Inhibition of Free Radical Polymerization: A Review
Abstract
1. Introduction
2. Styrene Polymerization Inhibition
| Inhibitor | Solvent Used in the Stock Solution | μo | ηo | ωo (eV) | No (eV) | Peak Surface Area | % Inhibition |
|---|---|---|---|---|---|---|---|
| TempoH | Methanol | −1.92 | 7.62 | 0.2418 | 0 | 467.7 | 12.5 |
| Amino carboxy | Toluene | −3.04 | 3.88 | 1.191 | 0.75 | 469.5 | 12.1 |
| Carboxy | THF (*) | −3.22 | 3.85 | 1.351 | 0.58 | 358.0 | 33 |
| Amino | Toluene | −3.05 | 3.9 | 1.193 | 0.73 | 334.5 | 37.4 |
| Tempo | Toluene | −3.04 | 3.89 | 1.192 | 0.74 | 321.2 | 39.9 |
| Acetamido | Ethanol | −3.22 | 3.84 | 1.350 | 0.59 | 281 | 47.4 |
| Butoxy | Toluene | −3.15 | 3.9 | 1.272 | 0.63 | 270.9 | 49.3 |
| Oxo | Toluene | −3.45 | 3.9 | 1.526 | 0.33 | 265.9 | 50.3 |
| Methacrylate | Toluene | −3.25 | 3.9 | 1.350 | 0.53 | 249.4 | 53.3 |
| Methoxy | Toluene | −3.14 | 3.88 | 1.270 | 0.65 | 241.6 | 54.8 |
| Benzoate | Toluene | −3.23 | 3.85 | 1.359 | 0.57 | 206.8 | 61.3 |
| Hydroxy | Toluene | −3.15 | 3.9 | 1.272 | 0.63 | 202.0 | 62.2 |
| Mono radical | −3.14 | 2.68 | 1.84 | 1.25 |
2.1. TEMPO Inhibitors
2.2. Phenolic Inhibitors
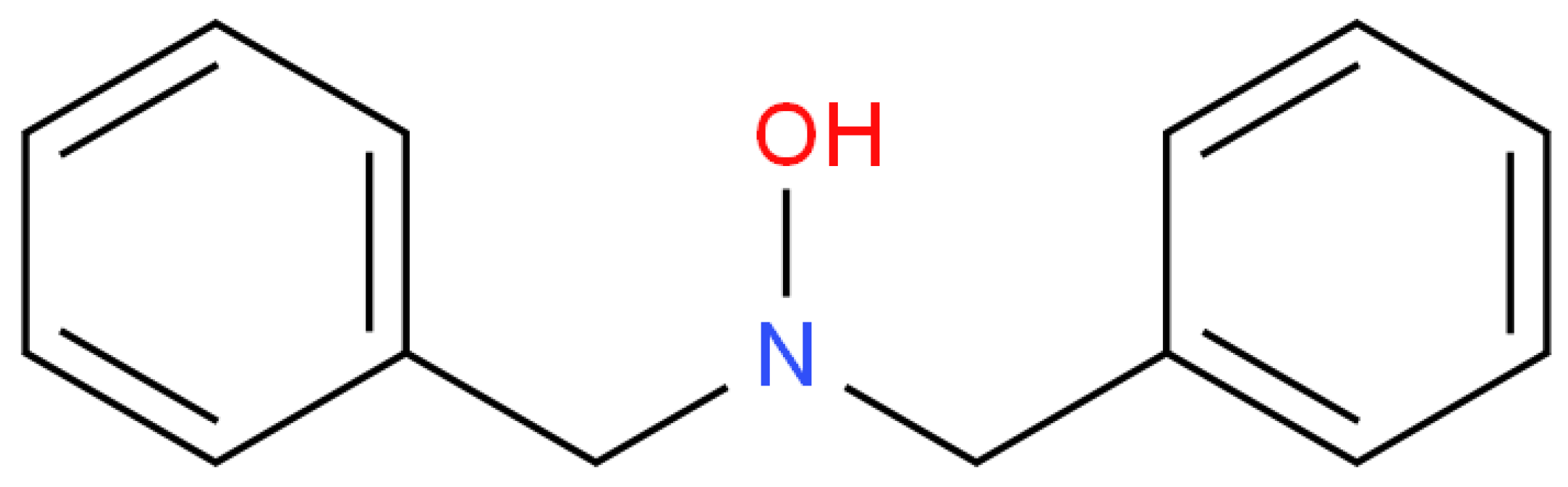
2.3. N,N-Dibenzyl hydroxylamine Inhibitor
2.4. 2,5-Di-tert-butyl-hydroquinone Inhibitor
3. Methyl Methacrylate Polymerization Inhibition
4. Acrylic Acid Polymerization Inhibition
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dakshinamoorthy, D.; Khopkar, A.R.; Louvar, J.F.; Ranade, V.V. CFD Simulations to Study Shortstopping Runaway Reactions in a Stirred Vessel. J. Loss Prev. Process Ind. 2004, 17, 355–364. [Google Scholar] [CrossRef]
- Ampelli, C.; di Bella, D.; Maschio, G.; Russo, A. Calorimetric Study of the Inhibition of Runaway Reactions during Methylmethacrylate Polymerization Processes. J. Loss Prev. Process Ind. 2006, 19, 419–424. [Google Scholar] [CrossRef]
- Ovejero, G.; Romero, M.D.; Díaz, I.; Mestanza, M.; Díez, E. Bentonite as an Alternative Adsorbent for the Purification of Styrene Monomer: Adsorption Kinetics, Equilibrium and Process Design. Adsorpt. Sci. Technol. 2010, 28, 101–123. [Google Scholar] [CrossRef]
