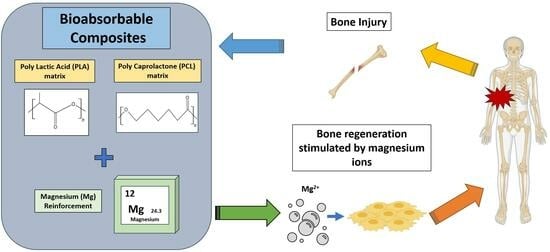
Bioabsorbable Composites Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg) for Use in Bone Regeneration Therapy: Physicochemical Properties and Biological Evaluation
Abstract
:1. Introduction
2. Polymers as Bioabsorbable Materials for Bone Tissue Engineering
2.1. Aliphatic Polyester as Bioabsorbable Material: PLA and PCL
2.1.1. Polylactic Acid (PLA)
2.1.2. Polycaprolactone (PCL)
| Material/Properties | PLA | PLLA | PDLLA | PCL |
|---|---|---|---|---|
| Mw(×103 g/mol) | 120–800 | 60–800 | 120–270 | 50.4–124 |
| Density (g/cm3) | 1.21–1.25 | 1.24–1.3 | 1.25–1.27 | 1.11–1.46 |
| Glass transition temperature, Tg (°C) | 45–60 | 55–65 | 50–60 | −60 |
| Melting point temperature, Tm (°C) | 150–162 | 170–200 | - | 56–65 |
| Young’s modulus, E (MPa) | 350–3500 | 2700–4140 | 1000–3450 | 252–440 |
| Tensile strength, (MPa) | 16.8–48 | 40–66.8 | 22.1–39.4 | 10.5–27.3 |
| Strain at break; (εb—%) | 2.5–6 | 3–10 | 1.5–20 | 80–800 |
3. Metals as Bioabsorbable Materials: Magnesium (Mg)
| Material/Properties | Cortical Bone | Trabecular Bone | Mg Pure | AZ91 | AZ31 | AZ61 | Mg6Zn |
|---|---|---|---|---|---|---|---|
| Density (g/cm3) | 1.8–2 | 1–1.4 | 1.74 | 1.81 | 1.77 | 1.80 | 1.84 |
| Young’s modulus, E (GPa) | 15–30 | 0.05–0.5 | 44 | 45 | 44.8 | 44.8 | 42.3 |
| Tensile strength, m (MPa) | 50–150 | 10–20 | 90 | 165–457 | 260 | 310 | 277–281 |
4. Bioabsorbable Composite Materials Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg)
4.1. Bioabsorbable Hybrid Materials Based on PLA Reinforced with Mg (PLA/Mg)
4.1.1. PLA Reinforced with Mg Particles
4.1.2. PLA Reinforced with Mg Wires
4.1.3. 3D Printing of PLA/Mg Particle Composites
4.2. Bioabsorbable Hybrid Materials Based on PCL Reinforced with Mg (PCL/Mg)
4.2.1. PCL Reinforced with Mg Particles
4.2.2. 3D Printing of PCL/Mg Composites
4.2.3. Mg-Reinforced PCL-Based Copolymers
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng.—Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, R.T.; Hong, X.; Schott, N.G.; Tiruchinapally, G.; Levi, B.; Stegemann, J.P. Injectable Osteogenic Microtissues Containing Mesenchymal Stromal Cells Conformally Fill and Repair Critical-Size Defects. Biomaterials 2019, 208, 32–44. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- García-Sobrino, R.; Casado-Losada, I.; Bruno-Pérez, L.; García, C.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A.; Martínez-Campos, E. Thermosensitive Hydrogels Functionalized with PH Sensitive COOH Groups for Bone Cell Harvesting. Eur. Polym. J. 2022, 169, 111131. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO Coatings Design for Mg-Ca Alloy for Cardiovascular Stent and Bone Regeneration Applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef]
- Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222. [Google Scholar] [CrossRef]
- Andalib, N.; Kehtari, M.; Seyedjafari, E.; Motamed, N.; Matin, M.M. In Vivo Bone Regeneration Using a Bioactive Nanocomposite Scaffold and Human Mesenchymal Stem Cells. Cell Tissue Bank. 2021, 22, 467–477. [Google Scholar] [CrossRef]
- Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Kakiuchi, Y.; Nakano, M.; Suzuki, S.; Handa, K.; Saito, M. Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy. Cells 2021, 10, 2687. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, V.; Brandalise, R.N.; Savaris, M. Composite Biomaterials. In Engineering of Biomaterials. Topics in Mining, Metallurgy and Materials Engineering; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2018; pp. 85–98. [Google Scholar] [CrossRef]
- Khan, H.; Barkham, B.; Trompeter, A. The Use of Bioabsorbable Materials in Orthopaedics. Orthop. Trauma 2021, 35, 289–296. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Raveendran, N.T.; Fournier, B.; Ivanovski, S. Fabrication of Biocompatible and Bioabsorbable Polycaprolactone/Magnesium Hydroxide 3D Printed Scaffolds: Degradation and in Vitro Osteoblasts Interactions. Compos. Part B Eng. 2020, 197, 108158. [Google Scholar] [CrossRef]
- DataIntelo. Bioabsorbable Implants Market Report|Global Forecast from 2022 to 2030. Available online: https://dataintelo.com/report/bioabsorbable-implants-market/ (accessed on 1 April 2023).
