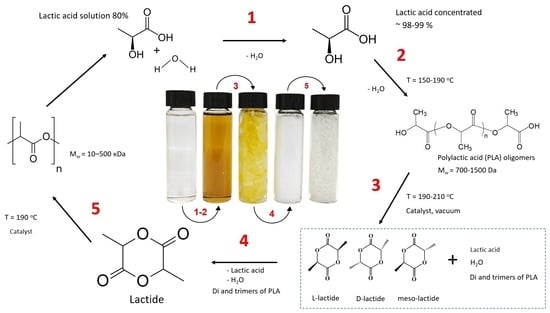
Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lactic Acid Concentration
2.3. Oligomerization of Lactic Acid
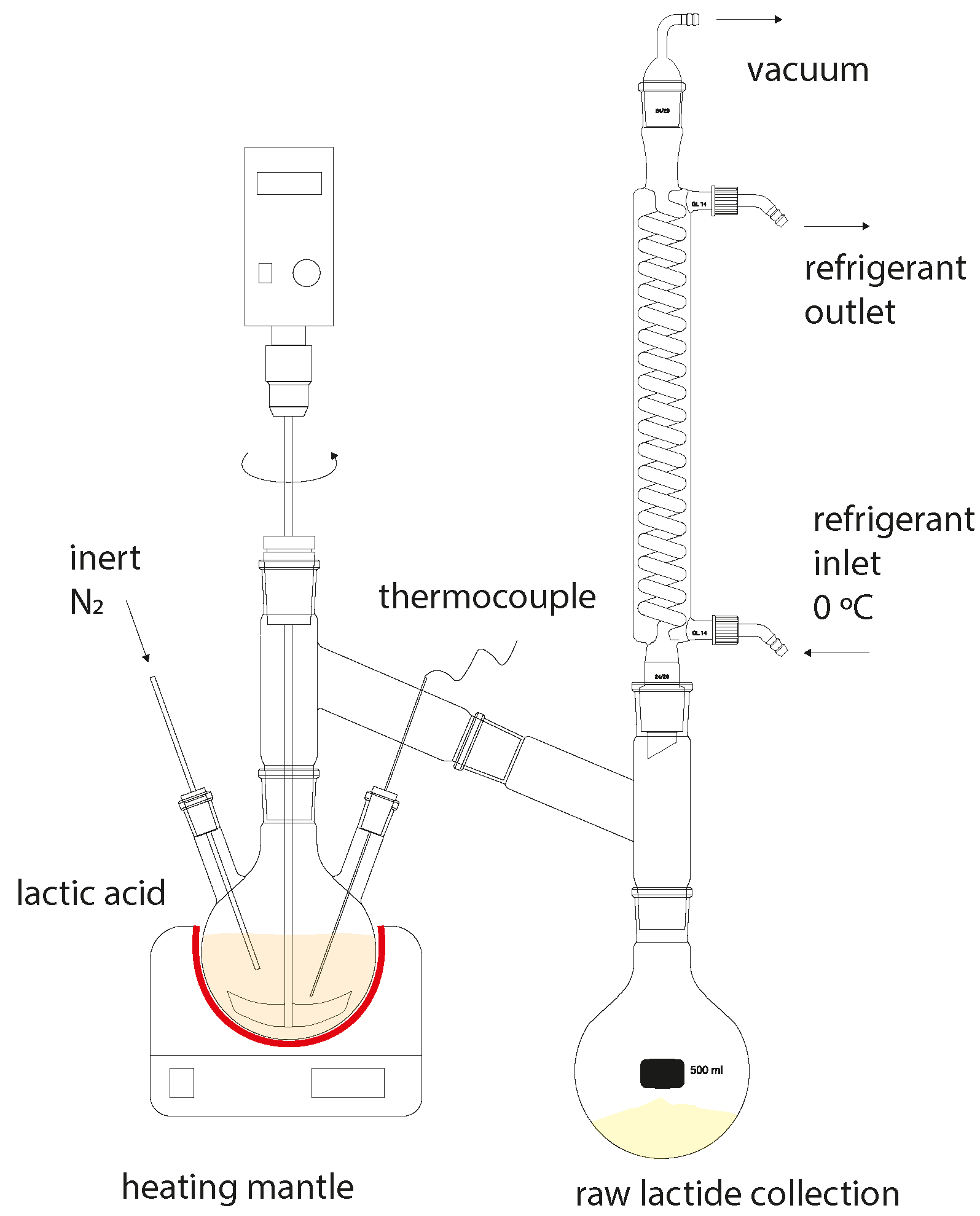
2.4. Synthesis of Lactide
2.5. Purification of Raw Lactide
2.6. Synthesis of Polylactide
2.7. Differential Scanning Calorimetry (DSC)
2.8. Gel Permeation Chromatography (GPC)
2.9. Nuclear Magnetic Resonance (NMR)
2.10. Determination of Refractive Index
3. Results and Discussion
3.1. Lactic Acid Concentration
3.2. Synthesis of Lactide and Its Characterization
3.3. Purification of Raw Lactide and Its Characterization
3.4. Synthesis of Polylactide and Its Characterization
4. Conclusions
- L-lactide was synthesized by depolymerization of PLA oligomers with the introduction of tin octoate at different concentrations into the system as a catalyst. It was found that the optimal amount of catalyst is about 0.50 wt.%.
- Several types of solvents were investigated for the recrystallization stage. It was determined that butyl acetate is the most suitable for purifying raw lactide from lactic acid and oligomers. The yield after four-fold recrystallization was 47%, and the presence of lactic acid was not detected. It was also shown, by the DSC method, that after four recrystallization iterations, the melting point of lactide stopped changing and amounted to 97.2 °C. The thermograms also lack peaks not associated with the melting of L-lactide.
- The data obtained by GPC confirmed that the purity degree of the obtained L-lactide is sufficient for the synthesis of high-molecular-weight PLA, where Mw > 200 kDa. The weight average molecular weight of the synthesized lactide was 228 kDa, and the polydispersity index was 1.94. The resulting molecular weight of the polymer is sufficient to synthesize copolymers of various molecular weights and structures based on purified lactide.
- An effective laboratory installation for the production of PLA pellets with a total loading of lactide from 100 to 400 g was developed. It makes it possible to obtain and test PLA with different molecular weights and compositions. In subsequent works, the purification of lactide will be examined in more detail, and its efficiency will be increased. Moreover, copolymers will also be synthesized in a laboratory installation in order to obtain granules with subsequent processing into materials of various forms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DSC | differential scanning calorimetry |
| GPC | gel permeation chromatography |
| NMR | nuclear magnetic resonance |
| PLA | polylactic acid or polylactide |
