Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Materials
2.2. Evaluation of Antibacterial Effects
2.2.1. Bacterial Culture and Growth Conditions
2.2.2. Morphological Assessment of the Bacteria Attached to the Specimens
2.2.3. Assessment of the Antibacterial Effect by Counting Colony-Forming Units
2.2.4. Evaluation of Bacterial Viability
2.3. Analysis of pH Variations
2.4. Evaluation of Zinc Ion Release
2.5. Assessment of the Depth of Cure
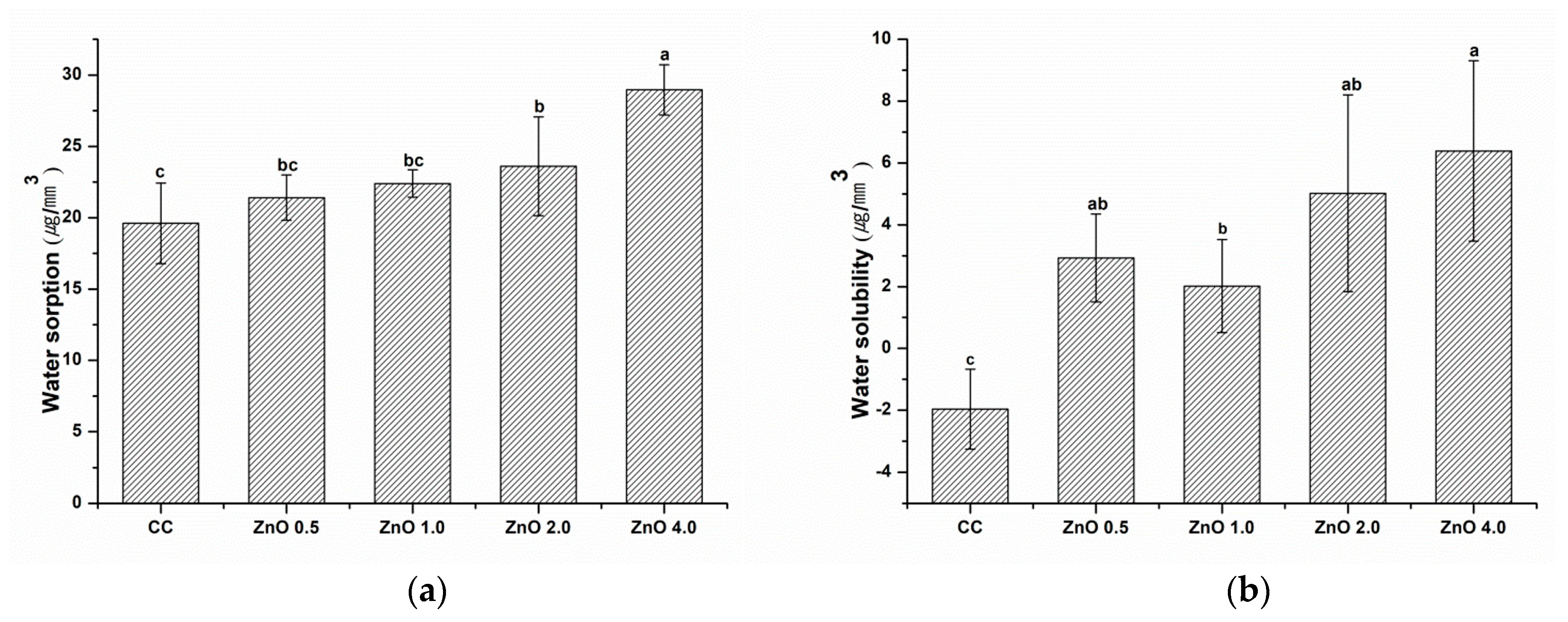
2.6. Water Sorption and Solubility
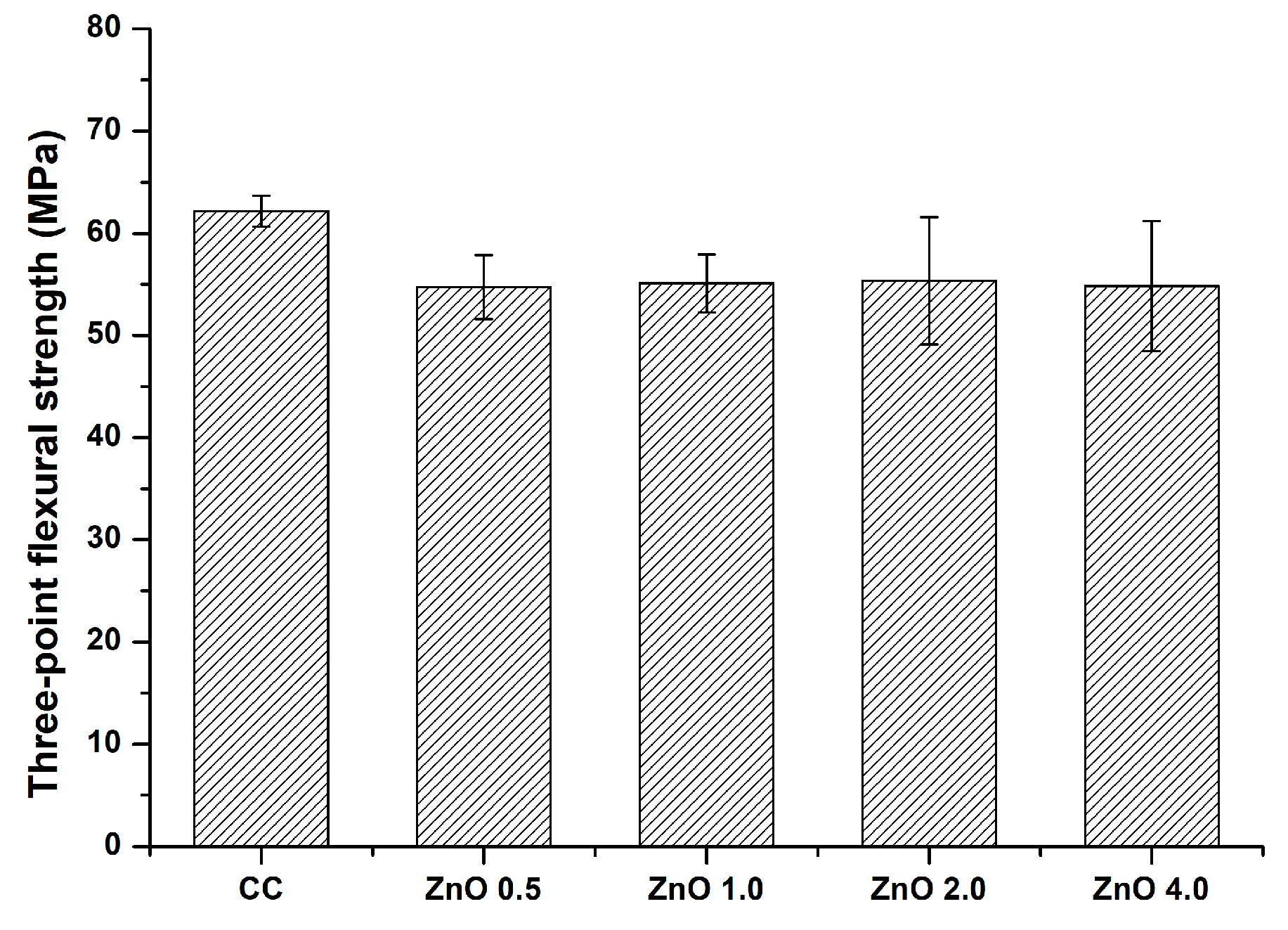
2.7. Three-Point Flexural Strength Assessment
2.8. Statistical Analysis
3. Results
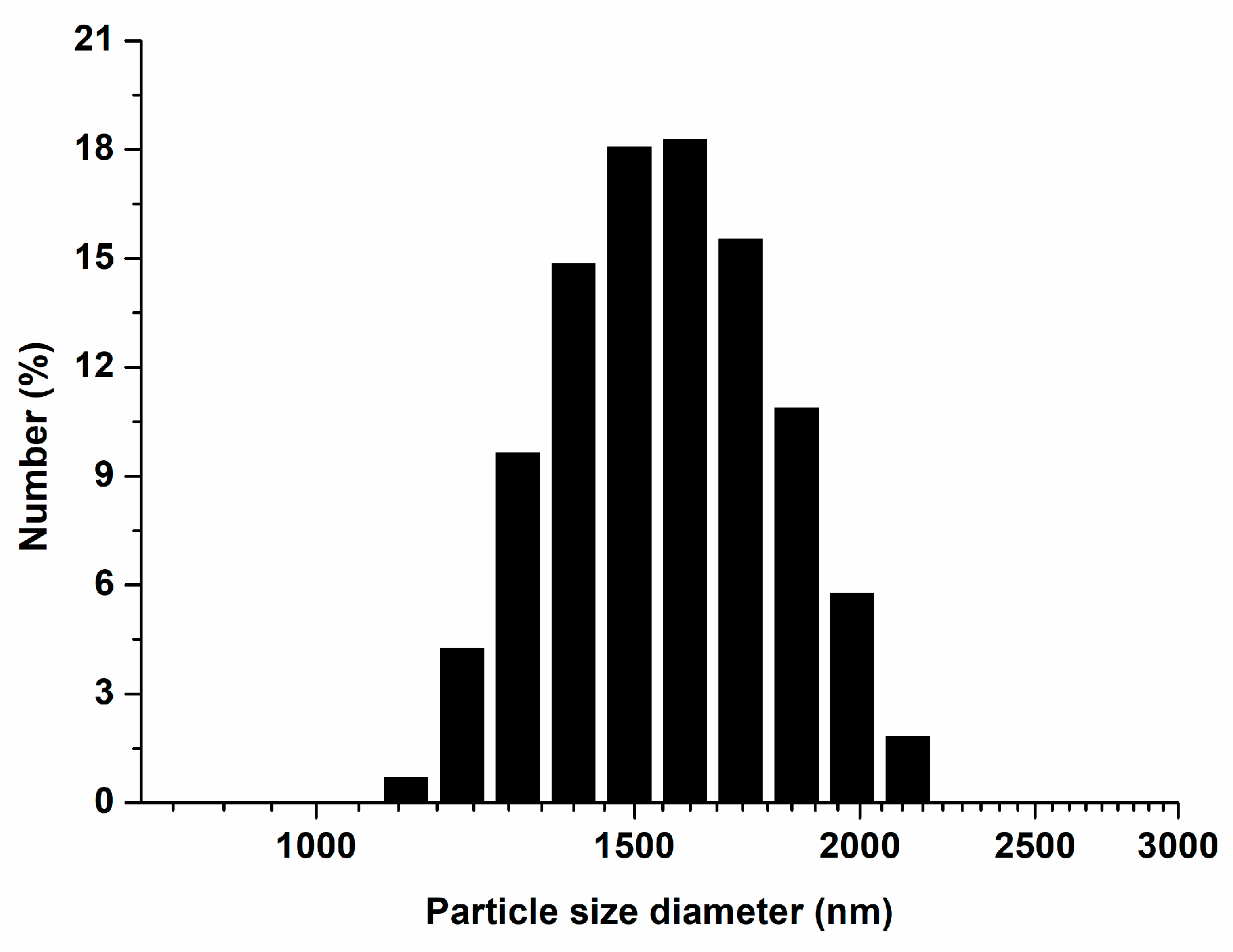
3.1. Characterization of ZnO NPs
3.2. Antibacterial Effects
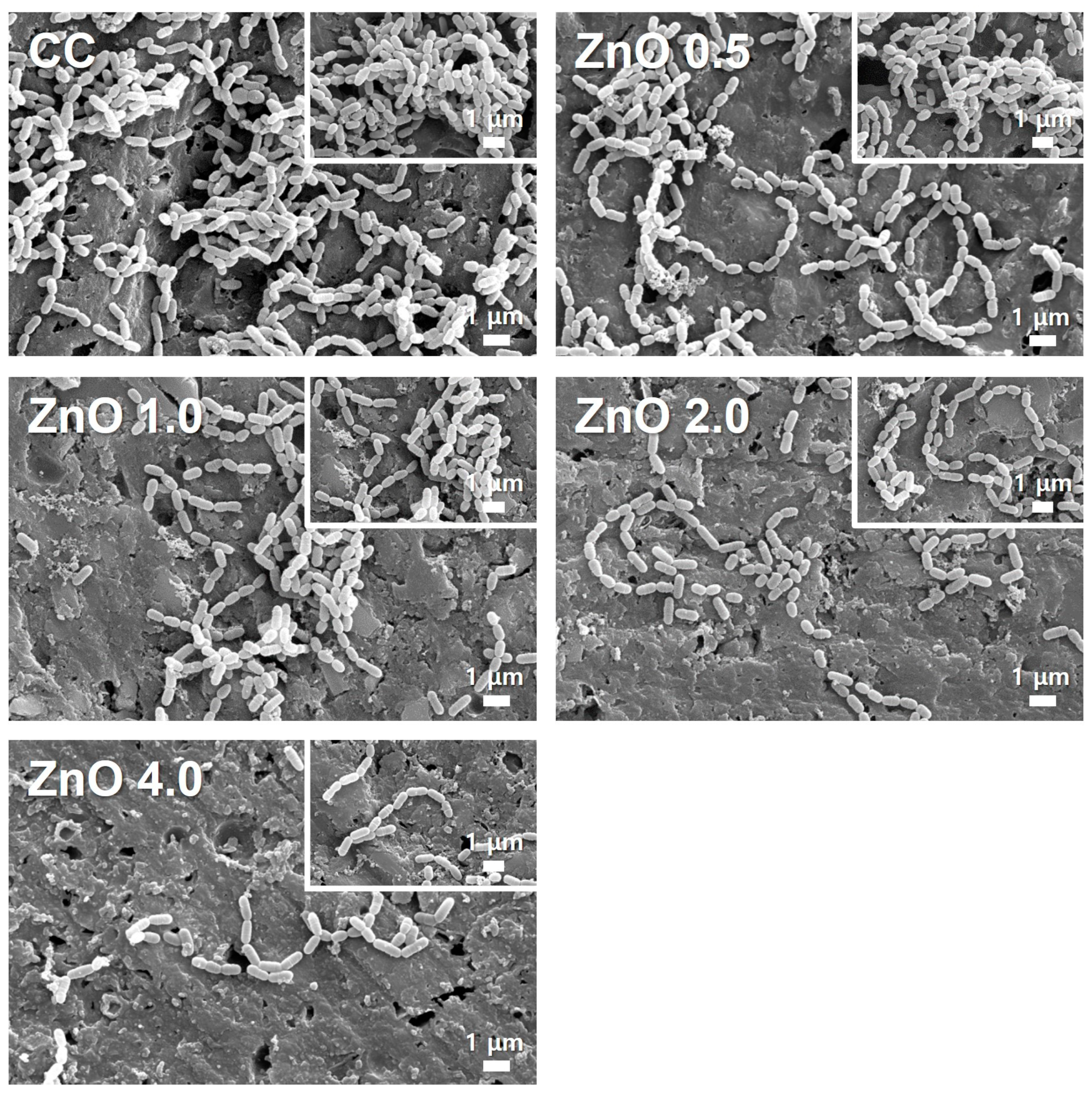
3.2.1. Morphology of Attached Bacteria on the Specimen
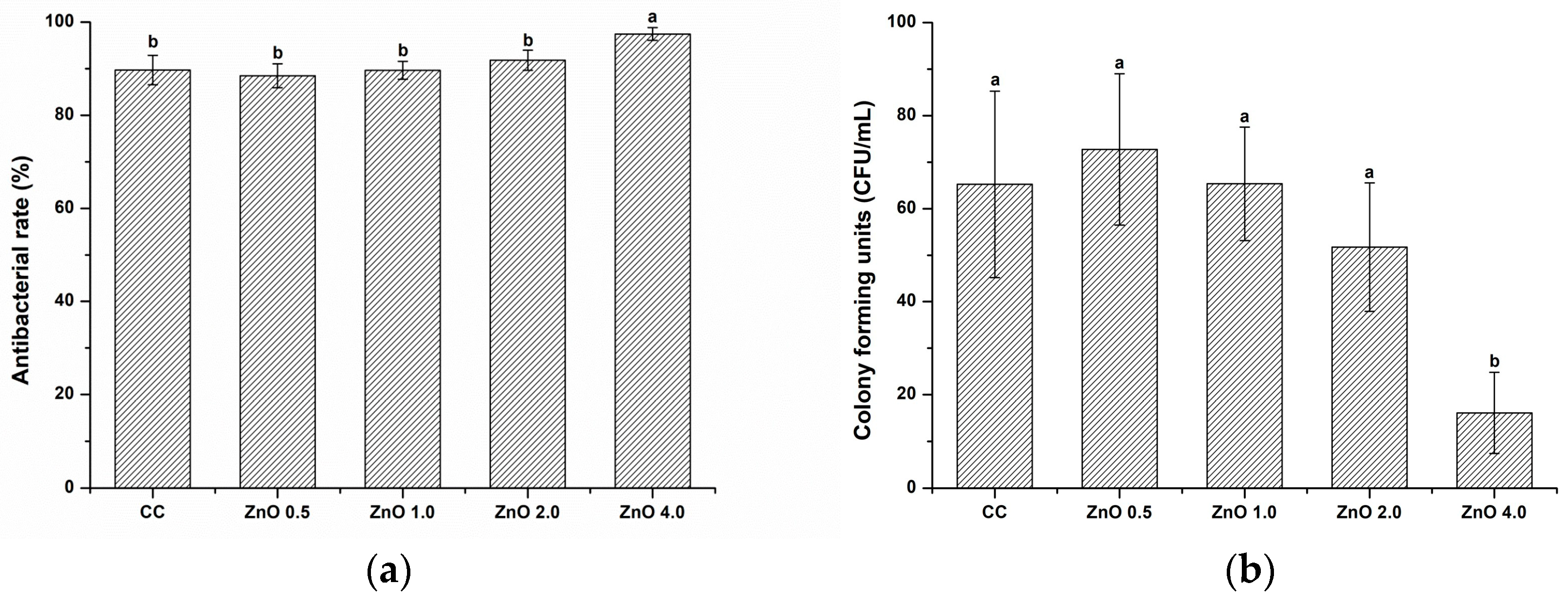
3.2.2. Evaluation of antibacterial effects by counting colony-forming units
3.2.3. Bacterial Viability
3.3. pH Variations
3.4. Zinc Ion Release
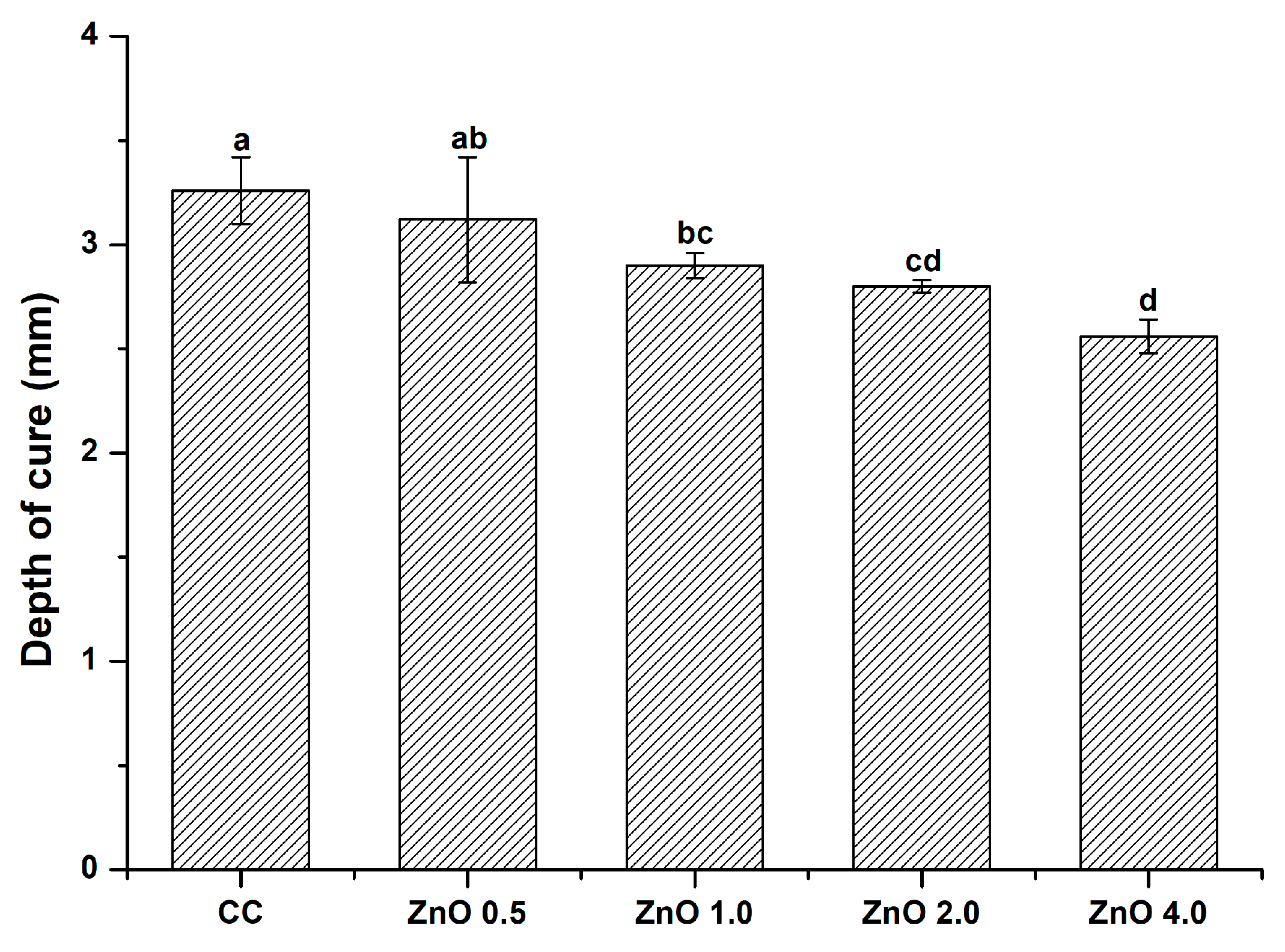
3.5. Depth of Cure
3.6. Water Sorption and Solubility
3.7. Three−Point Flexural Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.Y.; Barraza, J.P.; Arthur, R.A.; Hara, A.; Lewis, K.; Liu, Y.; Hajishengallis, E.; Whiteley, M.; Koo, H. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA 2020, 117, 12375–12386. [Google Scholar] [CrossRef] [PubMed]