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable Materials. Orthop. Trauma 2017, 31, 316–320. [Google Scholar] [CrossRef]
- Pearson, J.J.; Gerken, N.; Bae, C.; Lee, K.B.; Satsangi, A.; McBride, S.; Appleford, M.R.; Dean, D.D.; Hollinger, J.O.; Ong, J.L.; et al. In Vivo Hydroxyapatite Scaffold Performance in Infected Bone Defects. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2020, 108, 1157–1166. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A New Ag-Nanostructured Hydroxyapatite Porous Scaffold: Antibacterial Effect and Cytotoxicity Study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef]
- Balcon, R.; Beyar, R.; Chierchia, S.; De Scheerder, I.; Hugenholtz, P.G.; Kiemeneij, F.; Meier, B.; Meyer, J.; Monassier, J.P.; Wijns, W. Recommendations on Stent Manufacture, Implantation and Utilization. Eur. Heart J. 1997, 18, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A. Bioabsorbable Polymeric Materials for Biofilms and Other Biomedical Applications: Recent and Future Trends. Mater. Today Proc. 2021, 44, 2447–2453. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mather, P.T. POSS Polymers: Physical Properties and Biomaterials Applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Bonilla, C.E.P.; Perilla, J.E. The Past, Present and near Future of Materials for Use in Biodegradable Orthopaedic Implants. Ing. Investig. 2011, 31, 124–133. [Google Scholar] [CrossRef]
- Pawelec, K.M.; White, A.A.; Best, S.M. Properties and Characterization of Bone Repair Materials, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Verdier, C. Rheological Properties of Living Materials. From Cells to Tissues. J. Theor. Med. 2003, 5, 67–91. [Google Scholar] [CrossRef]
- Palmero, P. Ceramic-Polymer Nanocomposites for Bone-Tissue Regeneration; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Liu, C. Collagen–Hydroxyapatite Composite Scaffolds for Tissue Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Salama, A. Cellulose/Calcium Phosphate Hybrids: New Materials for Biomedical and Environmental Applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites. Handb. Bioceram. Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–1386. [Google Scholar] [CrossRef]
- Multigner, M.; Muñoz, M.; Pulido-González, N.; Torres, B.; Cifuentes, S.C. Mg as Bioabsorbable Material. Encycl. Mater. Met. Alloy. 2021, 1, 113–122. [Google Scholar] [CrossRef]
- Han, H.S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.C.; Seok, H.K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current Status and Outlook on the Clinical Translation of Biodegradable Metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, Q.W.; Chen, Q.; Cui, C.; Duan, S.; Kang, Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S.; et al. Biomedical Polymers: Synthesis, Properties, and Applications. Sci. China Chem. 2022, 65, 1010–1075. [Google Scholar] [CrossRef]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-Based Materials with Novel Characteristics for Tissue Engineering Applications—A Review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Biswal, T. Biopolymers for Tissue Engineering Applications: A Review. Mater. Today Proc. 2019, 41, 397–402. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, M.; Albertsson, A.C. Degradation Products of Aliphatic and Aliphatic-Aromatic Polyesters. Adv. Polym. Sci. 2008, 211, 85–116. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic Polyester Polymer Stars: Synthesis, Properties and Applications in Biomedicine and Nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Zhou, S.; Shanmugam, K.T.; Yomano, L.P.; Grabar, T.B.; Ingram, L.O. Fermentation of 12% (w/v) Glucose to 1.2 M Lactate by Escherichia Coli Strain SZ194 Using Mineral Salts Medium. Biotechnol. Lett. 2006, 28, 663–670. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, S.C.; Benavente, R.; Lieblich, M.; González-Carrasco, J.L. Biodegradable and Bioabsorbable Materials for Osteosynthesis Applications: State-of-the-Art and Future Perspectives. Handb. Compos. from Renew. Mater. 2017, 1–8, 109–143. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer Biodegradation and Biodegradable Polymers—A Review. Polish J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Pan, P.; Liang, Z.; Zhu, B.; Dong, T.; Inoue, Y. Roles of Physical Aging on Crystallization Kinetics and Induction Period of Poly(L-Lactide). Macromolecules 2008, 41, 8011–8019. [Google Scholar] [CrossRef]