| ROP | ring-opening polymerization |
| THF | tetrahydrofuran |
| TMS | tetramethylsilane |
References
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Sedush, N.G.; Kalinin, K.T.; Azarkevich, P.N.; Gorskaya, A.A. Physicochemical Characteristics and Hydrolytic Degradation of Polylactic Acid Dermal Fillers: A Comparative Study. Cosmetics 2023, 10, 110. [Google Scholar] [CrossRef]
- Gomzyak, V.I.; Sedush, N.G.; Puchkov, A.A.; Polyakov, D.K.; Chvalun, S.N. Linear and Branched Lactide Polymers for Targeted Drug Delivery Systems. Polym. Sci. Ser. B 2021, 63, 257–271. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Kedik, S.A.; Zhavoronok, E.S.; Sedishev, I.P.; Panov, A.V.; Suslov, V.V.; Petrova, E.A.; Sapelnikov, M.D.; Shatalov, D.O.; Eremin, D.N. Polymers for the prolonged drug delivery systems (review). Polymers and copolymers based on lactic and glycolic acids. Drug Dev. Regist. 2013, 2, 18–32. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymer 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Aliev, G.; Naumova, A.; Melnikov, P.; Gervald, A. Recent Developments and Perspectives of Recycled Poly(ethylene terephthalate)-Based Membranes: A Review. Membranes 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kirshanov, K.A.; Gervald, A.Y.; Toms, R.V.; Lobanov, A.N. Obtaining Phthalate Substituted Post-Consumer Polyethylene Terephthalate and Its Isothermal Crystallization. Fine Chem. Technol. 2022, 17, 164–171. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V.; Balashov, M.S.; Golubkov, S.S.; Melnikov, P.V.; Gervald, A.Y. Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics. Polymers 2023, 15, 3146. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 1, 70–83. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 6, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Chen, B. Study on Synthesis and Thermal Properties of Polylactic Acid. J. Phys. Conf. Ser. 2019, 1176, 1–7. [Google Scholar] [CrossRef]
- Glotova, V.; Zamanova, M.; Yarkova, A.; Krutas, D.; Izhenbina, T.N.; Novikov, V.T. Influence of Storage Conditions on the Stability of Lactide. Procedia Chem. 2014, 10, 252–257. [Google Scholar] [CrossRef]
- Steinborn-Rogulska, I.; Rokicki, G. Solid-State Polycondensation (SSP) as a Method to Obtain High Molecular Weight Polymers. Part I. Parameters Influencing the SSP Process. Polimery 2013, 58, 3–13. [Google Scholar] [CrossRef]
- Xavier, A.M.M. Study of Lactic Acid Polycondensation and Lactide Production. Master’s Thesis, Swiss Federal Institute of Technology Zurich, Zurich, Switzerland, 2010. [Google Scholar]
