial Bioplastics. Biotechnol. Adv. 2022, 58, 2022–2023. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Karbasi, S.; Toloue, E.B. Modified Poly(3-Hydroxybutyrate)-Based Scaffolds in Tissue Engineering Applications: A Review. Int. J. Biol. Macromol. 2021, 166, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, M.F.; Álvarez, G.; Chuhuaicura, P.; Godoy, K.; Alarcón, J.; Acevedo, F.; Gareis, I.; Dias, F.J. Polyhydroxybutyrate (PHB) Scaffolds for Peripheral Nerve Regeneration: A Systematic Review of Animal Models. Biology 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as Biomaterial for Electrospun Scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The Influence of Electrospun Fibre Size on Schwann Cell Behaviour and Axonal Outgrowth. Mater. Sci. Eng. C 2015, 48, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Tresco, P.A. Effect of Filament Diameter and Extracellular Matrix Molecule Precoating on Neurite Outgrowth and Schwann Cell Behavior on Multifilament Entubulation Bridging Device in Vitro. J. Biomed. Mater. Res.—Part A 2006, 76, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; McCarthy, C.W.; Gilbert, R.J. Varying the Diameter of Aligned Electrospun Fibers Alters Neurite Outgrowth and Schwann Cell Migration. Acta Biomater. 2010, 6, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Roca, F.; André, F.M.; Más Estellés, J.; Monleón Pradas, M.; Mir, L.M.; Martínez-Ramos, C. BDNF-Gene Transfected Schwann Cell-Assisted Axonal Extension and Sprouting on New PLA–PPy Microfiber Substrates. Macromol. Biosci. 2021, 21, 2000391. [Google Scholar] [CrossRef] [PubMed]
- Apablaza, J.A.; Lezcano, M.F.; Lopez Marquez, A.; Godoy Sánchez, K.; Oporto, G.H.; Dias, F.J. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review. Polymers 2021, 13, 2563. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes—Different Cell Effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Cheng, D.; Xu, S.; Du, C.; Xie, L.; Zhao, W. Applications of Electrospun Scaffolds with Enlarged Pores in Tissue Engineering. Biomater. Sci. 2022, 10, 1423–1447. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, Y.; Lim, C.T. Fabrication of Large Pores in Electrospun Nanofibrous Scaffolds for Cellular Infiltration: A Review. Tissue Eng.—Part B Rev. 2012, 18, 77–87. [Google Scholar] [CrossRef]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(l-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, J.H. Fabrication and Characterization of Hydrophilized Porous PLGA Nerve Guide Conduits by a Modified Immersion Precipitation Method. J. Biomed. Mater. Res. Part A 2007, 80A, 530–538. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional Neuronal Cell Growth and Differentiation on Aligned Polyhydroxyalkanoate Blend Microfibres with Varying Diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, L.; Prabhakaran, M.; Ding, X.; Ramakrishna, S. Fabrication of Nerve Growth Factor Encapsulated Aligned Poly(ε-Caprolactone) Nanofibers and Their Assessment as a Potential Neural Tissue Engineering Scaffold. Polymers 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.F.B.; Pawar, K.C.; Claeyssens, F.; Ryan, A.J.; Haycock, J.W. An Aligned 3D Neuronal-Glial Co-Culture Model for Peripheral Nerve Studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, J.A.; Lezcano, M.F.; Sánchez, K.G.; Oporto, G.H.; Dias, F.J. Optimal Morphometric Characteristics of a Tubular Polymeric Scaffold to Promote Peripheral Nerve Regeneration: A Scoping Review. Polymers 2022, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann Cell Plasticity Involved in Peripheral Nerve Repair after Injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann Cells Direct Axon Regeneration within the Peripheral Nerve Bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-Step Electrospun Scaffold of Dual-Sized Gelatin/Poly-3-Hydroxybutyrate Nano/Microfibers for Skin Regeneration in Diabetic Wound. Mater. Sci. Eng. C 2021, 119, 111602. [Google Scholar] [CrossRef]
- Acevedo, F.; Villegas, P.; Urtuvia, V.; Hermosilla, J.; Navia, R.; Seeger, M. Bacterial Polyhydroxybutyrate for Electrospun Fiber Production. Int. J. Biol. Macromol. 2018, 106, 692–697. [Google Scholar] [CrossRef]
- Han, D.; Cheung, K.C. Biodegradable Cell-Seeded Nanofiber Scaffolds for Neural Repair. Polymers 2011, 3, 1684–1733. [Google Scholar] [CrossRef]
- Hadlock, T.A.; Sundback, C.A.; Hunter, D.A.; Vacanti, J.P.; Cheney, M.L. A New Artificial Nerve Graft Containing Rolled Schwann Cell Monolayers. Microsurgery 2001, 21, 96–101. [Google Scholar] [CrossRef]
- Biazar, E.; Heidari Keshel, S. A Nanofibrous PHBV Tube with Schwann Cell as Artificial Nerve Graft Contributing to Rat Sciatic Nerve Regeneration across a 30-Mm Defect Bridge. Cell Commun. Adhes. 2013, 20, 41–49. [Google Scholar] [CrossRef]