- Díaz, I.; Langston, P.; Ovejero, G.; Romero, M.D.; Díez, E. Purification Process Design in the Production of Styrene Monomer. Chem. Eng. Process. Process Intensif. 2010, 49, 367–375. [Google Scholar] [CrossRef]
- Merck. Available online: https://www.sigmaaldrich.com/IN/en (accessed on 1 January 2023).
- Middle, K.V.; Bussey, R.; Cusco, L.; Kerr, D.; Snee, T.J. Reaction Inhibition in the Control of Exothermic Runaway; WIT Press: Ashurst, UK, 2003. [Google Scholar]
- Louvar, J.F.; Crowl, D.A. Chemical Process Safety: Fundamentals with Applications, 4th ed.; Pearson Education: London, UK, 2020. [Google Scholar]
- Saada, R.; Patel, D.; Saha, B. Causes and Consequences of Thermal Runaway Incidents—Will They Ever Be Avoided? Process Saf. Environ. Prot. 2015, 97, 109–115. [Google Scholar] [CrossRef]
- Sales, J.; Mushtaq, F.; Christou, M.D.; Nomen, R. Study of Major Accidents Involving Chemical Reactive Substances. Process Saf. Environ. Prot. 2007, 85, 117–124. [Google Scholar] [CrossRef]
- Barton, J.A.; Nolan, P.F. Hazards X: Process Safety in Fine and Speciality Chemical Plants. In I Chem E Symposium Series; CRC Press: Boca Raton, FL, USA, 1989; pp. 3–18. [Google Scholar]
- The US Chemical Safety and Hazard Investigation Board. Available online: https://www.csb.gov/ (accessed on 1 November 2022).
- Chen, C.-C.; Shu, C.-M.; Chang, R.-S.; Shyu, M.-L.; Chen, S.-C. Thermal Hazard Analysis of Styrene Monomer at Low Temperature Conditions during Storage and Transportation. In Proceedings of the Conference of NATAS, Pittsburg, PA, USA, 1–4 September 2002; pp. 10–25. [Google Scholar]
- Liao, C.C.; Wu, S.H.; Su, T.S.; Shyu, M.L.; Shu, C.M. Thermokinetics Evaluation and Simulations for the Polymerization of Styrene in the Presence of Various Inhibitor Concentrations. J. Therm. Anal. Calorim. 2006, 85, 65–71. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration (OSHA). Available online: https://www.osha.gov/ (accessed on 3 November 2022).
