Chitosan/Gelatin Scaffolds Loaded with Jatropha mollissima Extract as Potential Skin Tissue Engineering Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Development of the Ethanolic Extract of Jatropha mollissima
2.2.2. Scaffolds Preparation
2.3. Characterization
2.3.1. High Performance Liquid Chromatography coupled to Diode Array Detector
2.3.2. Determination of Phenolic, Flavonoid and Condensed Tannins Contents
2.3.3. Fourier Transform Infrared Spectroscopy
2.3.4. Swelling Degree
2.3.5. Enzymatic Biodegradation
2.3.6. Scanning Electron Microscopy
2.3.7. Cytotoxicity Assay
2.3.8. Statistical Analysis
3. Results and Discussion
3.1. High Performance Liquid Chromatography coupled to Diode Array Detector
3.2. Total Concentration of Phenols, Flavonoids and Condensed Tannins
3.3. Fourier Transform Infrared Spectroscopy
3.4. Swelling Degree
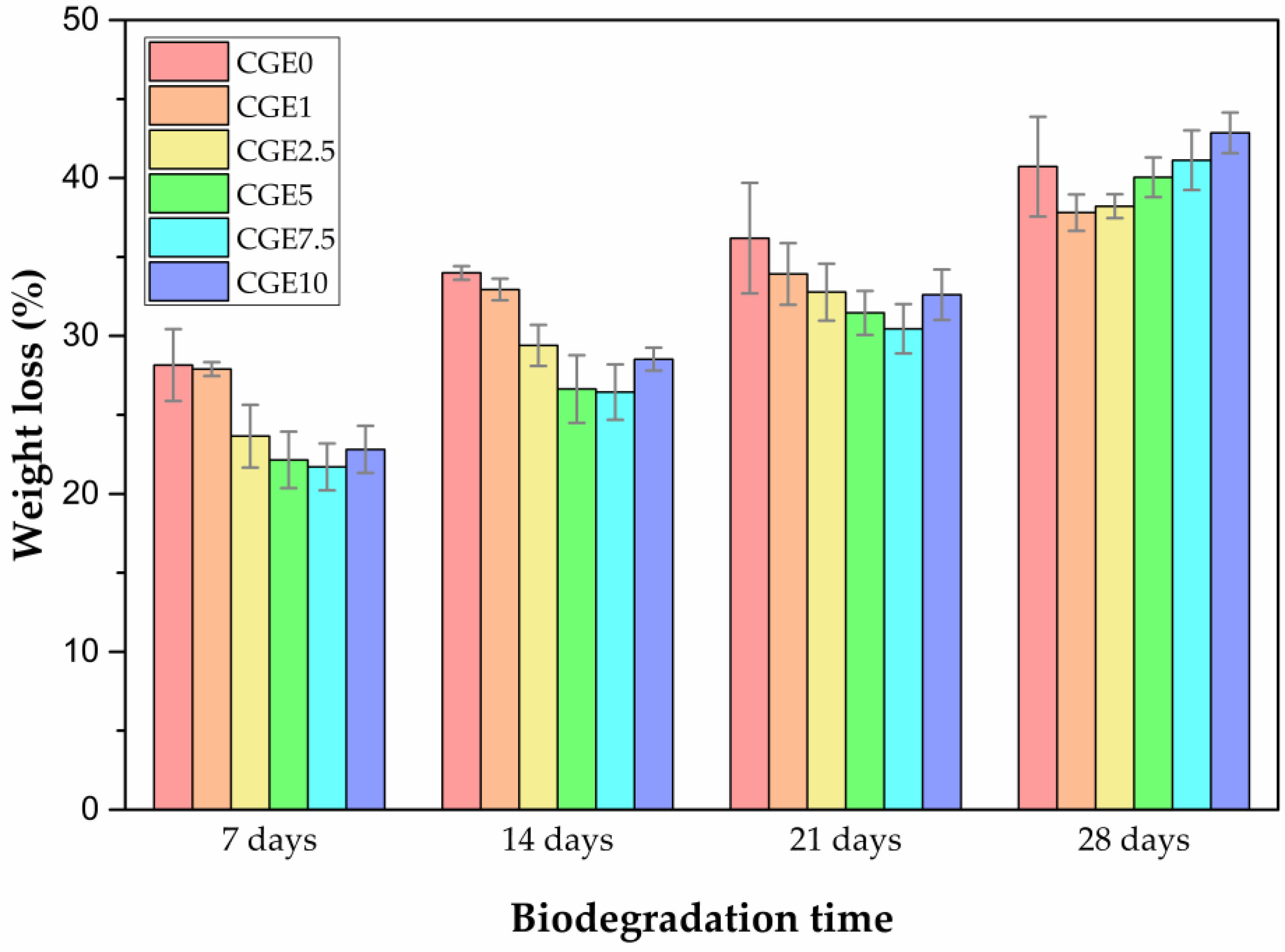
3.5. Enzymatic Biodegradation
3.6. Scanning Eelectron Microscopy (SEM)
3.7. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghieh, S.; Sarker, M.; Izadifar, M.; Chen, X. Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications-A brief review. J. Mech. Behav. Biomed. Mater. 2018, 78, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, P.; Kacarevic, Z.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikka, M.P.; Midha, V.K. 16-The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 463–488. [Google Scholar]
- Cheng, Y.-H.; Hung, K.-H.; Tsai, T.-H.; Lee, C.-J.; Ku, R.-Y.; Chiu, A.W.-H.; Chiou, S.-H.; Liu, C.J.-L. Sustained delivery of latanoprost by thermosensitive chitosan–gelatin-based hydrogel for controlling ocular hypertension. Acta Biomater. 2014, 10, 4360–4366. [Google Scholar] [CrossRef]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Amir Afshar, H.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. Part B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Rosellini, E.; Zhang, Y.S.; Migliori, B.; Barbani, N.; Lazzeri, L.; Shin, S.R.; Dokmeci, M.R.; Cascone, M.G. Protein/polysaccharide-based scaffolds mimicking native extracellular matrix for cardiac tissue engineering applications. J. Biomed. Mater. Res. Part A 2018, 106, 769–781. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.d.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Heimbuck, A.M.; Priddy-Arrington, T.R.; Padgett, M.L.; Llamas, C.B.; Barnett, H.H.; Bunnell, B.A.; Caldorera-Moore, M.E. Development of responsive chitosan–genipin hydrogels for the treatment of wounds. ACS Appl. Bio Mater. 2019, 2, 2879–2888. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 2020, 31, 350–375. [Google Scholar] [CrossRef] [PubMed]
- Kildeeva, N.; Chalykh, A.; Belokon, M.; Petrova, T.; Matveev, V.; Svidchenko, E.; Surin, N.; Sazhnev, N. Influence of genipin crosslinking on the properties of chitosan-based films. Polymers 2020, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Yang, Y.; Xing, X.; Yang, F.; Shan, P. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 73–85. [Google Scholar] [CrossRef]
