Superhydrophobic Modification of Sansevieria trifasciata Natural Fibres: A Promising Reinforcement for Wood Plastic Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sansevieria trifasciata Fibres Preparation
2.3. Pre-Treatment
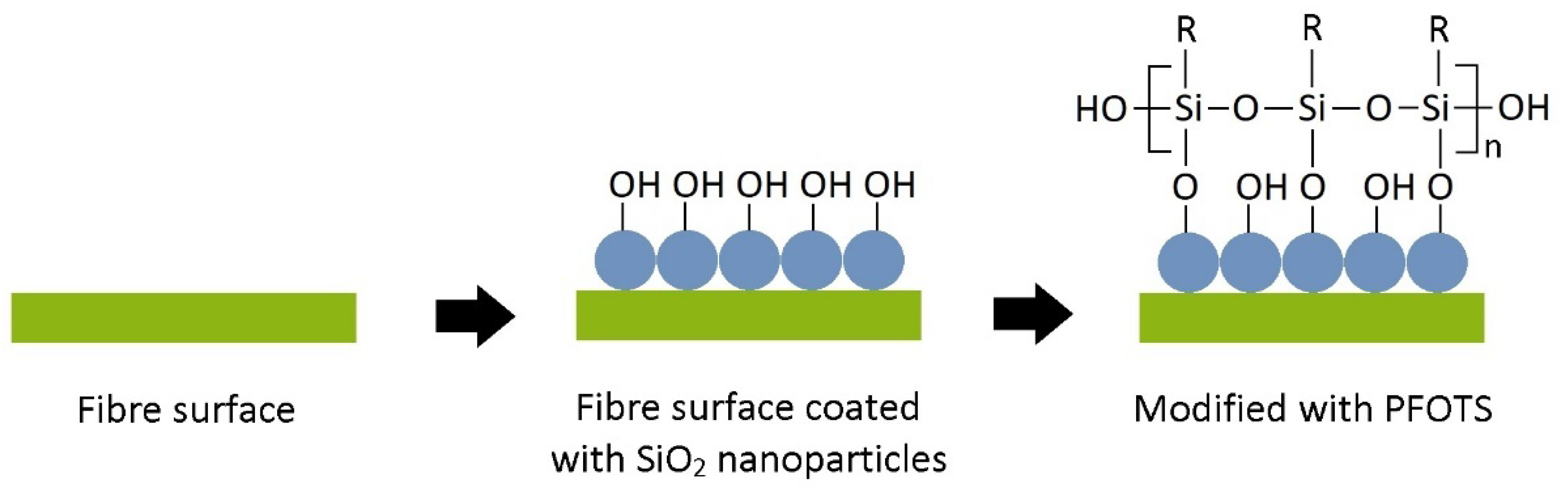
2.4. SiO2-Mediated Hydrophobic Treatment
2.5. Fluorosilane Hydrophobic Treatment
2.6. Characterization of STF
3. Results and Discussion
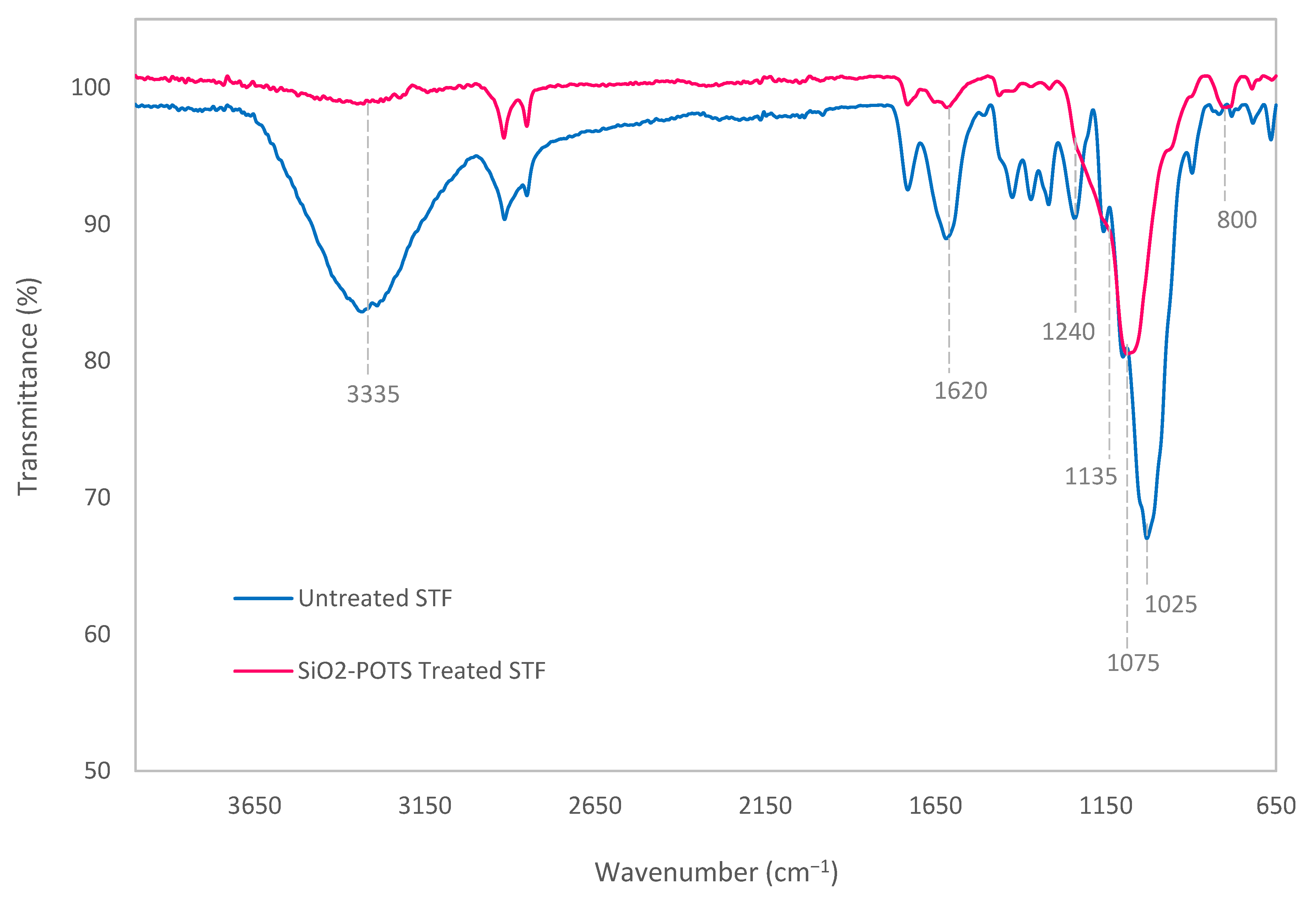
3.1. Chemical Changes on STF Surface
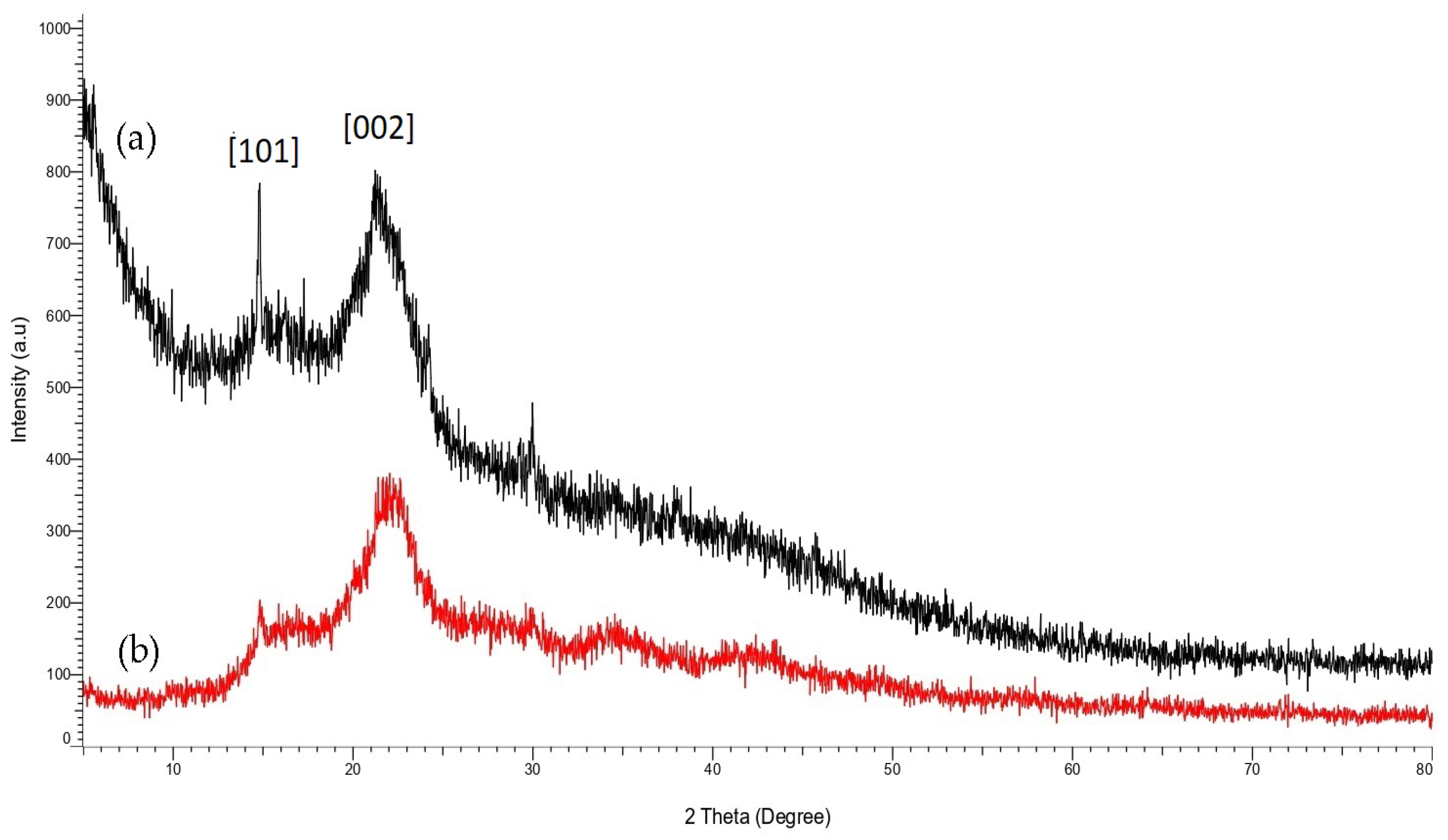
3.2. Crystallization Properties of STF
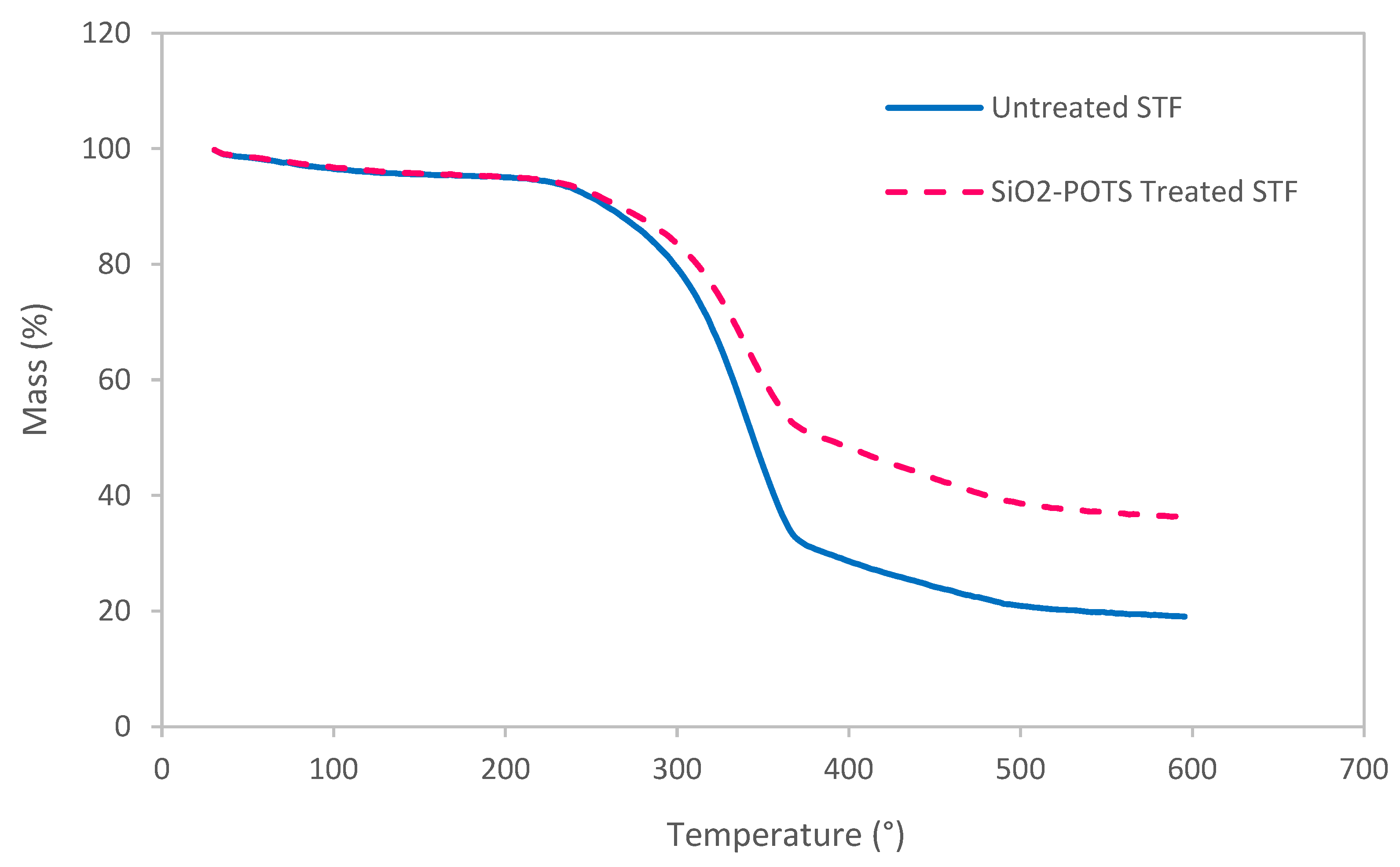
3.3. Thermal Decomposition Behaviour of STF
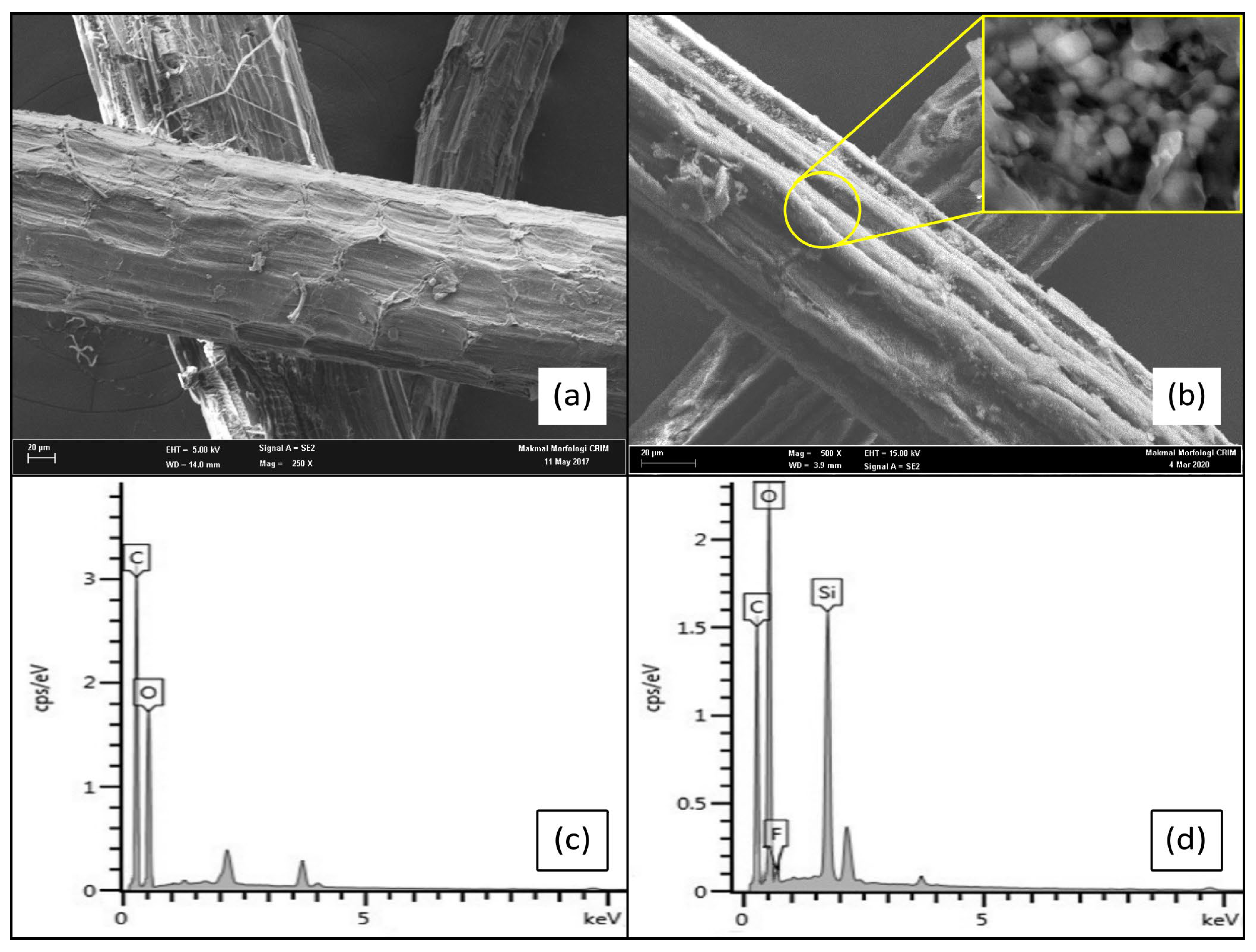
3.4. Surface Morphology of STF
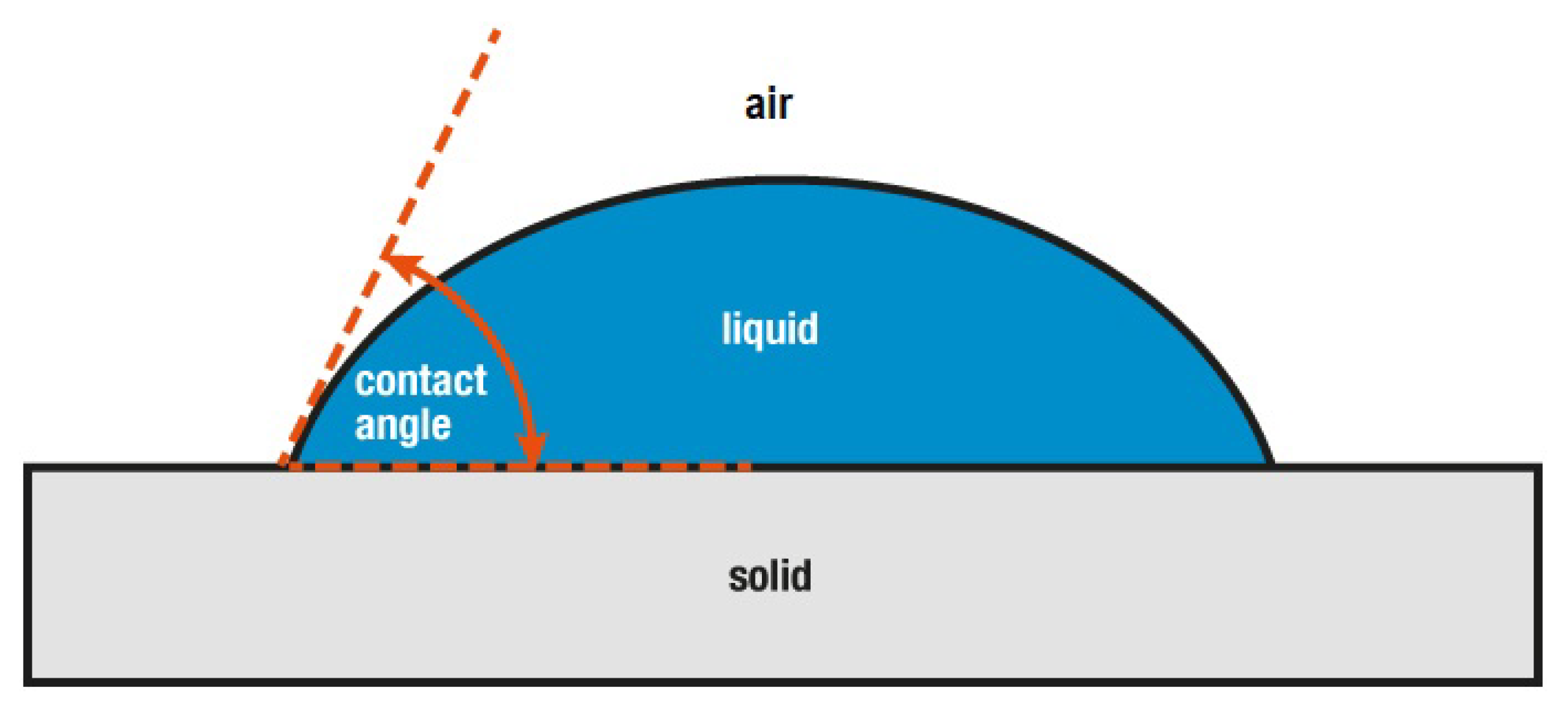
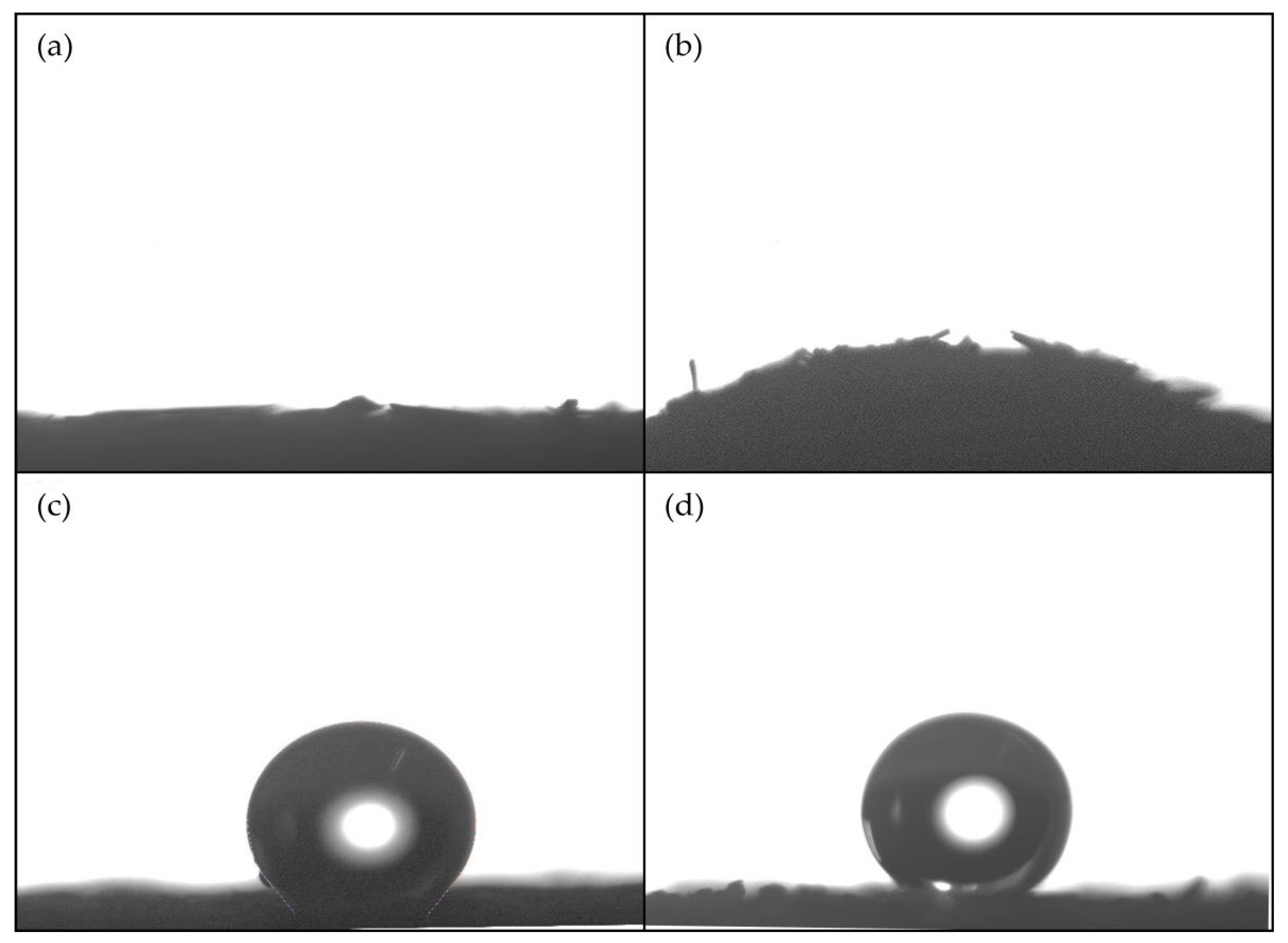
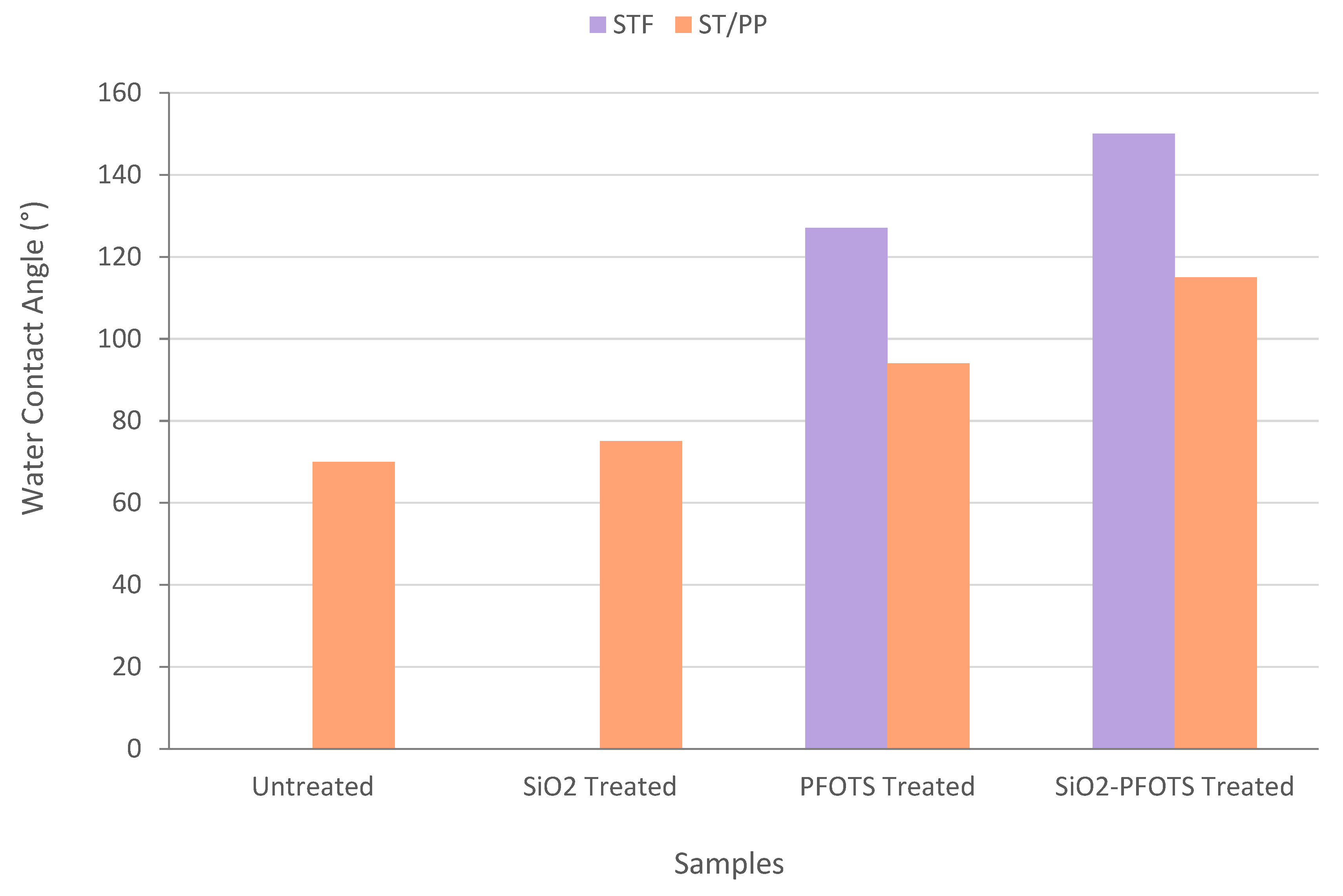
3.5. Wetting Properties of STF
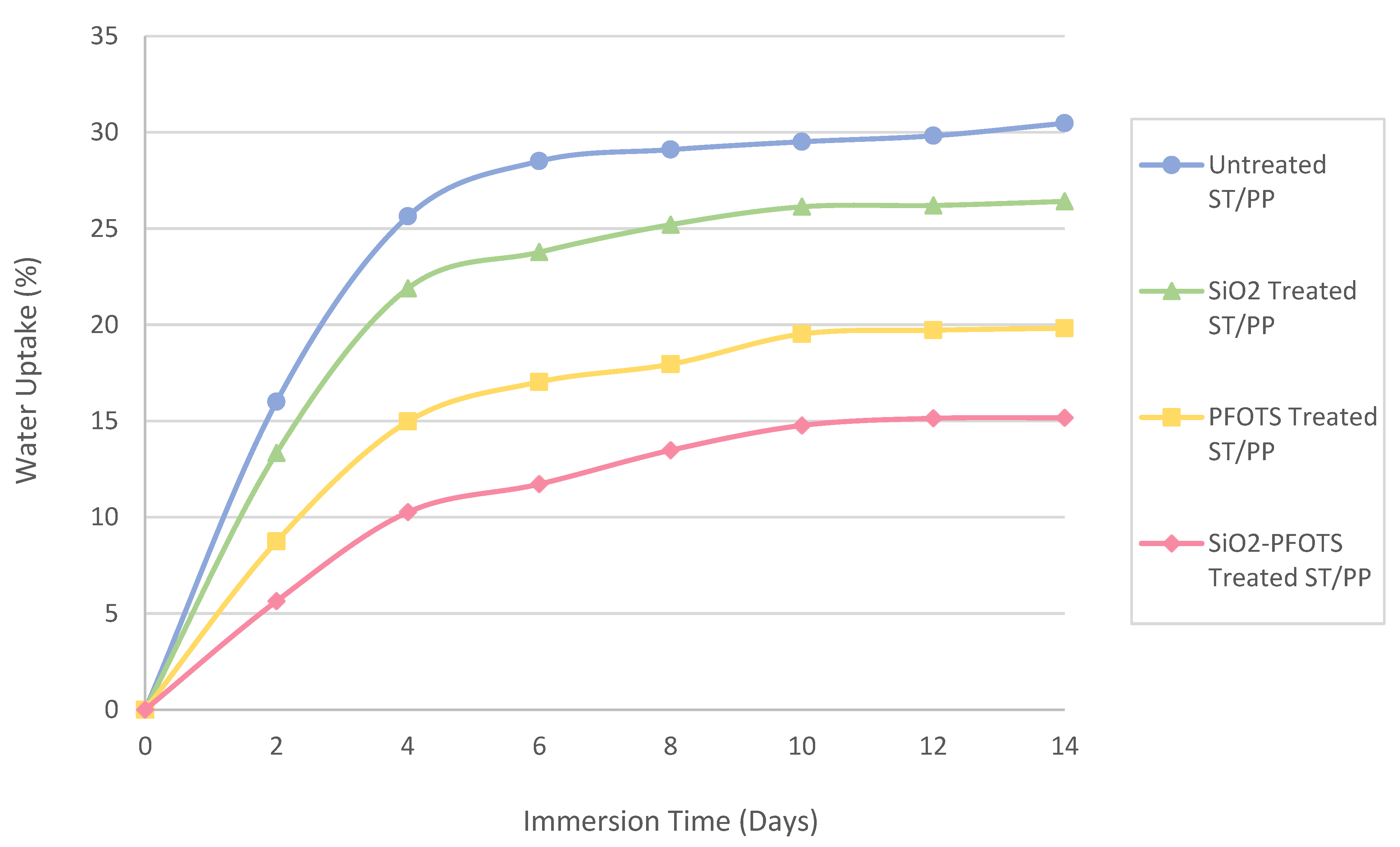
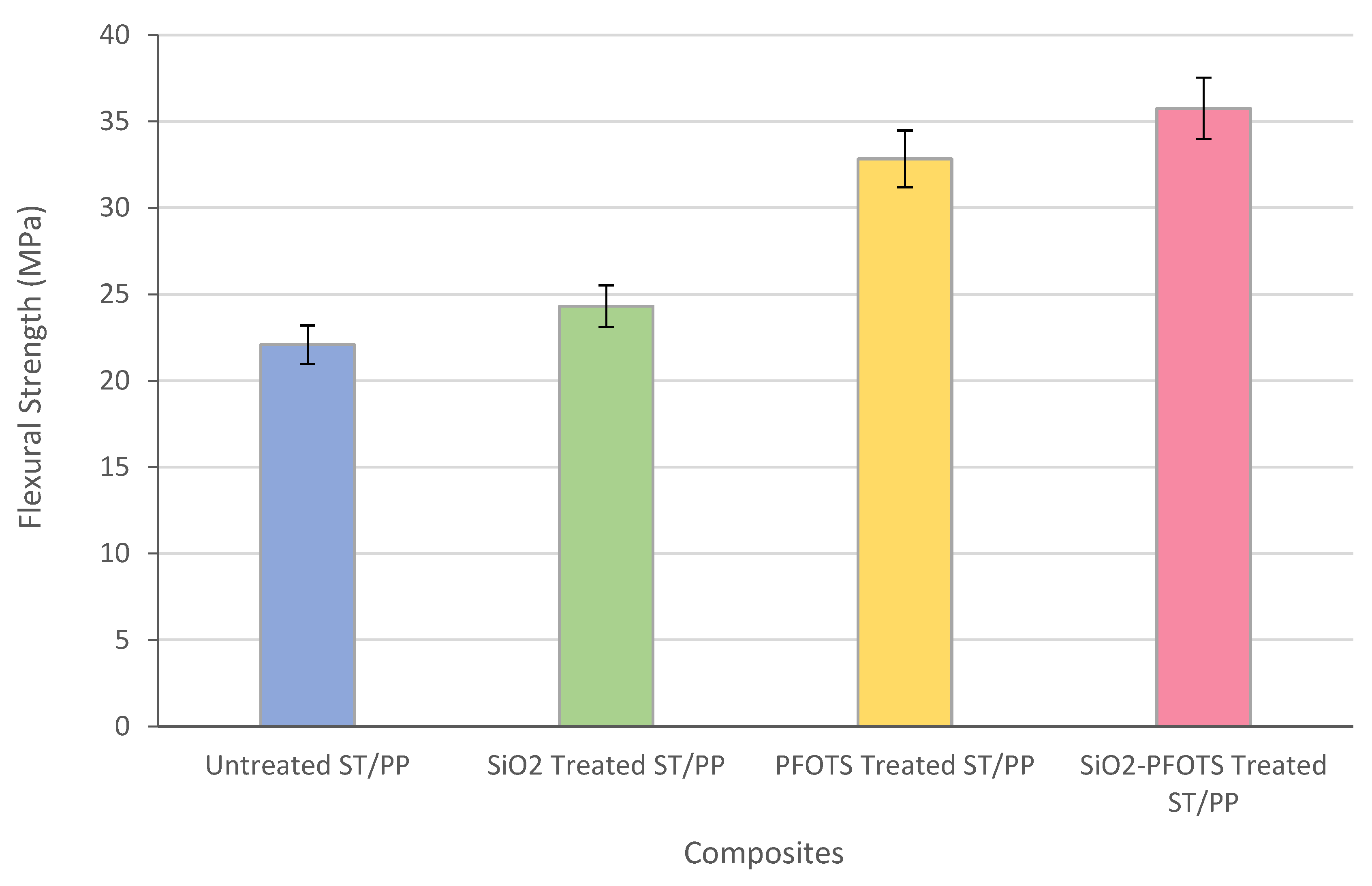
3.6. Application and Performance of Superhydrophobic STF in Wood Plastic Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aref, Y.M.; Baharum, A. Effect of fibre treatment using fluorosilane on Sansevieria trifasciata/polypropylene composite. In Proceedings of the 2017 UKM FST Postgraduate Colloquium, Selangor, Malaysia, 12–13 July 2017; AIP Publishing LLC: Melville, NY, USA, 2018. [Google Scholar]