- Sinclair, R.G.; Markle, R.A.; Smith, R.K. Lactide Production from Dehydration of Aqueous Lactic Acid Feed. U.S. Patent 5,274,127, 28 December 1993. [Google Scholar]
- Vink, E.T.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks polylactide (PLA) production. Polym. Degrad. Stab. 2003, 3, 403–419. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Makshina, E.; Sels, B.F. Catalytic Gas-Phase Production of Lactide from Renewable Alkyl Lactates. Angew. Chem. Int. Ed. Engl. 2018, 12, 3074–3078. [Google Scholar] [CrossRef] [PubMed]
- Upare, P.P.; Hwang, Y.K.; Chang, J.S. Synthesis of lactide from alkyl lactate via a prepolymer route. Ind. Eng. Chem. Res. 2012, 13, 4837–4842. [Google Scholar] [CrossRef]
- Ehsani, M.; Khodabakhshi, K.; Asgari, M. Lactide Synthesis Optimization: Investigation of the Temperature, Catalyst and Pressure Effects. E-Polymer 2014, 14, 353–361. [Google Scholar] [CrossRef]
- Kaihara, S.; Matsumura, S.; Mikos, A.G.; Fisher, J.P. Synthesis of Poly(L-Lactide) and Polyglycolide by Ring-Opening Polymerization. Nat. Protoc. 2007, 2, 2767–2771. [Google Scholar] [CrossRef]
- Yoo, D.K.; Dukjoon, K.; Lee, D.S. Synthesis of Lactide from Oligomeric PLA: Effects of Temperature, Pressure, and Catalyst. Macromol. Res. 2006, 14, 510–516. [Google Scholar] [CrossRef]
- Cunha, B.L.C.; Bahú, J.O.; Xavier, L.F.; Crivellin, S.; de Souza, S.D.A.; Lodi, L.; Jardini, A.L.; Filho, R.M.; Schiavon, M.I.R.B.; Concha, V.O.C.; et al. Lactide: Production Routes, Properties, and Applications. Bioengineering 2022, 4, 164. [Google Scholar] [CrossRef]
- McNeill, I.C.; Leiper, H.A. Degradation studies of some polyesters and polycarbonates—1. Polylactide: General features of the degradation under programmed heating conditions. Polym. Degrad. Stab. 1985, 11, 267–285. [Google Scholar] [CrossRef]
- Nishida, H.; Mori, T.; Hoshihara, S.; Fan, Y. Effect of tin on poly (l-lactic acid) pyrolysis. Polym. Degrad. Stab. 2003, 3, 515–523. [Google Scholar] [CrossRef]
- Feng, L.; Feng, S.; Bian, X.; Li, G. Pyrolysis mechanism of Poly (lactic acid) for giving lactide under the catalysis of tin. Polym. Degrad. Stab. 2018, 157, 212–223. [Google Scholar] [CrossRef]
- Kolstad, J.J. Crystallization kinetics of poly (L-lactide-co-meso-lactide). J. Appl. Polym. Sci. 1996, 7, 1079–1091. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 7, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.H. High molecular weight polylactic acid polymers. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; pp. 367–411. ISBN 978-3-642-08341-9. [Google Scholar]