- JST Failure Knowledge Database. Available online: http://www.sozogaku.com/fkd/en/ (accessed on 4 November 2022).
- Wu, Y.-C.; Laiwang, B.; Shu, C.-M. Investigation of an Explosion at a Styrene Plant with Alkylation Reactor Feed Furnace. Appl. Sci. 2019, 9, 503. [Google Scholar] [CrossRef]
- Saunders, K.J. Polystyrene and Styrene Copolymers. In Organic Polymer Chemistry; Springer: Dordrecht, The Netherlands, 1988; pp. 76–89. [Google Scholar]
- Brighton, C.A.; Pritchard, G. Styrene Polymers: Technology and Environmental Aspects; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Kruse, T.M.; Woo, O.S.; Broadbelt, L.J. Detailed Mechanistic Modeling of Polymer Degradation: Application to Polystyrene. Chem. Eng. Sci. 2001, 56, 971–979. [Google Scholar] [CrossRef]
- Braun, D. Poly(Vinyl Chloride) on the Way from the 19th Century to the 21st Century. J. Polym. Sci. A Polym. Chem. 2004, 42, 578–586. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastics Materials; Butterworth-Heinemann: Oxford, UK, 1999. [Google Scholar]
- Fox, T.G.; Flory, P.J. The Glass Temperature and Related Properties of Polystyrene. Influence of Molecular Weight. J. Polym. Sci. 1954, 14, 315–319. [Google Scholar] [CrossRef]
- Cox, W.P.; Merz, E.H. Correlation of Dynamic and Steady Flow Viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- ULLMANN’S Editorial Team. Ullmann′s Polymers and Plastics: Products and Processes 4 Volume Set, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 1. [Google Scholar]
- Mandal, B.M. Fundamentals of Polymerization; World Scientific Publishing Co Pvt Ltd.: Singapore, 2013. [Google Scholar]
- Moad, G.; Solomon, D.H. The Chemistry of Radical Polymerization, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Mayo, F.R.; Gregg, R.A. Effects of Inhibitors on the Polymerization of Styrene. J. Am. Chem. Soc. 1948, 70, 1284–1286. [Google Scholar] [CrossRef]
- Scott, G. (Ed.) Developments in Polymer Stabilisation—8; Springer: Dordrecht, The Netherlands, 1987; ISBN 978-94-010-8034-7. [Google Scholar]
- Conte, M.; Ma, Y.; Loyns, C.; Price, P.; Rippon, D.; Chechik, V. Mechanistic Insight into TEMPO-Inhibited Polymerisation: Simultaneous Determination of Oxygen and Inhibitor Concentrations by EPR. Org. Biomol. Chem. 2009, 7, 2685. [Google Scholar] [CrossRef]
- Hemmerich, R.H. Method of Minimizing Styrene Polymerization during Storage. U.S. Patent US2867672A, 6 January 1959. [Google Scholar]
- Roling, P.V. Methods and Compositions for Inhibiting Styrene Polymerization. U.S. Patent US4929778, 29 May 1990. [Google Scholar]
- Campbell, D.N. Polymerization Inhibitor. U.S. Patent US2965685A, 20 December 1960. [Google Scholar]
- Watson, J.M. Polymerization Inhibitor for Vinyl Aromatic Compounds. U.S. Patent US4105506A, 8 August 1978. [Google Scholar]
- Butler, J.R.; Watson, J.M.; Kendall, D.L.; Mikkelson, K.A. Polymerization Inhibition Process for Vinyl Aromatic Compounds. Canada Patent CA1224811A, 28 July 1987. [Google Scholar]
- Wadhwa, K.; Hennissen, J.; Shetty, S.; Pensini, E.; Frissen, M.; Leen, S.; Kwakkenbos, G.; Geijselaers, C. Influence of Substitution of Various Functional Groups on Inhibition Efficiency of TEMPO Analogues on Styrene Polymerization. J. Polym. Res. 2017, 24, 201. [Google Scholar] [CrossRef]
- Boutevin, B.; Bertin, D. Controlled Free Radical Polymerization of Styrene in the Presence of Nitroxide Radicals I. Thermal Initiation. Eur. Polym. J. 1999, 35, 815–825. [Google Scholar] [CrossRef]