- Abdullah, N.M.; Ahmad, I. Effect of chemical treatment on mechanical and water-sorption coconut fibre-unsaturated polyester from recycled PET. Int. Sch. Res. Netw. 2012, 2012, 1–8. [Google Scholar]
- Ghasemi, I.; Farsi, M. Interfacial behaviour of wood plastic composite: Effect of chemical treatment on wood fibres, Iran. Polym. J. 2010, 19, 811–818. [Google Scholar]
- Tan, J.Y.; Ang, W.L.; Mohammad, A.W. Hydrophobic polyvinylidene fluoride membrane modified with silica nanoparticles and silane for succinic acid purification using osmotic distillation process. J. Kejuruter. 2021, 33, 89–101. [Google Scholar]
- Sajab, M.S.; Jauhari, W.N.W.A.R.; Chia, C.H.; Kaco, H.Z.H.; Noor, A.M. Oleophilicity and oil-water separation by reduced graphene oxide grafted oil palm empty fruit bunch fibres. Sains Malays. 2018, 47, 1891–1896. [Google Scholar] [CrossRef]
- Nakajima, A.; Hashimoto, K.; Watanabe, T. Recent studies on super-hydrophobic films. Mon. Fuer Chem. 2001, 132, 31–41. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef]
- Dorrer, C.; Rühe, J. Some thoughts on superhydrophobic wetting. Soft Matter 2008, 5, 51–61. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Stepien, M.; Saarinen, J.J.; Teisala, H.; Tuominen, M.; Aromaa, M.; Kuusipalo, J.; Mäkelä, J.M.; Toivakka, M. Adjustable wettability of paperboard by liquid flame spray nanoparticle deposition. Appl. Surf. Sci. 2011, 257, 1911–1917. [Google Scholar] [CrossRef]
- Levkin, P.A.; Svec, F.; Frechet, J.M.J. Porous polymer coatings: A versatile approach to superhydrophobic surfaces. Adv. Funct. Mater. 2009, 19, 1993–1998. [Google Scholar] [CrossRef]