- Barber, F.A.; Dockery, W.D. Long-Term Absorption of Poly-L-Lactic Acid Interference Screws. Arthrosc.—J. Arthrosc. Relat. Surg. 2006, 22, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric Materials for Bone and Cartilage Repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. 32—Biodegradation of Biopolymers; Elsevier B.V.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2019, 10, 381–388. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-Based (Bio)degradable Polymers as Environmentally Friendly Materials for Sustainable Development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Odaira, T.; Xu, S.; Hirata, K.; Xu, X.; Omori, T.; Ueki, K.; Ueda, K.; Narushima, T.; Nagasako, M.; Harjo, S.; et al. Flexible and Tough Superelastic Co–Cr Alloys for Biomedical Applications. Adv. Mater. 2022, 34, e2202305. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Ryu, H.; Seo, M.H.; Rogers, J.A. Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices. Adv. Healthc. Mater. 2021, 10, 2002236. [Google Scholar] [CrossRef] [PubMed]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-Term Biocompatibility of a Corrodible Peripheral Iron Stent in the Porcine Descending Aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current Status and Perspectives of Zinc-Based Absorbable Alloys for Biomedical Applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Witte, F. Reprint of: The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2015, 23, S28–S40. [Google Scholar] [CrossRef]
- Song, G. Recent Progress in Corrosion and Protection of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent Progress in Mg-Based Alloys as a Novel Bioabsorbable Biomaterials for Orthopedic Applications. J. Magnes. Alloys 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Díaz-tocados, J.M.; Herencia, C.; Martínez-moreno, J.M.; Montes, A.; Oca, D.; Rodríguez-ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S. Magnesium Chloride Promotes Osteogenesis through Notch Signaling Activation and Expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef]
- Hung, C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. Acta Biomaterialia The Role of Magnesium Ions in Bone Regeneration Involves the Canonical Wnt Signaling Pathway Q. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In Vitro and in Vivo Studies on the Degradation of High-Purity Mg (99.99 wt.%) Screw with Femoral Intracondylar Fractured Rabbit Model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jamel, M.M.; Jamel, M.M.; Lopez, H.F. Designing Advanced Biomedical Biodegradable Mg Alloys: A Review. Metals 2022, 12, 85. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; Stjohn, D.H. The Role of Solute in Grain Refinement of Magnesium. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Abd El-Rahman, S.S. Neuropathology of Aluminum Toxicity in Rats (Glutamate and GABA Impairment). Pharmacol. Res. 2003, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium Based Implants for Functional Bone Tissue Regeneration—A Review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Currey, J. The Structure and Mechanical Properties of Bone; Woodhead Publishing Limited: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Frutos, E.; González-Carrasco, J.L.; Muñoz, M.; Multigner, M.; Chao, J.; Benavente, R.; Lieblich, M. Novel PLLA/Magnesium Composite for Orthopedic Applications: A Proof of Concept. Mater. Lett. 2012, 74, 239–242. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Lieblich, M.; López, F.A.; Benavente, R.; González-Carrasco, J.L. Effect of Mg Content on the Thermal Stability and Mechanical Behaviour of PLLA/Mg Composites Processed by Hot Extrusion. Mater. Sci. Eng. C 2017, 72, 18–25. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Frutos, E.; Benavente, R.; Lorenzo, V.; González-Carrasco, J.L. Assessment of Mechanical Behavior of PLA Composites Reinforced with Mg Micro-Particles through Depth-Sensing Indentations Analysis. J. Mech. Behav. Biomed. Mater. 2017, 65, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, S.C.; Bensiamar, F.; Gallardo-Moreno, A.M.; Osswald, T.A.; González-Carrasco, J.L.; Benavente, R.; González-Martín, M.L.; García-Rey, E.; Vilaboa, N.; Saldaña, L. Incorporation of Mg Particles into PDLLA Regulates Mesenchymal Stem Cell and Macrophage Responses. J. Biomed. Mater. Res.—Part A 2016, 104, 866–878. [Google Scholar] [CrossRef]