- Connolly, T.J.; Scaiano, J.C. Reactions of the “Stable” Nitroxide Radical TEMPO. Relevance to “Living” Free Radical Polymerizations and Autopolymerization of Styrene. Tetrahedron Lett. 1997, 38, 1133–1136. [Google Scholar] [CrossRef]
- Shim, S.E.; Oh, S.; Chang, Y.H.; Jin, M.-J.; Choe, S. Solvent Effect on TEMPO-Mediated Living Free Radical Dispersion Polymerization of Styrene. Polymer 2004, 45, 4731–4739. [Google Scholar] [CrossRef]
- Cunningham, M.F. Controlled/Living Radical Polymerization in Aqueous Dispersed Systems. Prog. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Alam, M.N.; Zetterlund, P.B.; Okubo, M. TEMPO-Mediated Radical Polymerization of Styrene in Aqueous Miniemulsion: Macroinitiator Concentration Effects. Polymer 2008, 49, 3428–3435. [Google Scholar] [CrossRef]
- Cunningham, M.F.; Ng, D.C.T.; Milton, S.G.; Keoshkerian, B. Low Temperature TEMPO-Mediated Styrene Polymerization in Miniemulsion. J. Polym. Sci. A Polym. Chem. 2006, 44, 232–242. [Google Scholar] [CrossRef]
- Knoop, C.A.; Studer, A. Hydroxy- and Silyloxy-Substituted TEMPO Derivatives for the Living Free-Radical Polymerization of Styrene and n-Butyl Acrylate: Synthesis, Kinetics, and Mechanistic Studies. J. Am. Chem. Soc. 2003, 125, 16327–16333. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, K.; Smith, J.-A.; Cunningham, M.F. Synthesis of Polystyrene-Block-Poly(Butyl Acrylate) Copolymers Using Nitroxide-Mediated Living Radical Polymerization in Miniemulsion. Macromol. Rapid Commun. 2001, 22, 957–961. [Google Scholar] [CrossRef]
- Greszta, D.; Matyjaszewski, K. Mechanism of Controlled/“Living” Radical Polymerization of Styrene in the Presence of Nitroxyl Radicals. Kinetics and Simulations. Macromolecules 1996, 29, 7661–7670. [Google Scholar] [CrossRef]
- Jupp, A.R.; Johnstone, T.C.; Stephan, D.W. The Global Electrophilicity Index as a Metric for Lewis Acidity. Dalton Trans. 2018, 47, 7029–7035. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C. A New Equation for Calculation of Chemical Hardness of Groups and Molecules. Mol. Phys. 2015, 113, 1311–1319. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. Global and Local Reactivity Indices for Electrophilic/Nucleophilic Free Radicals. Org. Biomol. Chem. 2013, 11, 4350–4358. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A Further Exploration of a Nucleophilicity Index Based on the Gas-Phase Ionization Potentials. J. Mol. Struct. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
- de Vleeschouwer, F.; van Speybroeck, V.; Waroquier, M.; Geerlings, P.; de Proft, F. Electrophilicity and Nucleophilicity Index for Radicals. Org. Lett. 2007, 9, 2721–2724. [Google Scholar] [CrossRef]
- Darvishi, A.; Rahimpour, M.R.; Raeissi, S. A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization. Processes 2019, 7, 677. [Google Scholar] [CrossRef]
- Perez, V.V.; Martin, J.F.; Roling, P.V. Inhibiting Polymerization of Vinyl Aromatic Compounds. European Patent EP0240297A1, 7 October 1987. [Google Scholar]
- Klemchuk, P. Substituted Hydroxylamine Anti-Oxidants. U.S. Patent US3778464A, 11 December 1973. [Google Scholar]
- N,N-Dibenzylhydroxylamine. Available online: https://www.guidechem.com/encyclopedia/n-n-dibenzylhydroxylamine-dic5957.html (accessed on 15 November 2022).