- Song, J.; Rojas, O.J. Approaching super-hydrophobicity from cellulosic materials. Nord. Pulp Pap. Res. J. 2013, 28, 216–238. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Bressler, A.H.; Abolghasemibizaki, M.; Mohammadi, R. Rapid synthesis of inherently robust and stable superhydrophobic carbon soot coatings. Appl. Surf. Sci. 2016, 369, 341–347. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Fedchenko, Y.I.; Gyoshev, S.D.; Lazarov, Y.; Chaushev, T.A.; Grakov, T. On the development of ultradurable extremely water-repellent and oleophobic soot-based fabrics with direct relevance to sperm cryopreservation. ACS Appl. Biol. Mater. 2022, 5, 3519–3529. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Mahltig, B.; Swaboda, C.; Roessler, A.; Böttcher, H. Functionalising wood by nanosol application. J. Mater. Chem. 2008, 18, 3180–3192. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M.; Nematollahi, B.; Baghalha, M. Effect of Fe-containing supports prepared by a novel sol-gel method in the CO methanation reaction: CO elimination and synthetic natural gas production. Energy Technol. 2019, 7, 1900410. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Liu, G.; Zhang, M.; Li, J.; Wang, C. Fabrication of superhydrophobic wood surface by a sol-gel process. Appl. Surf. Sci. 2011, 258, 806–810. [Google Scholar] [CrossRef]