- Li, Y.; Lv, S.; Yuan, H.; Ye, G.; Mu, W.; Fu, Y.; Zhang, X.; Feng, Z.; He, Y.; Chen, W. Peripheral Nerve Regeneration with 3D Printed Bionic Scaffolds Loading Neural Crest Stem Cell Derived Schwann Cell Progenitors. Adv. Funct. Mater. 2021, 31, 2010215. [Google Scholar] [CrossRef]
- Schaakxs, D.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Wiberg, M.; Kingham, P.J. Poly-3-Hydroxybutyrate Strips Seeded with Regenerative Cells Are Effective Promoters of Peripheral Nerve Repair. J. Tissue Eng. Regen. Med. 2017, 11, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Erba, P.; Mahay, D.; Wiberg, M.; Pierer, G.; Terenghi, G. Schwann Cell Strip for Peripheral Nerve Repair. J. Hand Surg. Eur. Vol. 2008, 33, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, H.; Amoabediny, G.; Nik, N.S.; Heydari, M.; Yosefifard, M.; Siadat, S.O.R.; Mottaghy, K. The Role of Biodegradable Engineered Scaffolds Seeded with Schwann Cells for Spinal Cord Regeneration. Neurochem. Int. 2009, 54, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Novikova, L.N.; Pettersson, J.; Brohlin, M.; Wiberg, M.; Novikov, L.N. Biodegradable Poly-β-Hydroxybutyrate Scaffold Seeded with Schwann Cells to Promote Spinal Cord Repair. Biomaterials 2008, 29, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Armati, P.J. The Biology of Schwann Cells; Armati, P., Ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780521850209. [Google Scholar]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the Pore Size of Electrospun Scaffolds. Tissue Eng.—Part B Rev. 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef]
- Thorat Gadgil, B.S.; Killi, N.; Rathna, G.V.N. Polyhydroxyalkanoates as Biomaterials. Medchemcomm 2017, 8, 1774–1787. [Google Scholar] [CrossRef]
- Miu, D.M.; Eremia, M.C.; Moscovici, M. Polyhydroxyalkanoates (PHAs) as Biomaterials in Tissue Engineering: Production, Isolation, Characterization. Materials 2022, 15, 1410. [Google Scholar] [CrossRef]
- Suwantong, O.; Waleetorncheepsawat, S.; Sanchavanakit, N.; Pavasant, P.; Cheepsunthorn, P.; Bunaprasert, T.; Supaphol, P. In Vitro Biocompatibility of Electrospun Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Fiber Mats. Int. J. Biol. Macromol. 2007, 40, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tanideh, N.; Azarpira, N.; Sarafraz, N.; Zare, S.; Rowshanghiyas, A.; Farshidfar, N.; Iraji, A.; Zarei, M.; Fray, M. El Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers 2020, 12, 2588. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V. The Properties of Human Schwann Cells: Lessons from in Vitro Culture and Transplantation Studies. Glia 2020, 68, 797–810. [Google Scholar] [CrossRef] [PubMed]
| Scaffold | PHB (%) | Voltage (kV) | Flow Rate (mL/h) | Needle–Collector Distance (cm) | Collector Diameter (cm) | Collector Speed (rpm) | Time (min) |
|---|---|---|---|---|---|---|---|
| S1 | 10 | 25 | 1 | 15 | 3.1 | 50 | 150 |
| S2 | 10 | 20 | 1 | 15 | 3.1 | 50 | 150 |
| S3 | 15 | 25 | 1 | 15 | 3.1 | 50 | 150 |
| S4 | 15 | 20 | 1 | 15 | 3.1 | 50 | 150 |
| S5 | 15 | 25 | 2 | 15 | 3.1 | 50 | 150 |
| S6 | 15 | 20 | 2 | 15 | 3.1 | 50 | 150 |
| Scaffold | Fiber Thickness (μm) | Pore Size (μm) |
|---|---|---|
| S1 | 2.4 ± 0.4 | 16.7 ± 9.7 |
| S2 | 3.1 ± 0.4 | 22.4 ± 9.4 |
| S3 | 4.3 ± 0.5 | 27.8 ± 11.0 |
| S4 | 1.8 ± 0.5 | 18.5 ± 6.6 |
| S5 | 5.0 ± 0.8 | 25.5 ± 10.3 |
| S6 | 5.3 ± 1.3 | 37.0 ± 18.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lezcano, M.F.; Martínez-Rodríguez, P.; Godoy, K.; Hermosilla, J.; Acevedo, F.; Gareis, I.E.; Dias, F.J. Exploring Schwann Cell Behavior on Electrospun Polyhydroxybutyrate Scaffolds with Varied Pore Sizes and Fiber Thicknesses: Implications for Neural Tissue Engineering. Polymers 2023, 15, 4625. https://doi.org/10.3390/polym15244625
Lezcano MF, Martínez-Rodríguez P, Godoy K, Hermosilla J, Acevedo F, Gareis IE, Dias FJ. Exploring Schwann Cell Behavior on Electrospun Polyhydroxybutyrate Scaffolds with Varied Pore Sizes and Fiber Thicknesses: Implications for Neural Tissue Engineering. Polymers. 2023; 15(24):4625. https://doi.org/10.3390/polym15244625
Chicago/Turabian StyleLezcano, María Florencia, Paulina Martínez-Rodríguez, Karina Godoy, Jeyson Hermosilla, Francisca Acevedo, Iván Emilio Gareis, and Fernando José Dias. 2023. "Exploring Schwann Cell Behavior on Electrospun Polyhydroxybutyrate Scaffolds with Varied Pore Sizes and Fiber Thicknesses: Implications for Neural Tissue Engineering" Polymers 15, no. 24: 4625. https://doi.org/10.3390/polym15244625
APA StyleLezcano, M. F., Martínez-Rodríguez, P., Godoy, K., Hermosilla, J., Acevedo, F., Gareis, I. E., & Dias, F. J. (2023). Exploring Schwann Cell Behavior on Electrospun Polyhydroxybutyrate Scaffolds with Varied Pore Sizes and Fiber Thicknesses: Implications for Neural Tissue Engineering. Polymers, 15(24), 4625. https://doi.org/10.3390/polym15244625