- Baldassarri, C. Hydroxylamine-Based Inhibitors of Auto-Initiated Styrene Polymerization; University of York: York, UK, 2014. [Google Scholar]
- Aleksandrov, A.L. Methyl-and Tert-Butyl-Substituted Hydroquinones and Semiquinone Radicals: Bond Strength Estimates, Enthalpy of Formation, and the Rate Constants of Their Reactions with Peroxy Radicals. Kinet. Catal. 2006, 47, 672–676. [Google Scholar] [CrossRef]
- 2,5-Di-Tert-Butyl-Hydroquinone. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8258782.htm (accessed on 15 May 2022).
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The Unusual Reaction of Semiquinone Radicals with Molecular Oxygen. J. Org. Chem. 2008, 73, 1830–1841. [Google Scholar] [CrossRef]
- Engel, P.S.; Park, H.J.; Mo, H.; Duan, S. The Reaction of α-Phenethyl Radicals with 1,4-Benzoquinone and 2,6-Di-Tert-Butyl-1,4-Benzoquinone. Tetrahedron 2010, 66, 8805–8814. [Google Scholar] [CrossRef]
- Shushunova, N.Y.; Chesnokov, S.A. Inhibition of Polymerization of Methyl Methacrylate by an Ortho-Benzoquinone-Amine System. Polym. Sci. Ser. B 2009, 51, 427–437. [Google Scholar] [CrossRef]
- Acrylic Acid. Available online: https://www.alliedmarketresearch.com/acrylic-acid-market (accessed on 8 January 2023).
- Acrylic Acid Market Analysis. Available online: https:/www.chemanalyst.com/industry-report/acrylic-acid-market-287 (accessed on 3 June 2022).
- Schulze, S.; Vogel, H. Aspects of the Safe Storage of Acrylic Monomers: Kinetics of the Oxygen Consumption. Chem. Eng. Technol. 1998, 21, 829–837. [Google Scholar] [CrossRef]
- Li, R.; Schork, F.J. Modeling of the Inhibition Mechanism of Acrylic Acid Polymerization. Ind. Eng. Chem. Res. 2006, 45, 3001–3008. [Google Scholar] [CrossRef]
- Barnes, C.E. Mechanism of Vinyl Polymerization. I. Role of Oxygen. J. Am. Chem. Soc. 1945, 67, 217–220. [Google Scholar] [CrossRef]
- Barnes, C.E.; Elofson, R.M.; Jones, G.D. Role of Oxygen in Vinyl Polymerization. II. Isolation and Structure of the Peroxides of Vinyl Compounds. J. Am. Chem. Soc. 1950, 72, 210–215. [Google Scholar] [CrossRef]
- Levy, L.B. Inhibition of Acrylic Acid Polymerization by Phenothiazine and P-Methoxyphenol. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 1505–1515. [Google Scholar] [CrossRef]
- Levy, L.B. Inhibition of Acrylic Acid Polymerization by Phenothiazine and P-Methoxyphenol. II. Catalytic Inhibition by Phenothiazine. J. Polym. Sci. A Polym. Chem. 1992, 30, 569–576. [Google Scholar] [CrossRef]
- Cutie, S.S.; Henton, D.E.; Powell, C.; Reim, R.E.; Smith, P.B.; Staples, T.L. The Effects of MEHQ on the Polymerization of Acrylic Acid in the Preparation of Superabsorbent Gels. J. Appl. Polym. Sci. 1997, 64, 577–589. [Google Scholar] [CrossRef]