- Baik, A.; Almoudi, N.; El-Housseiny, A.; Altuwirqi, A. Fluoride varnishes for preventing occlusal dental caries: A review. Dent. J. 2021, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.; Holban, A.M.; Păuna, M.R.; Imre, M.; Farcașiu, A.T.; Farcașiu, C. Review of professionally applied fluorides for preventing dental caries in children and adolescents. Appl. Sci. 2022, 12, 1054. [Google Scholar] [CrossRef]
- Fei, X.; Li, Y.; Weir, M.D.; Baras, B.H.; Wang, H.; Wang, S.; Sun, J.; Melo, M.; Ruan, J.; Xu, H. Novel pit and fissure sealant containing nano-CaF2 and dimethylaminohexadecyl methacrylate with double benefits of fluoride release and antibacterial function. Dent. Mater. 2020, 36, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, A.; Aghazadeh, Z.; Sadrabad, Z.K.; Aghazadeh, M.; Alizadeh, V.; Esmaili, Z.; Ashraf, M.P. Evaluation of the success rate of pit and fissure sealants on first molars: 12 months follow-up study. Int. J. Dent. Hyg. 2022, 20, 465–470. [Google Scholar] [CrossRef]
- Sudarsan, R.; Ravindran, V. Evaluation of commonly treated maxillary teeth with preventive resin sealant among children with primary dentition. J. Med. Dent. Sci. 2022, 10, 271–276. [Google Scholar]
- Li, K.Y.; Tsai, C.C.; Fang, C.H.; Wang, Y.L.; Lin, F.H.; Lin, C.P. Fluorinated montmorillonite composite resin as a dental pit and fissure sealant. Polymers 2019, 11, 1535. [Google Scholar] [CrossRef]
- Ishigure, H.; Kawaki, H.; Shintani, K.; Ueno, K.; Mizuno-Kamiya, M.; Takayama, E.; Hotta, M.; Kondoh, N.; Nikaido, T. Effects of multi-components released from S-PRG filler on the activities of human dental pulp-derived stem cells. Dent. Mater. J. 2021, 40, 1329–1337. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sayed, M.; Hiraishi, N.; Husain, N.A.H.; Tagami, J.; Özcan, M.; Shimada, Y. Effect of surface pre-reacted glass ionomer containing dental sealant on the inhibition of enamel demineralization. J. Funct. Biomater. 2022, 13, 189. [Google Scholar] [CrossRef]
- Shinkai, K.; Yoshii, D. Effect of the S-PRG filler content in the multi-ion releasing paste on the acid resistance of the enamel surface after polishing with the paste. Dent. Mater. J. 2021, 40, 1136–1141. [Google Scholar] [CrossRef]
- Zhou, Y.; Hiraishi, N.; Shimada, Y.; Wang, G.; Tagami, J.; Feng, X. Evaluation of tooth demineralization and interfacial bacterial penetration around resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2021, 37, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Miki-Oka, S.; Mayanagi, G.; Abiko, Y.; Takahashi, N.; Imazato, S. Inhibitory effect of resin composite containing S-PRG filler on Streptococcus mutans glucose metabolism. J. Dent. 2018, 70, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Maruo, Y.; Sano, H.; Yoshida, Y.; Meerbeek, B.V. Bacterial adhesion not inhibited by ion-releasing bioactive glass filler. Dent. Mater. 2017, 33, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Cho, M.Y.; Lee, E.S.; Jung, H.I.; Kim, B.I. Effects of short-time exposure of surface pre-reacted glass-ionomer eluate on dental microcosm biofilm. Sci. Rep. 2020, 10, 14425. [Google Scholar] [CrossRef]