- McKeen, L.W. Fluorinated Coatings and Finishes Handbook: The Definitive User’s Guide, 1st ed.; Wiliam Andrew Publication: New York, NY, USA, 2006. [Google Scholar]
- Textor, T.; Mahltig, B. A sol-gel based surface treatment for preparation of water repellent antistatic textiles. Appl. Surf. Sci. 2010, 256, 1668–1674. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, hydrophilicity, and silanes. Paint Coat. Ind. 2006, 22, 114. [Google Scholar]
- Hsieh, C.T.; Chen, J.M.; Huang, Y.H.; Kuo, R.R.; Li, C.T.; Shih, H.C.; Lin, T.S.; Wu, C.F. Influence of fluorine/carbon atomic ratio on superhydrophobic behavior of carbon nanofiber arrays. J. Vac. Sci. Technol. B 2006, 24, 113–117. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-step coating of fluoro-containing silica nanoparticles for universal generation of surface superhydrophobicity. Chem. Commun. 2008, 7, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Jia, S.T.; Zhang, J.; Tian, L.Q. Superhydrophobic surfaces on cotton textiles by complex coating of silica nanoparticles and hydrophobization. Thin Solid Film. 2009, 517, 4593–4598. [Google Scholar] [CrossRef]
- Wang, X.; Chai, Y.; Liu, J. Formation of highly hydrophobic wood surfaces using silica nanoparticles modified with long-chain alkylsilane. Holzforschung 2013, 67, 667–672. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Gurav, A.B.; Latthe, S.S.; Vhatkar, R.S. Sol-gel-processed porous water-repellent silica microbowls. Surf. Innov. 2013, 1, 157–161. [Google Scholar] [CrossRef]