- Mosnáček, J.; Nicolaÿ, R.; Kar, K.K.; Fruchey, S.O.; Cloeter, M.D.; Harner, R.S.; Matyjaszewski, K. Efficient Polymerization Inhibition Systems for Acrylic Acid Distillation: New Liquid-Phase Inhibitors. Ind. Eng. Chem. Res. 2012, 51, 3910–3915. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Valgimigli, L.; Gigmes, D.; Tordo, P. Bond Dissociation Energies of the N−H Bond and Rate Constants for the Reaction with Alkyl, Alkoxyl, and Peroxyl Radicals of Phenothiazines and Related Compounds. J. Am. Chem. Soc. 1999, 121, 11546–11553. [Google Scholar] [CrossRef]
- Becker, H.; Vogel, H. Phenothiazine as Stabilizer for Acrylic Acid. Chem. Eng. Technol. 2006, 29, 931–936. [Google Scholar] [CrossRef]





















| Date | Location | Fatalities | Injuries | Hazard | Chemical(s) |
|---|---|---|---|---|---|
| 06/27/1998 | Channahol, IL, USA | 0 | 1 | 1. leakage | 1. ethylbenzene 2. styrene |
| 06/23/1999 | Pasadena, TX, USA | 2 | 4 | 1. fire 2. explosion | 1. styrene 2. butadiene |
| 07/25/1999 | Hong Kong, China | 0 | 0 | 1. explosion | 1. styrene 2. trichloroethylene |
| 09/13/1999 | Newton, MA, USA | 0 | 22 | 1. leakage | 1. styrene |
| 02/05/2000 | Hong Kong, China | 0 | 0 | 1. leakage | 1. styrene |
| 03/14/2000 | Fredericton, NB, Canada | 0 | 0 | 1. leakage | 1. alcohol 2. styrene |
| 03/27/2000 | Pasadena, TX, USA | 1 | 71 | 1. leakage 2. fire 3. explosion | 1. butadiene 2. Cyclohexane 3. styrene |
| 10/31/2000 | Channel Islands, France | 0 | 0 | 1. leakage | 1. isopropyl alcohol 2. methyl ethyl ketone 3. styrene |
| 04/04/2001 | Zhejiang, China | 0 | 0 | 1. leakage | 1. styrene |
| 04/17/2001 | Shanghai, China | 0 | 0 | 1. leakage | 1. styrene |
| 10/29/2001 | Marietta, OH, USA | 0 | 0 | 1. leakage | 1. styrene |
| 02/13/2003 | Hangzhou, China | 0 | 0 | 1. leakage | 1. styrene |
| 03/12/2003 | Yeochon, Republic of Korea | 1 | 0 | 1. explosion | 1. styrene |
| 04/08/2004 | Jiangsu, China | 6 | 8 | 1. leakage | 1. styrene |
| 06/07/2004 | Canada, USA | 0 | 0 | 1. leakage | 1. styrene |
| Date | Location | Consequences | |
|---|---|---|---|
| Injury | Fatality | ||
| 01/21/1998 | Kaohsiung, Taiwan | 4 | 0 |
| 12/24/1998 | Kanagawa, Japan | 0 | 0 |
| 06/27/1998 | Channahon, IL, USA | 1 | 0 |
| 06/23/1999 | Pasadena, TX, USA | 21 | 2 |
| 10-06-1999 | Chiayi, Taiwan | 1 | 0 |
| 03/27/2000 | Pasadena, TX, USA | 71 | 1 |
| 04-02-2003 | Addyston, OH, USA | 0 | 1 |
| 04-08-2004 | Jiangsu, China | 8 | 6 |
| 06/30/2005 | Mesa, AZ, USA | 0 | 1 |
| 07/11/2006 | Mainland China | Only Equipment Damage | |
| 03/05/2008 | Mainland China | Only Equipment Damage | |
| 21/09/2014 | Fairfield, AL, USA | 1 | 2 |
| 06/02/2017 | Taiwan | 4 | 0 |
| 29/01/2018 | Taiwan | Only Equipment Damage | |
| 07/05/2020 | Visakhapatnam, India | 585 | 13 |