- Radhika Rajasree, S.R.; Gobalakrishnan, M.; Aranganathan, L.; Karthih, M.G. Fabrication and characterization of chitosan based collagen/gelatin composite scaffolds from big eye snapper Priacanthus hamrur skin for antimicrobial and anti oxidant applications. Mater. Sci. Eng. C 2020, 107, 110270. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.K.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C 2020, 109, 110618. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Ferreira, L.M.; Santos, P.M.P.; Leal, J.P.; Rodrigues, G.; Iria, I.; Alves, S.; Pais, D.; Casal, D. Chitosan-Based Membranes for Skin Wound Repair in a Dorsal Fold Chamber Rat Model. Pharmaceutics 2022, 14, 2736. [Google Scholar] [CrossRef]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT-Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Ashraf, S.S. Cinnamon extract loaded electrospun chitosan/gelatin membrane with antibacterial activity. Int. J. Biol. Macromol. 2021, 173, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.V.; Martins, V.C.A.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M.G. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef] [PubMed]
- Sabandar, C.W.; Ahmat, N.; Jaafar, F.M.; Sahidin, I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Dias, W.L.F.; do Vale Junior, E.P.; das Dores Alves de Oliveira, M.; Barbosa, Y.L.P.; do Nascimento Silva, J.; da Costa Júnior, J.S.; de Almeida, P.M.; Martins, F.A. Cytogenotoxic effect, phytochemical screening and antioxidant potential of Jatropha mollissima (Pohl) Baill leaves. S. Afr. J. Bot. 2019, 123, 30–35. [Google Scholar] [CrossRef]
- Queiroz Neto, R.F.d.; Araújo Júnior, H.N.d.; Freitas, C.I.A.; Costa, K.M.d.F.M.; Abrantes, M.R.; Almeida, J.G.L.d.; Torres, T.M.; Moura, G.H.F.; Batista, J.S. The Jatropha mollissima (Pohl) Baill: Chemical and pharmacological activities of the latex and its extracts. Semin. Ciências Agrárias 2019, 40, 2613–2624. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.R.C.; Andrade, F.D.d.; Medeiros, M.d.C.d.; Camboim, A.d.S.; Pereira Júnior, F.A.; Athayde, A.C.R.; Rodrigues, O.G.; Silva, W.W. Estudo da atividade anti-helmíntica do extrato etanólico de Jatropha mollissima (Pohl) Baill.(Euphorbiaceae) sob Haemonchus contortus em ovinos no semiárido paraibano. Pesqui. Veterinária Bras. 2014, 34, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Dantas, M.V.O.; Nogueira, P.L.; Lima, F.O.; Oliveira, D.C.P.; Gomes, E.N.S.; Rodrigues, J.F.B.; Amoah, S.K.S.; Rosendo, R.A.; da Penha, E.S.; Dantas, A.F.M.; et al. In vivo Hemostatic Activity of Jatropha mollissima: A Triple-Blinded, Randomized, Controlled Trial in an Animal Model. Eur. J. Dent. 2021, 15, 741–745. [Google Scholar] [CrossRef]
- Patel, B.; Wene, D.; Fan, Z.T. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Guleria, K.; Sehgal, A.; Bhat, I.A.; Singh, S.K.; Vamanu, E.; Singh, M.P. Impact of Altering the Ratio of Black Tea Granules and Ocimum gratissimum Leaves in a Binary Infusion on Radical Scavenging Potential Employing Cell Free Models and Ex Vivo Assays. Appl. Sci. 2022, 12, 10632. [Google Scholar] [CrossRef]