- Ferrandez-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. New Approach to Improve Polymer-Mg Interface in Biodegradable PLA/Mg Composites through Particle Surface Modification. Surf. Coat. Technol. 2020, 383, 125285. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L.; Ferrari, B. Study of the Matrix-Filler Interface in PLA/Mg Composites Manufactured by Material Extrusion Using a Colloidal Feedstock. Addit. Manuf. 2020, 33, 101142. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L. In Vitro Degradation of Biodegradable Polylactic Acid/Magnesium Composites: Relevance of Mg Particle Shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Lieblich, M.; Saldaña, L.; González-Carrasco, J.L.; Benavente, R. In Vitro Degradation of Biodegradable Polylactic Acid/Mg Composites: Influence of Nature and Crystalline Degree of the Polymeric Matrix. Materialia 2019, 6, 100270. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Zhang, X. Development of PLA/Mg Composite for Orthopedic Implant: Tunable Degradation and Enhanced Mineralization. Compos. Sci. Technol. 2017, 147, 8–15. [Google Scholar] [CrossRef]
- Lee, H.; Shin, D.Y.; Na, Y.; Han, G.; Kim, J.; Kim, N.; Bang, S.J.; Kang, H.S.; Oh, S.K.; Yoon, C.B.; et al. Antibacterial PLA/Mg Composite with Enhanced Mechanical and Biological Performance for Biodegradable Orthopedic Implants. Biomater. Adv. 2023, 152, 213523. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, E.B.; Jeong, C.J.; Sharker, S.M.; In, I.; Park, S.Y. Light Controllable Surface Coating for Effective Photothermal Killing of Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 15600–15606. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Manivasagan, P.; Jeong, M.S.; Choi, M.; Jang, E.S.; Priya, V.V.; Wang, M.H. Photothermally Responsive Chitosan-Coated Iron Oxide Nanoparticles for Enhanced Eradication of Bacterial Biofilms. Biomater. Adv. 2022, 141, 213129. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdeljawad, M.; Carette, X.; Argentati, C.; Martino, S.; Gonon, M.F.; Odent, J.; Morena, F.; Mincheva, R.; Raquez, J.M. Interfacial Compatibilization into Pla/Mg Composites for Improved in Vitro Bioactivity and Stem Cell Adhesion. Molecules 2021, 26, 5944. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, C.; Zhou, L.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. Fully Degradable PLA-Based Composite Reinforced with 2D-Braided Mg Wires for Orthopedic Implants. Compos. Sci. Technol. 2017, 142, 180–188. [Google Scholar] [CrossRef]
- Ali, W.; Mehboob, A.; Han, M.G.; Chang, S.H. Effect of Fluoride Coating on Degradation Behaviour of Unidirectional Mg/PLA Biodegradable Composite for Load-Bearing Bone Implant Application. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105464. [Google Scholar] [CrossRef]
- Li, X.; Yu, W.; Han, L.; Chu, C.; Bai, J.; Xue, F. Degradation Behaviors of Mg Alloy Wires/PLA Composite in the Consistent and Staged Dynamic Environments. Mater. Sci. Eng. C 2019, 103, 109765. [Google Scholar] [CrossRef]
- Ali, W.; Mehboob, A.; Han, M.G.; Chang, S.H. Experimental Study on Degradation of Mechanical Properties of Biodegradable Magnesium Alloy (AZ31) Wires/Poly(Lactic Acid) Composite for Bone Fracture Healing Applications. Compos. Struct. 2019, 210, 914–921. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, Y.; Meng, J.; Li, X.; Xue, F.; Chu, C.; Tao, L.; Bai, J. Enhanced Fully-Biodegradable Mg/PLA Composite Rod: Effect of Surface Modification of Mg-2Zn Wire on the Interfacial Bonding. Surf. Coat. Technol. 2018, 350, 722–731. [Google Scholar] [CrossRef]