- Tombesi, A.; Li, S.; Sathasivam, S.; Page, K.; Heale, F.L.; Pettinari, C.; Carmalt, C.J.; Parkin, I.P. Aerosol-assisted chemical vapour deposition of transparent superhydrophobic film by using mixed functional alkoxysilane. Sci. Rep. 2019, 9, 7549. [Google Scholar] [CrossRef]
- Yu, C.; Wang, F.; Lucia, L.A.; Fu, S. Induction of superhydrophobicity in a cellulose substrate by LbL assembly of covalently linked dual-sized silica nanoparticle layers. Adv. Mater. Phys. Chem. 2017, 7, 395–410. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lei, Q.; Wu, Y.; Li, W. Fabrication of superhydrophobic composite coating based on fluorosilicone resin and silica nanoparticles. R. Soc. Open Sci. 2018, 5, 180598. [Google Scholar] [CrossRef]
- Kanimozhi, M. Investigating the physical characteristics of Sansevieria trifasciata Fibre. Int. J. Sci. Res. Publ. 2011, 1, 30–33. [Google Scholar]
- Perera, H.J.; Goyal, A.; Alhassan, S.M. Surface properties of alkylsilane treated date palm fibre. Sci. Rep. 2022, 12, 9760. [Google Scholar] [CrossRef]
- Morshed, M.; Alam, M.M.; Daniels, S. Plasma treatment of natural jute fibre by RIE plus plasma tool. Plasma Sci. Technol. 2010, 12, 325–329. [Google Scholar] [CrossRef]