- Jin, Z.; Tian, Y.; Wang, J. Chemistry and thermodynamic properties of lactic acid and lactide and solvent miscibility. In Poly(Lactic Acid) Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 19–25. ISBN 9780470293669. [Google Scholar]
- Muller, M.; Hess, J.; Schnell, W.; Bendix, D.; Entenmann, G. Meso-Lactide. U.S. Patent 5,214,159, 25 May 1993. [Google Scholar]
- Glotova, V.M. Improving the Technology of Synthesis and Purification of Lactide. Ph.D. Thesis, National Research Tomsk Polytechnic University, Tomsk, Russia, 2016. [Google Scholar]
- Sodergard, A.; Stolt, M. Properties of Lactic Acid Based Polymers and Their Correlation with Composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Rodgers, L.; Fry, T. Polyester/poly(methyl methacrylate) Articles and Methods to Make Them. WIPO Patent WO2021262974A1, 30 December 2021. [Google Scholar]
- Kricheldorf, H.; Weidner, S.; Scheliga, F. SnOct 2-Catalyzed and Alcohol-Initiated ROPS of L-Lactide–Control of the Molecular Weight and the Role of Cyclization. Macromol. Chem. Phys. 2022, 223, 2100464. [Google Scholar] [CrossRef]
- Gomzyak, V.I.; Bychkov, N.V.; Aduev, A.S.; Ivanova, V.A.; Koshelev, A.D.; Chvalun, S.N. Polymerization of D,L-lactide in the presence of Boltorn™ polyester polyol. Fine Chem. Technol. 2022, 17, 242–252. [Google Scholar] [CrossRef]
- Zhang, X.; Macdonald, D.A.; Goosen, M.F.A.; Mcauley, K.B. Mechanism of lactide polymerization in the presence of stannous octoate: The effect of hydroxy and carboxylic acid substances. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2965–2970. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing Technology for Poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Kricheldorf, H.; Weidner, S.; Meyer, A. High Tm poly(L-lactide)s via REP or ROPPOC of l-lactide. Polym. Chem. 2020, 11, 2182–2193. [Google Scholar] [CrossRef]
- Kricheldorf, H.; Weidner, S. Syntheses of polylactides by means of tin catalysts. Polym. Chem. 2022, 13, 1618–1647. [Google Scholar] [CrossRef]
- Kricheldorf, H.; Weidner, S. High molar mass cyclic poly(l-lactide) obtained by means of neat tin(ii) 2-ethylhexanoate. Polym. Chem. 2020, 11, 5249–5260. [Google Scholar] [CrossRef]
- Kricheldorf, H.; Weidner, S.; Falkenhagen, J. Reversible polycondensations outside the Jacobson–Stockmayer theory and a new concept of reversible polycondensations. Polym. Chem. 2021, 12, 5003–5016. [Google Scholar] [CrossRef]
- Puchkov, A.A.; Sedush, N.G.; Buzin, A.I.; Bozin, T.N.; Bakirov, A.V.; Borisov, R.S.; Chvalun, S.N. Synthesis and characterization of well-defined star-shaped poly(L-lactides). Polymer 2023, 264, 125573. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Nowicki, M.; Vuković-Kwiatkowska, I.; Nowakowska, S. Crosslinked blends of poly(lactic acid) and polyacrylates: AFM, DSC and XRD studies. J. Polym. Res. 2013, 20, 91. [Google Scholar] [CrossRef]