- Li, X.; Qi, C.; Han, L.; Chu, C.; Bai, J.; Guo, C.; Xue, F.; Shen, B.; Chu, P.K. Influence of Dynamic Compressive Loading on the in Vitro Degradation Behavior of Pure PLA and Mg/PLA Composite. Acta Biomater. 2017, 64, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Meng, J.; Li, X.; Xue, F.; Chu, C.; Guo, C.; Bai, J. In Vitro Degradation Behavior of Mg Wire/Poly(Lactic Acid) Composite Rods Prepared by Hot Pressing and Hot Drawing. Acta Biomater. 2019, 98, 125–141. [Google Scholar] [CrossRef]
- Sun, S.; Gao, L.; Liang, B.; Yin, Z.; Pan, S.; Shi, C.; Guo, C.; Huang, Z.; Chu, C.; Dong, Y. Long-Term and Uniform Release of Magnesium Ions from PLA Porous Composite Materials Oriently Reinforced by Mg Wires for Potential Bone Repair Application. Surf. Interfaces 2023, 40, 103018. [Google Scholar] [CrossRef]
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manuf. 2017, 11, 545–554. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Additive Manufacturing Applications in Industry 4.0: A Review. J. Ind. Integr. Manag. 2019, 4, 1930001. [Google Scholar] [CrossRef]
- Garcia, C.; Gallardo, A.; López, D.; Elvira, C.; Azzahti, A.; Lopez-Martinez, E.; Cortajarena, A.L.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Smart PH-Responsive Antimicrobial Hydrogel Scaffolds Prepared by Additive Manufacturing. ACS Appl. Bio Mater. 2018, 1, 1337–1347. [Google Scholar] [CrossRef]
- Wang, S.; Daelemans, L.; D’hooge, D.R.; Couck, L.; Van Den Broeck, W.; Cornillie, P.; Gou, M.; De Clerck, K.; Cardon, L. Lifting the Quality of Fused Filament Fabrication of Polylactic Acid Based Composites. Compos. Part B Eng. 2021, 210, 108613. [Google Scholar] [CrossRef]
- Bakhshi, R.; Mohammadi-Zerankeshi, M.; Mehrabi-Dehdezi, M.; Alizadeh, R.; Labbaf, S.; Abachi, P. Additive Manufacturing of PLA-Mg Composite Scaffolds for Hard Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2023, 138, 105655. [Google Scholar] [CrossRef]
- Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef]
- Li, X.; Chu, C.; Wei, Y.; Qi, C.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. In Vitro Degradation Kinetics of Pure PLA and Mg/PLA Composite: Effects of Immersion Temperature and Compression Stress. Acta Biomater. 2017, 48, 468–478. [Google Scholar] [CrossRef]
- Ali, F.; Kalva, S.N.; Mroue, K.H.; Keyan, K.S.; Tong, Y.; Khan, O.M.; Koç, M. Degradation Assessment of Mg-Incorporated 3D Printed PLA Scaffolds for Biomedical Applications. Bioprinting 2023, 35, e00302. [Google Scholar] [CrossRef]
- Zeynivandnejad, M.; Moradi, M.; Sadeghi, A. Mechanical, Physical, and Degradation Properties of 3D Printed PLA + Mg Composites. J. Manuf. Process. 2023, 101, 234–244. [Google Scholar] [CrossRef]
- Schmidt, F.; Weishaupt, O.; Radwan, M.; Willeke, M.; Frerich, S. PLA-Mg Composites by Laser-Based Powder Bed Fusion—A Preliminary Study. Addit. Manuf. Lett. 2023, 6, 100148. [Google Scholar] [CrossRef]
- Karl, D.; Jastram, B.; Kamm, P.H.; Schwandt, H.; Gurlo, A.; Schmidt, F. Evaluating Porous Polylactide-Co-Glycolide/Bioactive Glass Composite Microsphere Powders for Laser Sintering of Scaffolds. Powder Technol. 2019, 354, 289–300. [Google Scholar] [CrossRef]
- Kusoglu, I.M.; Doñate-buendía, C.; Barcikowski, S.; Gökce, B. Laser Powder Bed Fusion of Polymers: Quantitative Research Direction Indices. Materials 2021, 14, 1169. [Google Scholar] [CrossRef]
- Wong, H.M.; Wu, S.; Chu, P.K.; Cheng, S.H.; Luk, K.D.K.; Cheung, K.M.C.; Yeung, K.W.K. Low-Modulus Mg/PCL Hybrid Bone Substitute for Osteoporotic Fracture Fixation. Biomaterials 2013, 34, 7016–7032. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kessler, M.R. Pultruded Glass Fiber/Bio-Based Polymer: Interface Tailoring with Silane Coupling Agent. Compos. Part A Appl. Sci. Manuf. 2014, 65, 83–90. [Google Scholar] [CrossRef]
- Wong, H.M.; Chu, P.K.; Leung, F.K.L.; Cheung, K.M.C.; Luk, K.D.K.; Yeung, K.W.K. Engineered Polycaprolactone-Magnesium Hybrid Biodegradable Porous Scaffold for Bone Tissue Engineering. Prog. Nat. Sci. Mater. Int. 2014, 24, 561–567. [Google Scholar] [CrossRef]