- Lai, C.C.; Lin, C.P.; Wang, Y.L. Development of antibacterial composite resin containing chitosan/fluoride microparticles as pit and fissure sealant to prevent caries. J. Oral. Microbiol. 2022, 14, 2008615. [Google Scholar] [CrossRef] [PubMed]
- Shafik, O. Comparison between nanocomposites and conventional pit and fissure sealant-an in vitro study. J. Orofac. Res. 2022, 11, 34–37. [Google Scholar]
- Ferreira, I.; Oliveira, T.T.; Reis, A.C. Antimicrobial activity in pit and fissure sealants: A systematic review. Clin. Lab. Res. Den. 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Fiorati-Aguiar, S.M.; Lucisano, M.P.; Silva, L.A.B.; Silva, R.A.B.; Spadaro, A.C.C.; Borsatto, M.C.; Nelson-Filho, P. Mechanical, chemical and antimicrobial properties of a bisphenol A-free pit-and-fissure sealant. Am. J. Dent. 2018, 31, 279–284. [Google Scholar]
- Saqib, S.; Nazeer, A.; Ali, M.; Zaman, W.; Younas, M.; Sunera, S.; Nisar, M. Catalytic potential of endophytes facilitates synthesis of biometallic zinc oxide nanoparticles for agricultural application. BioMetals 2022, 35, 967–985. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; He, H.; Wu, X.; Cao, X.; Jin, J.; Sun, Q.J.; Roy, V.A.L.; Li, R.K.Y. Rhelogical and antibacterial performance of sodium alginate/zinc oxide composite coating for cellulosic paper. Colloid. Surf. B 2018, 167, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Kachoei, M.; Divband, B.; Rahbar, M.; Esmaeilzadeh, M.; Ghanizadeh, M.; Alam, M. A novel developed bioactive composite resin containing silver/zinc oxide (Ag/ZnO) nanoparticles as an antimicrobial material against Streptococcus mutans, Lactobacillus, and Candida albicans. Evid. Based. Complement. Alternat. Med. 2021, 2021, 4743411. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, F.; Amiri, M.; Daneshkazemi, A.; Zandi, H.; Javadi, Z.S. Antimicrobial effect of different sizes of nano zinc oxide on oral microorganisms. Front. Dent. 2019, 16, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kachoei, M.; Nourian, A.; Divband, B.; Kachoei, Z.; Shirazi, S. Zinc-oxide nanocoating for improvement of the antibacterial and frictional behavior of nickel-titanium alloy. Nanomed. J. 2016, 11, 2511–2527. [Google Scholar] [CrossRef] [PubMed]
- Izgis, H.; Ilhan, E.; Kalkandelen, C.; Celen, E.; Guncu, M.M.; Sasmazel, H.T.; Gunduz, O.; Ficai, D.; Ficai, A.; Constantinescu, G. Manufacturing of zinc oxide nanoparticle (ZnO NP)-loaded polyvinyl alcohol (PVA) nanostructured mats using ginger extract for tissue engineering applications. Nanomaterials 2022, 12, 3040. [Google Scholar] [CrossRef] [PubMed]
- ISO-6874; Polymer-Based Pit and Fissure Sealants. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO-4049; Polymer-Based Restorative Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Zhang, L.; Weir, M.D.; Chow, L.C.; Antonucci, J.M.; Chen, J.; Xu, H.H.K. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater. 2016, 32, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, M.J.; Kim, J.Y.; Lee, D.; Kwon, J.S.; Choi, S.H. Incorporation of zwitterionic materials into light-curable fluoride varnish for biofilm inhibition and caries prevention. Sci. Rep. 2019, 9, 19550. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef]