- Palacios, C.E.; Nagai, A.; Torres, P.; Rodrigues, J.A.; Salatino, A. Contents of tannins of cultivars of sorghum cultivated in Brazil, as determined by four quantification methods. Food Chem. 2021, 337, 127970. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Shen, S.; Johnson, S.; Fang, Z. Comprehensive profiling of phenolic compounds by HPLC-DAD-ESI-QTOF-MS/MS to reveal their location and form of presence in different sorghum grain genotypes. Food Res. Int. 2020, 137, 109671. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Giocaliere, E.; Guccione, C.; Pieraccini, G.; Gallo, E.; Vannacci, A.; Bilia, A.R. Combined HPLC-DAD–MS, HPLC–MSn and NMR spectroscopy for quality control of plant extracts: The case of a commercial blend sold as dietary supplement. J. Pharm. Biomed. Anal. 2014, 88, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Manjón, E.; Recio-Torrado, A.; Ramos-Pineda, A.M.; García-Estévez, I.; Escribano-Bailón, M.T. Effect of different yeast mannoproteins on the interaction between wine flavanols and salivary proteins. Food Res. Int. 2021, 143, 110279. [Google Scholar] [CrossRef]
- Cavalcante, N.B.; da Conceição Santos, A.D.; da Silva Almeida, J.R.G. The genus Jatropha (Euphorbiaceae): A review on secondary chemical metabolites and biological aspects. Chem. Biol. Interact. 2020, 318, 108976. [Google Scholar] [CrossRef] [PubMed]
- Granados, S.; Balcázar, N.; Guillén, A.; Echeverri, F. Evaluation of the Hypoglycemic Effects of Flavonoids and Extracts from Jatropha gossypifolia L. Molecules 2015, 20, 6181–6193. [Google Scholar] [CrossRef] [Green Version]
- Rahu, M.I.; Naqvi, S.H.A.; Memon, N.H.; Idrees, M.; Kandhro, F.; Pathan, N.L.; Sarker, M.N.I.; Bhutto, M.A. Determination of antimicrobial and phytochemical compounds of Jatropha curcas plant. Saudi J. Biol. Sci. 2021, 28, 2867–2876. [Google Scholar] [CrossRef]
- Tinco-Jayo, J.A.; Aguilar-Felices, E.J.; Enciso-Roca, E.C.; Arroyo-Acevedo, J.L.; Herrera-Calderon, O. Phytochemical Screening by LC-ESI-MS/MS and Effect of the Ethyl Acetate Fraction from Leaves and Stems of Jatropha macrantha Müll Arg. on Ketamine-Induced Erectile Dysfunction in Rats. Molecules 2021, 27, 115. [Google Scholar] [CrossRef]
- Braquehais, I.D.; Vasconcelos, F.R.; Ribeiro, A.R.C.; Da Silva, A.R.A.; Franca, M.G.A.; De Lima, D.R.; De Paiva, C.F.; Guedes, M.I.F.; Magalhães, F.E.A. Estudo preliminar toxicológico, antibacteriano e fitoquímico do extrato etanólico das folhas de Jatropha mollissima (Pohl) Baill.(pinhão-bravo, Euphorbiaceae), coletada no Município de Tauá, Ceará, Nordeste Brasileiro. Rev. Bras. Plantas Med. 2016, 18, 582–587. [Google Scholar] [CrossRef] [Green Version]
- De Sousa Araújo, T.A.; Alencar, N.L.; de Amorim, E.L.C.; de Albuquerque, U.P. A new approach to study medicinal plants with tannins and flavonoids contents from the local knowledge. J. Ethnopharmacol. 2008, 120, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.d.S.; Félix-Silva, J.; Morais Fernandes, J.; Geraldo Amaral, J.; Lopes, N.P.; Tabosa do Egito, E.S.; da Silva-Júnior, A.A.; Maria Zucolotto, S.; Fernandes-Pedrosa, M.d.F. Aqueous leaf extract of Jatropha mollissima (Pohl) bail decreases local effects induced by Bothropic venom. BioMed Res. Int. 2016, 2016, 6101742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.O.; Yahya, E.B. In vivo assessment of reversing aminoglycoside antibiotics nephrotoxicity using Jatropha mollissima crude extract. Tissue Cell 2021, 72, 101525. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.K.; Froeder, A.L.F.; Janovik, V.; Venturini, T.P.; Pereira, R.P.; Boligon, A.A.; de Brum, T.F.; Alves, S.H.; da Rocha, J.B.T.; Athayde, M.L. Antioxidant capacity, antimicrobial activity and triterpenes isolated from Jatropha isabellei Müll Arg. Nat. Prod. Res. 2013, 27, 1049–1059. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Wisetkomolmat, J.; Suppakittpaisarn, P.; Sommano, S.R. Detergent plants of Northern Thailand: Potential sources of natural saponins. Resources 2019, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, L.G.; Veras, G.; de Oliveira Alves, J.V.; Oliveira de Veras, B.; da Silva, M.V.; Bacalhau Rodrigues, J.F.; Lia Fook, M.V.; Sagoe Amoah, S.K.; da Conceicao de Menezes Torres, M. Chemodiversity and Antibacterial Activity of the Essential Oil of Leaves of Croton argyrophyllus. Chem. Biodivers. 2020, 17, e2000575. [Google Scholar] [CrossRef]
- Badhe, R.V.; Bijukumar, D.; Chejara, D.R.; Mabrouk, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering. Carbohydr. Polym. 2017, 157, 1215–1225. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 1–13. [Google Scholar] [CrossRef]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M. How can genipin assist gelatin/carbohydrate chitosan scaffolds to act as replacements of load-bearing soft tissues? Carbohydr. Polym. 2013, 93, 635–643. [Google Scholar] [CrossRef]
- Peng, J.; Wang, X.; Lou, T. Preparation of chitosan/gelatin composite foam with ternary solvents of dioxane/acetic acid/water and its water absorption capacity. Polym. Bull. 2020, 77, 5227–5244. [Google Scholar] [CrossRef]
- Mohonta, S.K.; Maria, K.H.; Rahman, S.; Das, H.; Hoque, S.M. Synthesis of hydroxyapatite nanoparticle and role of its size in hydroxyapatite/chitosan–gelatin biocomposite for bone grafting. Int. Nano Lett. 2021, 11, 381–393. [Google Scholar] [CrossRef]
- Ajuong, E.; Birkinshaw, C. The effects of acetylation on the extractives of Sitka Spruce (Picea sitchensis) and Larch (Larix leptoleptis) wood. Holz Als Roh-Und Werkst. 2004, 62, 189–196. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Rajan, T.; Muthukrishnana, S. Characterization of phenolic compounds in Pseudarthria viscida root extract by HPLC and FT-IR analysis. Asian J. Pharm. Clin. Res. 2013, 6, 274–276. [Google Scholar]
- Dos Santos Grasel, F.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Puică, N.M.; Pui, A.; Florescu, M. FTIR spectroscopy for the analysis of vegetable tanned ancient leather. Eur. J. Sci. Theol. 2006, 2, 49–53. [Google Scholar]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Recent advances on the role of process variables affecting gelatin yield and characteristics with special reference to enzymatic extraction: A review. Food Hydrocoll. 2017, 63, 85–96. [Google Scholar] [CrossRef]
- Cowen, S.; Al-Abadleh, H.A. DRIFTS studies on the photodegradation of tannic acid as a model for HULIS in atmospheric aerosols. Phys. Chem. Chem. Phys. 2009, 11, 7838–7847. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Ragavendran, P.; Sophia, D.; Arul Raj, C.; Gopalakrishnan, V.K. Functional group analysis of various extracts of Aerva lanata (L.) by FTIR spectrum. Pharmacologyonline 2011, 1, 358–364. [Google Scholar]