- Salaris, V.; Leonés, A.; López, D.; Kenny, J.M.; Peponi, L. A Comparative Study on the Addition of MgO and Mg(OH)2 Nanoparticles into PCL Electrospun Fibers. Macromol. Chem. Phys. 2023, 224, 2200215. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, B.J.; Lih, E.; Park, W.; Lee, S.H.; Joung, Y.K.; Han, D.K. Versatile Effects of Magnesium Hydroxide Nanoparticles in PLGA Scaffold–Mediated Chondrogenesis. Acta Biomater. 2018, 73, 204–216. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Sharma Kopparthy, S.D.; Gupta, M. A Study on the Effect of Low-Cost Eggshell Reinforcement on the Immersion, Damping and Mechanical Properties of Magnesium–Zinc Alloy; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 182. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa Glasses without Clinically Observable Hydrogen Evolution for Biodegradable Implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, E.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.N.; Snega, S.; Praseetha, P.K. Green Fabricated MgO Nanoparticles as Antimicrobial Agent: Characterization and Evaluation. Mater. Today Proc. 2021, 45, 5579–5583. [Google Scholar] [CrossRef]
- Imani, M.M.; Safaei, M. Optimized Synthesis of Magnesium Oxide Nanoparticles as Bactericidal Agents. J. Nanotechnol. 2019, 2019, 6063832. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, K.; Guo, Y.; Tan, J.; Wu, J.; Yang, Y.; Fu, P.; Wang, L.; Jiang, W.; Hao, Y. Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 5120–5131. [Google Scholar] [CrossRef]
- Wolfenson, H.; Lavelin, I.; Geiger, B. Review Dynamic Regulation of the Structure and Functions of Integrin Adhesions. Dev. Cell 2013, 24, 447–458. [Google Scholar] [CrossRef]
- Hoshiba, T.; Yoshikawa, C.; Sakakibara, K. Characterization of Initial Cell Adhesion on Charged Polymer Substrates in Serum-Containing and Serum-Free Media. Langmuir 2018, 34, 4043–4051. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-Printed Mg-Incorporated PCL-Based Scaffolds: A Promising Approach for Bone Healing. Mater. Sci. Eng. C 2021, 129, 112372. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Huang, Y.; Han, D.; Zhang, J.; Cao, J.; Jin, X.; Huang, J. Scaffolds Incorporating Bone Marrow Mesenchymal Stem Cells for the Repair of Bone Defects. Biomed. Mater. 2016, 11, 025005. [Google Scholar] [CrossRef]
- No, Y.J.; Lu, Z.; Ng, P.Y.; Chen, Y.; Shi, J. A Bioceramic with Enhanced Osteogenic Properties to Regulate the Function of Osteoblastic and Osteocalastic Cells for Bone Tissue Regeneration. Biomed. Mater. 2016, 11, 035018. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Lin, H.Y.; Chen, Y.W.; Lin, C.Y.; Hsu, T.T.; Kao, C.T. Laser Sintered Magnesium-Calcium Silicate/Poly-ε-Caprolactone Scaffold for Bone Tissue Engineering. Materials 2017, 10, 65. [Google Scholar] [CrossRef]
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D Printed Biomedical Devices and Their Applications: A Review on State-of-the-Art Technologies, Existing Challenges, and Future Perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The Roles of Ions on Bone Regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Xiao, Y. The Effect of Silicate Ions on Proliferation, Osteogenic Differentiation and Cell Signalling Pathways (WNT and SHH) of Bone Marrow Stromal Cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, W.; Zhai, X.; Chen, B.; Qiao, W.; Li, W.; Li, P.; Zhao, Y.; Meng, Y.; Qian, S.; et al. 3D-Printed Nanocomposite Scaffolds with Tunable Magnesium Ionic Microenvironment Induce in Situ Bone Tissue Regeneration. Appl. Mater. Today 2019, 16, 493–507. [Google Scholar] [CrossRef]
- Wang, F.; Xia, D.; Wang, S.; Gu, R.; Yang, F.; Zhao, X. Bioactive Materials Photocrosslinkable Col/PCL/Mg Composite Membrane Providing Spatiotemporal Maintenance and Positive Osteogenetic Effects during Guided Bone Regeneration. Bioact. Mater. 2022, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]









| Matrix | Mg Wires | Content (vol.%) | Surface Treatment | Tensile/Bending Strength (MPa) | Degradation Conditions | Degraded Bending Strength | Year, Reference |