| 14/04/2022 | Andhra Pradesh, India | 12 | 6 |
| Inhibitor | Global Chemical Potential (μo) | Chemical Hardness (ηo) | Electrophilicity (ωo) |
|---|---|---|---|
| BHT | −2.6654 | 2.9900 | 1.1880 |
| TBC | −3.2072 | 2.7878 | 1.8448 |
| TBHQ | −3.2376 | 2.6937 | 1.9457 |
| DTBMP | −2.5161 | 2.8021 | 1.1297 |
| MEHQ | −3.2282 | 2.6478 | 1.9680 |
| After 4 h of Operation | ||||||
|---|---|---|---|---|---|---|
| Inhibitor | Weight (g) | Growth Percentage | Outlet Mass Fraction (wt.%) | Conversion (%) | ||
| Styrene | Dimer | Trimer | ||||
| BHT | 0.285 | 42.50 | 99.839 | 0.022 | 0.010 | 0.111 |
| TBC | 0.305 | 52.65 | 99.811 | 0.028 | 0.012 | 0.139 |
| TBHQ | 0.363 | 81.25 | 99.749 | 0.034 | 0.015 | 0.201 |
| DTBMP | 0.233 | 16.40 | 99.902 | 0.012 | 0.005 | 0.048 |
| MEHQ | 0.387 | 93.35 | 99.730 | 0.053 | 0.023 | 0.251 |
| After 8 h of Operation | ||||||
| BHT | 0.399 | 99.50 | 99.713 | 0.036 | 0.015 | 0.237 |
| TBC | 0.470 | 135.08 | 99.643 | 0.038 | 0.016 | 0.307 |
| TBHQ | 0.526 | 162.81 | 99.568 | 0.054 | 0.023 | 0.382 |
| DTBMP | 0.319 | 59.47 | 99.812 | 0.019 | 0.008 | 0.138 |
| MEHQ | 0.630 | 215.16 | 99.493 | 0.062 | 0.027 | 0.491 |
| Inhibitor Mixture | (BQ)] × 103, mol/L | (Amine), mol/L | r × 104, mol/(l s) |
|---|---|---|---|
| BQ-1–DMA | 4.25 | 0.08 | 2.8 |
| 4.25 | 0.21 | 2.8 | |
| 4.25 | 0.425 | 2.8 | |
| 2.12 | 0.21 | 3.0 | |
| 3.19 | 0.21 | 3.0 | |
| 8.5 | 0.21 | 2.5 | |
| BQ-1–DMPA | 4.25 | 0.08 | 2.1 |
| 4.25 | 0.21 | 2.1 | |
| 4.25 | 0.425 | 2.1 | |
| 2.12 | 0.21 | 2.6 | |
| 3.19 | 0.21 | 2.6 | |
| 8.5 | 0.21 | 1.8 | |
| BQ-2–DMA | 4.25 | 0.425 | 3.0 |
| BQ-3–DMA | 4.25 | 0.425 | 3.0 |
| BQ-4–DMA | 4.25 | 0.425 | 3.0 |
| BQ-5–DMA | 4.25 | 0.425 | 2.7 |
| S. No. | Free Radical Inhibitor | Monomer | Advantages | Disadvantages |
|---|---|---|---|---|
| 1. | TEMPO and TEMPO-derivatives | Ethylene, Butadiene, Vinyl monomers, and MMA. | 1. High efficacy. 2. Cheap cost. 3. High stability. | 1.It shows inhibitory properties at high concentration. 2. Toxic. |
| 2. | 4-methoxyphenol (MEHQ) | Styre1ne, Acrylic Acid. | 1. Stable at higher temperatures. | 1. Not effective in the absence of oxygen. |
| 3. | Phenothiazine (PTZ) | Acrylic Acid (AA). | 1. Highly effective for AA. 2. Effective even in absence of oxygen. 3. Highly efficient. | 1. Toxic. |
| 4. | Hydroquinone (Hq) | Methyl methacrylate (MMA), Vinyl acetate, Acrylic Acid. | 1. Oxygen-independent inhibitor. | 1. Toxic. |
| 5. | Ortho-benzoquinone | Methyl methacrylate (MMA). | 1. Oxygen-independent inhibitor. | 1. Toxic. |
| 6. | N,N-dimethylaniline (DMA) | Methyl methacrylate (MMA). | 1. Stable at high temperatures. | 1. Quite expensive. |
| 7. | N,N-dimethylisopropanolamine (DMPA) | Methyl methacrylate (MMA). | 1. Less corrosive. | 1. Toxic. |