- Rajeshkumar, G. Characterization of surface modified Phoenix sp. fbers for composite reinforcement. J. Nat. Fibers 2020, 18, 2033–2044. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Cardona, F.; Aravinthan, T. Effect of chemical treatment on the mechanical and thermal properties hemp fibre reinforced thermoset sandwich composites. Inc. Sustain. Pract. Mech. Struct. Mater. 2010, 2010, 439–444. [Google Scholar]
- Chen, H.; Zhang, W.; Wang, X.; Wang, H.; Wu, Y.; Zhong, T.; Fei, B. Effect of alkali treatment on wettability and thermal stability of individual bamboo fibers. J. Wood Sci. 2018, 64, 398–405. [Google Scholar] [CrossRef]
- Mittal, M.; Chaudhary, R. Experimental investigation on the thermal behaviour of untreated and alkali-treated pineapple leaf and coconut husk fibers. Int. J. Appl. Sci. Eng. 2019, 76, 1–16. [Google Scholar]
- Reddy, G.R.; Kumar, M.A.; Jayaramudu, J. Biodegradable Sansevieria cylindrica leaf fiber/Tamarind fruit fiber based polymer hybrid composites on characterizations. Int. Lett. Chem. Phys. Astronony 2014, 39, 116–128. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Contact angle measurements. Membr. Charact. 2017, 2017, 219–255. [Google Scholar]
- Peng, L.; Qisui, W.; Xi, L.; Zhang, C. Investigation of the states of water and OH groups on the surface of silica. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 112–115. [Google Scholar] [CrossRef]
- Jose, J.P.; Malhotra, S.K.; Thomas, S.; Joseph, K.; Goda, K.; Sreekala, M.S. Advances in polymer composites: Macro- and microcomposites—State of the art, new challenges, and opportunities. Polym. Compos. 2012, 1, 3–16. [Google Scholar]