|---|---|---|---|---|---|---|---|
| PLA (90,000–120,000 g/mol) | AZ31 | 0 | 59/- | 2018, [99] | |||
| 20 | 85/131 | ||||||
| 30 | 109/177 | ||||||
| 40 | 135/196 | ||||||
| 50 | 164/245 | PBS (35 days at 37 °C) | 64 | ||||
| PBS (14 days at 50 °C) | 42 | ||||||
| PLA (90,000–120,000 g/mol) | AZ31 | 50 | MgF2 | 164/255 | PBS (56 days at 37 °C) | 108 | 2019, [97] |
| PLA (density 1.24 g/cm3) | AZ31 | 10 | MAO | -/110 | KBM (21 days at 37 °C) Dynamic tests (1 Hz) | 2017, [101] | |
| 0 MPa | 90 | ||||||
| 0.1 MPa | 82 | ||||||
| 0.3 MPa | 75 | ||||||
| 0.9 MPa | 65 | ||||||
| PLA (density 1.24 g/cm3) | AZ31 | 20 | MAO | -/156 | KBM (30 Days at 37 °C) Dynamic tests | 2019, [98] | |
| 1 Hz | |||||||
| 0 MPa | 128 | ||||||
| 0.2 MPa | 110 | ||||||
| 0.5 MPa | 100 | ||||||
| 1 MPa | 80 | ||||||
| 0.2 MPa | |||||||
| 0.5 Hz | 112 | ||||||
| 1 Hz | 108 | ||||||
| 2.5 Hz | 90 | ||||||
| PLA (78,000 g/mol) | Mg2Zn | 0 | -/103 | Hank’s solution (10 weeks at 37 °C) | 80 | 2019, [102] | |
| 5 | MAO | -/129 | 20 | ||||
| 5 | MAO and hot drawn | -/207 | 25 | ||||
| 10 | MAO | -/160 | 36 | ||||
| 10 | MAO and hot drawn | -/211 | 45 | ||||
| PLA Matrix | Reinforcement (Size)/Surfaces Modification | Reinf. wt.% in Polymer | Processing Method | Year, Reference |
|---|---|---|---|---|
| PLLA (1.25 g/cm3) | Mg (<250 µm)/- | 0 and 30 | Solvent casting and compression molding | 2012, [83] |
| PLLA (MFI: 35.8 g/10 min) | Mg (<50 µm)/- | 0, 0.5, 1, 3, 5 and 7 | Hot extrusion and compression molding | 2017, [84] |
| PLLA (95,000 g/mol) and PDLLA (103,000 g/mol) | Mg (25 µm)/- | 0, 1, 5, 10 and 15 | Hot extrusion and compression molding | 2017, [85] |
| PLA 2002D | Mg (25 µm)/- | 0, 0.2 and 1 | Injection molding | 2016, [86] |
| PLA 2003D | Mg (29.22 µm)/PEI and CTAB | 0, 5, 10, 30 and 50 | Colloidal processing | 2020, [88] |
| PLA 2002D | Mg (<50 µm) spherical and irregular particles/- | 0 and 10 | Hot extrusion and compression molding | 2016, [89] |
| PLLA (89,000 g/mol) and PDLLA (95,000 g/mol) | Mg (25 µm) irregular particles/- | 0 and 10 | Hot extrusion and compression molding | 2019, [90] |
| PLA (1.25 g/cm3) | Mg (100 µm)/- | 0, 2 and 5 | Solvent casting and compression molding | 2017, [91] |
| PLA (4032D) | Mg (120 µm)/- | 0, 15 and 30 vol.% | High shear processing | 2023, [92] |
| PLA | Mg (<100 µm) irregular particles/- | 0, 2, 4, 6, 8 and 10 | FFF (3D-Printing) | 2023, [108] |
| PLA (68,000 g/mol) | AZ61 (<100 µm)/- | 0, 5, 10 and 15 | FFF (3D-Printing) | 2023, [111] |
| PLA pellets | MgO (<40 µm)/- | 0, 10, 25 and 40 | PBF-LB (3D-Printing) | 2023, [113] |
| Sample | Preclinical Phase | Cell Line | Comments | Year/Reference |
|---|---|---|---|---|
| PDLLA with 0, 0.2 and 1 wt.% Mg | In vitro | hMSCs | Low presence of magnesium resulted in improved cell viability and macrophage responses | 2016, [86] |
| PLLA and PDLLA with 10 wt.% Mg | In vitro | hMSCs | Mg favored cytocompatibility evaluation. On the other hand, the structures with higher amorphous content (PDLLA) showed better cellular response | 2019, [90] |
| PLA with 0, 2 and 5 wt.% Mg | In vitro | MC3T3−E1 | Improved cell viability | 2017, [91] |
| PLA with 0, 15 and 30 vol.% Mg | In vitro | MC3T3−E1 | Improved cell activity in terms of cell adhesion and proliferation compared to control and 30 vol.%. This later was associated with an increase in pH | 2023, [92] |
| Mg ion antibacterial properties with respect to E. Coli and S. Aureus | Antibacterial capacity of magnesium ions under near infrared (NIR) emission (808 nm) | |||
| PLA (AZ31 wires with Ca-P) | In vitro | ADSCs | Mg improves cell adhesion and proliferation processes | 2023, [103] |
| PLA with 0, 2, 4, 6, 8 and 10 wt.% Mg | In vitro | Human fibroblast (L929) | Up to 6% positive effect in terms of bone cell culture | 2021, [108] |
| PLA with 0, 5, 10 and 15 wt.% (AZ61) | In vitro | MC7s epithelial line | Improved cell viability | 2023, [111] |
| PCL Matrix (g/mol) | Reinforcement (Size)/Surface Modification | Reinforcement wt.% in Polymer | Processing Method | Year, Reference |
|---|---|---|---|---|
| 80,000 | Mg (45–150 µm)/TSPM | 10 | Blending at 60 °C | 2013, [116] |