- Sreekumar, K.; Bindhu, B.; Veluraja, K. Perspectives of polylactic acid from structure to applications. Polym. Renew. 2021, 12, 60–74. [Google Scholar] [CrossRef]
- Zhai, W.; Ko, Y.; Zhu, W.; Wong, A.; Park, C.B. A study of the crystallization, melting, and foaming behaviors of polylactic acid in compressed CO2. Int. J. Mol. Sci. 2009, 12, 5381–5397. [Google Scholar] [CrossRef]
- Sedush, N.G.; Kadina, Y.A.; Razuvaeva, E.V.; Puchkov, A.A.; Shirokova, E.M.; Gomzyak, V.I.; Kalinin, K.T.; Kulebyakina, A.I.; Chvalun, S.N. Nanoformulations of Drugs Based on Biodegradable Lactide Copolymers with Various Molecular Structures and Archi-tectures. Nanobiotechnol. Rep. 2021, 16, 421–438. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, Z.; Wang, X.; Li, Q. Synthesis of Poly(l-lactide-co-ϵ-caprolactone) Copolymer: Structure, Toughness, and Elasticity. Polymers 2021, 13, 1270. [Google Scholar] [CrossRef] [PubMed]

























| Synthesis Number | Catalyst, % wt | Reaction Time, min | Mw | Yield of Raw Lactide, % |
|---|---|---|---|---|
| 1 | 0.25 | 78 | 220 | 69 |
| 100 | 300 | |||
| 180 | 860 | |||
| 2 | 0.50 | 78 | 360 | 68 |
| 125 | 570 | |||
| 186 | 1670 | |||
| 3 | 1.00 | 135 | 350 | 67 |
| 225 | 610 | |||
| 285 | 1995 | |||
| 345 | 7400 |
| Iteration Number | Initial TMelting, °C | |||
|---|---|---|---|---|
| Ethanol | Isopropanol | Ethyl Acetate | Butyl Acetate | |
| 1 | 84.5 | 92.9 | 83.4 | 90.6 |
| 2 | 95.6 | 94.9 | 96.0 | 95.8 |
| 3 | 94.8 | 96.7 | 96.9 | 96.9 |
| 4 | 97.1 | 97.2 | 97.2 | 97.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliev, G.; Toms, R.; Melnikov, P.; Gervald, A.; Glushchenko, L.; Sedush, N.; Chvalun, S. Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology. Polymers 2024, 16, 624. https://doi.org/10.3390/polym16050624
Aliev G, Toms R, Melnikov P, Gervald A, Glushchenko L, Sedush N, Chvalun S. Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology. Polymers. 2024; 16(5):624. https://doi.org/10.3390/polym16050624
Chicago/Turabian StyleAliev, Gadir, Roman Toms, Pavel Melnikov, Alexander Gervald, Leonid Glushchenko, Nikita Sedush, and Sergei Chvalun. 2024. "Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology" Polymers 16, no. 5: 624. https://doi.org/10.3390/polym16050624
APA StyleAliev, G., Toms, R., Melnikov, P., Gervald, A., Glushchenko, L., Sedush, N., & Chvalun, S. (2024). Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology. Polymers, 16(5), 624. https://doi.org/10.3390/polym16050624