- Masuelli, M.A. Introduction of fibre-reinforced polymers–polymers and composites: Concepts, properties and processes. In Fiber Reinforced Polymers: The Technology Applied for Concrete Repair; IntechOpen: London, UK, 2013; pp. 1–38. [Google Scholar]
- Ali, A.; Shaker, K.; Nawab, Y.; Ashraf, M.; Basit, A.; Shahid, S.; Umair, M. Impact of hydrophobic treatment of jute on moisture regain and mechanical properties of composite material. J. Reinf. Plast. Compos. 2015, 34, 2059–2068. [Google Scholar] [CrossRef]
- Doleza, P.I.; Arfaouia, M.A.; Dubéb, M.; David, E. Hydrophobic treatments for natural fibers based on metal oxide nanoparticles and fatty acids. Procedia Eng. 2017, 200, 81–88. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Q.; Li, X.; Meng, Y.; Ling, Z.; Ji, Z.; Chen, F. Preparation and characterization of degradable cellulose based paper with superhydrophobic, antibacterial, and barrier properties for food packaging. Int. J. Mol. Sci. 2022, 23, 11158. [Google Scholar] [CrossRef]

| Fabrication | Wettability | Mechanical Durability | Thermal Stability | References |
|---|---|---|---|---|
| SiO2 nanoparticles by sol-gel process, followed by modification with PFOTS | WCA = 150°, SA < 10° | Flexural strength increases from 22.09 MPa to 35.75 MPa | Main degradation temperature increases from 278 °C to 308 °C | This work |
| ZnO by the hydrothermal process followed by modification with stearic acid | WCA = 151°, SA < 5° | Breaking force decreases from 90 cN to 60 cN | Main degradation temperature decreases from 260 °C to 240 °C | [44] |