| 80,000 | Mg (45–150 µm)/TSPM | 0, 5, 10 and 15 | Salt leaching | 2014, [118] |
| 50,000 | Mg(OH)2 (10 nm) and MgO (20 nm) | 0, 0.5, 1, 5, 10 and 20 | Electrospinning | 2023, [119] |
| 68,000 | Mg (45 µm)/- | 0, 5, 10 and 15 | FFF (3D printing) | 2020, [125] |
| 80,000 | Mg (28.6 µm)/- | 0, 1, 3, 5, 7 and 9 | FFF (3D printing) | 2021, [128] |
| 45,000 | Mg(OH)2 (<50 nm)/- | 0, 5 and 20 | FFF (3D printing) | 2020, [18] |
| - | Powder from ball milling mix [132]/- | 0, 10, 20 and 30 | LS (3D printing) | 2017, [131] |
| Sample | Preclinical Phase | Cell line or Animal Model (Injury) | Comments | Year/Reference |
|---|---|---|---|---|
| PCL with 10 wt.% of Mg (treated with TMSPM) | In vitro | MC3T3−E1 | ALP protein marker showed better differentiation towards bone lineage in the presence of Mg | 2013, [116] |
| In vivo | SD rats (lateral epicondyle) | Micro-CT analysis demonstrated increased bone formation in the presence of Mg | ||
| PCL with 0, 5, 10 and 15 wt.% of Mg (treated with TMSPM) | In vitro | MC3T3−E1 | High Mg levels favored stages such as cell adhesion and proliferation with respect to the control sample. In addition, ALP marker showed greater capacity for bone differentiation | 2014, [118] |
| In vivo | SD rats (lateral epicondyle) | Micro-CT analysis demonstrated increased bone formation with Mg presence | ||
| PCL with 0, 5, 10 and 15 wt.% of Mg | In vitro | rBMSCs | Improvement of cellular processes up to 10 wt.%; from this percentage, a reduction in efficiency was observed due to an increase in the pH of the medium | 2020, [125] |
| In vivo | SD rats (skull model) | Micro-CT and X-ray measurements showed that the reinforced sample was significantly improved over the control sample | ||
| PCL with 0, 1, 3, 5, 7 and 9 wt.% of Mg | In vitro | rBMSCs | Improvement of cellular processes such as cell adhesion and proliferation effective up to 5 wt.%. In addition, ALP marker showed that 3 wt.% exhibited better differentiation capacity towards bone lineage | 2021, [128] |
| In vivo | Rabbits (medial tibial tubercle) | Analysis of 3 wt.% Mg improved in terms of bone repair with respect to the control sample | ||
| PCL with 0, 5 and 20 wt.% of Mg(OH)2 | In vitro | hOBs | Improved cell adhesion and proliferation with the presence of Mg. On the other hand, ALP also showed a greater capacity for differentiation towards bone lineage | 2020, [18] |
| PCL with 0, 10, 20 and 30 wt.% of CS-Mg | In vitro | hMSCs | Alizarin red staining and osteocalein showed higher bone matrix mineralization at 20 wt.% with respect to the control system. Synergy formed with the corresponding Mg and release of silicate ions | 2017, [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sobrino, R.; Muñoz, M.; Rodríguez-Jara, E.; Rams, J.; Torres, B.; Cifuentes, S.C. Bioabsorbable Composites Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg) for Use in Bone Regeneration Therapy: Physicochemical Properties and Biological Evaluation. Polymers 2023, 15, 4667. https://doi.org/10.3390/polym15244667
García-Sobrino R, Muñoz M, Rodríguez-Jara E, Rams J, Torres B, Cifuentes SC. Bioabsorbable Composites Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg) for Use in Bone Regeneration Therapy: Physicochemical Properties and Biological Evaluation. Polymers. 2023; 15(24):4667. https://doi.org/10.3390/polym15244667
Chicago/Turabian StyleGarcía-Sobrino, Rubén, Marta Muñoz, Elías Rodríguez-Jara, Joaquín Rams, Belén Torres, and Sandra C. Cifuentes. 2023. "Bioabsorbable Composites Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg) for Use in Bone Regeneration Therapy: Physicochemical Properties and Biological Evaluation" Polymers 15, no. 24: 4667. https://doi.org/10.3390/polym15244667
APA StyleGarcía-Sobrino, R., Muñoz, M., Rodríguez-Jara, E., Rams, J., Torres, B., & Cifuentes, S. C. (2023). Bioabsorbable Composites Based on Polymeric Matrix (PLA and PCL) Reinforced with Magnesium (Mg) for Use in Bone Regeneration Therapy: Physicochemical Properties and Biological Evaluation. Polymers, 15(24), 4667. https://doi.org/10.3390/polym15244667