| 8. | 4-tert-butylcatechol (TBC) | Styrene, Butadiene. | 1. Easy to remove prior to polymerization by alkalinewashing, by distillation, or by passing through an activated alumina column. | 1. Has low vacuum pressure and hence in gaseous processes. |
| 9. | Tert-butyl hydroquinone (TBHQ) | Styrene, Butadiene. | 1. Stable at high temperatures. 2. Non-toxic. 3. Does not cause discoloration. | 1. Fire hazard. |
| 10. | 2,6-di-tert-butyl-4-methoxyphenol (DTBMP) | Styrene. | 1. Easily handled liquid product. 2. Stable at higher temperatures. | 2. Toxic. |
| 11. | 2,6-Di-tert-butyl-4-methylphenol (BHT) | Styrene, Butadiene. MMA, Acrylic Acid. | 1. Non-toxic. | 1. Fire hazards. |
| 12. | N,N-Dibenzylhydroxylamine (DBHA) | Styrene. | 1. Non-toxic. | 1. Causes eye and skin irritation. 2. Not efficient in the absence of oxygen. |
| 13. | 2,5-Di-tert-butyl-hydroquinone (DTBHQ) | Styrene. | 1. Highly effective and widely used in industrial processes. | 1. Highly toxic. |
| 14. | 2,4-dinitrophenol (DNP) | Styrene. | 1. Effective even in absence of oxygen. | 1. Toxic. 2. Fire hazard. |
| 15. | 2,4-dinitro-6-sec-butyl phenol (DNBP) | Styrene. | 1. Effective even in the absence of oxygen. | 1. Toxic. |
| 16. | 2,6-Dinitro-p-cresol | Vinyl aromatic monomers, such as styrene monomer. | 1. Stable polymerization inhibiting performance. 2. Low unit consumption. 3. Low toxicity. | It is solid and can become unstable if subjected to temperatures above its melting point and may explode. |
| 17. | Phenylenediamines | Vinyl aromatic monomers, such as styrene monomer. | 1. Non-toxic. | 1. Ineffective in the absence of oxygen. |
| 18. | Aryl amines | Styrene. | 1. Efficient even at reduced concentration. | 1. Fire hazard. |
| 19. | p-Benzoquinone | Styrene, Acrylic Acid, Methyl methacrylate. | 1. Oxygen-independent inhibitor. | 1. It is very difficult to obtain color-free monomers when they have been inhibited with p-benzoquinone. |
| 20. | Oxygen | Styrene,Methyl methacrylate. | 1. Easily available. 2. Cheap. | 1. Needs instrumentation to control the amount of flow. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maafa, I.M. Inhibition of Free Radical Polymerization: A Review. Polymers 2023, 15, 488. https://doi.org/10.3390/polym15030488
Maafa IM. Inhibition of Free Radical Polymerization: A Review. Polymers. 2023; 15(3):488. https://doi.org/10.3390/polym15030488
Chicago/Turabian StyleMaafa, Ibrahim M. 2023. "Inhibition of Free Radical Polymerization: A Review" Polymers 15, no. 3: 488. https://doi.org/10.3390/polym15030488
APA StyleMaafa, I. M. (2023). Inhibition of Free Radical Polymerization: A Review. Polymers, 15(3), 488. https://doi.org/10.3390/polym15030488