| PLA/CIN coating followed by nano-SiO2-modified stearic acid | WCA = 156.3° SA = N/A | Breaking elongation decreases from 3.5% to 3.1% | Main degradation temperature increases from 355 °C to 360 °C | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Aref, Y.; Othaman, R.; Anuar, F.H.; Ku Ahmad, K.Z.; Baharum, A. Superhydrophobic Modification of Sansevieria trifasciata Natural Fibres: A Promising Reinforcement for Wood Plastic Composites. Polymers 2023, 15, 594. https://doi.org/10.3390/polym15030594
Mohd Aref Y, Othaman R, Anuar FH, Ku Ahmad KZ, Baharum A. Superhydrophobic Modification of Sansevieria trifasciata Natural Fibres: A Promising Reinforcement for Wood Plastic Composites. Polymers. 2023; 15(3):594. https://doi.org/10.3390/polym15030594
Chicago/Turabian StyleMohd Aref, Yanzur, Rizafizah Othaman, Farah Hannan Anuar, Ku Zarina Ku Ahmad, and Azizah Baharum. 2023. "Superhydrophobic Modification of Sansevieria trifasciata Natural Fibres: A Promising Reinforcement for Wood Plastic Composites" Polymers 15, no. 3: 594. https://doi.org/10.3390/polym15030594
APA StyleMohd Aref, Y., Othaman, R., Anuar, F. H., Ku Ahmad, K. Z., & Baharum, A. (2023). Superhydrophobic Modification of Sansevieria trifasciata Natural Fibres: A Promising Reinforcement for Wood Plastic Composites. Polymers, 15(3), 594. https://doi.org/10.3390/polym15030594