- Brandão, N.L.; Portela, M.B.; Maia, L.C.; Antônio, A.; Silva, E.M.D. Model resin composites incorporating ZnO-NP: Activity against S. mutans and physicochemical properties characterization. J. Appl. Oral Sci. 2018, 26, e20170270. [Google Scholar] [CrossRef]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids. Surf. B Biointerfaces. 2014, 117, 158–165. [Google Scholar] [CrossRef]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the protocol for the LIVE/DEAD BacLightTM bacterial viability kit for rapid determination of bacterial load. Front. Microbiol. 2019, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Hojati, S.; Alaghemand, H.; Hamze, F.; Ahmadian-Babaki, F.; Rajab-Nia, R.; Rezvani, M.B.; Kaviani, M.; Atai, M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent. Mater. 2013, 29, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kaga, N.; Nagano-Takebe, F.; Nezu, T.; Matsuura, T.; Endo, K.; Kaga, M. Protective effects of GIC and S-PRG filler restoratives on demineralization of bovine enamel in lactic acid solution. Materials. 2020, 13, 2140. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Choi, J.W.; Kim, K.M.; Kwon, J.S. Prevention of secondary caries using resin-based pit and fissure sealants containing hydrated calcium silicate. Polymers 2020, 12, 1200. [Google Scholar] [CrossRef]
- Cierech, M.; Wojnarowicz, J.; Kolenda, A.; Krawczyk-Balska, A.; Prochwicz, E.; Wozniak, B.; Łojkowski, W.; Mierzwińska-Nastalska, E. Zinc oxide nanoparticles cytotoxicity and release from newly formed PMMA-ZnO nanocomposites designed for denture bases. Nanomaterials 2019, 9, 1318. [Google Scholar] [CrossRef]
- Liu, Y.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Acidity-induced release of zinc ion from BioUnionTM filler and its inhibitory effects against Streptococcus mutans. Dent. Mater. J. 2020, 39, 547–553. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.B.; Scille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent Mater. 2018, 34, 324–333. [Google Scholar] [CrossRef]
- Alansy, A.S.; Saeed, T.A.; Al-Attab, R.; Guo, Y.; Yang, Y.; Liu, B.; Fan, Z. Boron nitride nanosheets modified with zinc oxide nanoparticles as novel fillers of dental resin composite. Dent Mater. 2022, 38, 266–274. [Google Scholar] [CrossRef]
- Mansouri, S.A.; Zidan, A.Z. Effect of water sorption and solubility on color stability of bulk-fill resin composite. J. Contemp. Dent. Pract. 2018, 19, 1129–1134. [Google Scholar]
- Methley, A.M.; Campbell, S.; Chew-Craham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Beun, S.; Bailly, C.; Devaux, J.; Leloup, G. Physical, mechanical and rheological characterization of resin-based pit and fissure sealants compared to flowable resin composites. Dent. Mater. 2012, 28, 349–359. [Google Scholar] [CrossRef] [PubMed]



| Group Code | Pit and Fissure Sealant | Zinc Oxide |
|---|---|---|
| CC | 100 | 0 |
| ZnO 0.5 | 99.5 | 0.5 |
| ZnO 1.0 | 99.0 | 1.0 |
| ZnO 2.0 | 98.0 | 2.0 |
| ZnO 4.0 | 96.0 | 4.0 |
| Group | 1 d | 2 d | 4 d | 7 d | 14 d | 21 d | 30 d | 60 d | 90 d |
|---|---|---|---|---|---|---|---|---|---|
| CC | 7.15 ± 0.12 Aab | 6.62 ± 0.28 CDa | 6.67 ± 0.14 Cda | 6.77 ± 0.11 BCb | 6.79 ± 0.11 BCb | 6.59 ± 0.06 Dea | 6.48 ± 0.11 Ec | 6.66 ± 0.11 Cdab | 6.87 ± 0.08 Bd |
| ZnO 0.5 | 7.05 ± 0.03 Abb | 6.59 ± 0.20 Ca | 6.74 ± 0.09 Ca | 6.98 ± 0.09 Aba | 6.94 ± 0.05 Bab | 6.71 ± 0.07 Ca | 6.55 ± 0.07 Dbc | 6.59 ± 0.22 CDb | 7.09 ± 0.06 Abc |
| ZnO 1.0 | 7.04 ± 0.04 Ab | 6.60 ± 0.07 Dea | 6.64 ± 0.05 Dea | 6.84 ± 0.13 Bcab | 6.88 ± 0.13 Abab | 6.69 ± 0.07 Cda | 6.52 ± 0.11 Ebc | 6.58 ± 0.19 Deb | 7.02 ± 0.08 Acd |
| ZnO 2.0 | 7.22 ± 0.19 Aa | 6.84 ± 0.11 Bca | 6.77 ± 0.10 Bca | 6.96 ± 0.10 Ba | 6.98 ± 0.14 Ba | 6.76 ± 0.09 Ca | 6.62 ± 0.04 Cab | 6.79 ± 0.15 Bcab | 7.22 ± 0.14 Aab |
| ZnO 4.0 | 7.13 ± 0.08 Bab | 6.72 ± 0.19 Da | 6.72 ± 0.16 Da | 6.85 ± 0.06 Cdab | 7.03 ± 0.11 Bca | 6.77 ± 0.17 Da | 6.68 ± 0.11 Da | 6.89 ± 0.20 Cda | 7.37 ± 0.18 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Yang, S.-Y. Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants. Polymers 2023, 15, 529. https://doi.org/10.3390/polym15030529
Choi J-W, Yang S-Y. Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants. Polymers. 2023; 15(3):529. https://doi.org/10.3390/polym15030529
Chicago/Turabian StyleChoi, Ji-Won, and Song-Yi Yang. 2023. "Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants" Polymers 15, no. 3: 529. https://doi.org/10.3390/polym15030529
APA StyleChoi, J.-W., & Yang, S.-Y. (2023). Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants. Polymers, 15(3), 529. https://doi.org/10.3390/polym15030529