- Sarika, P.R.; Cinthya, K.; Jayakrishnan, A.; Anilkumar, P.R.; James, N.R. Modified gum arabic cross-linked gelatin scaffold for biomedical applications. Mater. Sci. Eng. C 2014, 43, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Bi, S.; Yan, D.; Zhou, Z.; Sun, G.; Cheng, X.; Chen, X. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr. Polym. 2018, 184, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci. Rep. 2021, 11, 1–20. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, H.S. Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater. Sci. Eng. C 2017, 78, 690–705. [Google Scholar] [CrossRef]
- Yao, C.-H.; Chen, K.-Y.; Cheng, M.-H.; Chen, Y.-S.; Huang, C.-H. Effect of genipin crosslinked chitosan scaffolds containing SDF-1 on wound healing in a rat model. Mater. Sci. Eng. C 2020, 109, 110368. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Hasirci, V. Mimicking corneal stroma using keratocyte-loaded photopolymerizable methacrylated gelatin hydrogels. J. Tissue Eng. Regen. Med. 2018, 12, e1899–e1910. [Google Scholar] [CrossRef]
- Tanuma, H.; Saito, T.; Nishikawa, K.; Dong, T.; Yazawa, K.; Inoue, Y. Preparation and characterization of PEG-cross-linked chitosan hydrogel films with controllable swelling and enzymatic degradation behavior. Carbohydr. Polym. 2010, 80, 260–265. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Pan, S.-k.; Wu, S.-j.; Kim, J.-m. Preparation of glucosamine by hydrolysis of chitosan with commercial α-amylase and glucoamylase. J. Zhejiang Univ. Sci. B 2011, 12, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liu, Y.; Shin, H.-d.; Chen, R.; Li, J.; Du, G.; Chen, J. Microbial production of glucosamine and N-acetylglucosamine: Advances and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.-H.; Hsu, C.-N.; Chung, M.-Y.; Tsai, W.-L.; Liu, C.-H.J.J.o.F.; Analysis, D. Effect of Different Concentrations of Collagen, Ceramides, N-acetyl glucosamine, or Their Mixture on Enhancing the Proliferation of Keratinocytes, Fibroblasts and the Secretion of Collagen and/or the Expression of mRNA of Type I Collagen. J. Food Drug Anal. 2008, 16, 66–74. [Google Scholar] [CrossRef]
- Sayo, T.; Sakai, S.; Inoue, S. Synergistic Effect of N-Acetylglucosamine and Retinoids on Hyaluronan Production in Human Keratinocytes. Skin Pharmacol. Physiol. 2004, 17, 77–83. [Google Scholar] [CrossRef]
- Ashkani-Esfahani, S.; Emami, Y.; Esmaeilzadeh, E.; Bagheri, F.; Namazi, M.J. Glucosamine enhances tissue regeneration in the process of wound healing in rats as animal model: A stereological study. J. Cytol. Histol. 2012, 3, 1000150. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Tondera, C.; Hauser, S.; Krüger-Genge, A.; Jung, F.; Neffe, A.T.; Lendlein, A.; Klopfleisch, R.; Steinbach, J.; Neuber, C.; Pietzsch, J.J.T. Gelatin-based hydrogel degradation and tissue interaction in vivo: Insights from multimodal preclinical imaging in immunocompetent nude mice. Theranostics 2016, 6, 2114. [Google Scholar] [CrossRef] [PubMed]
- Ullm, S.; Krüger, A.; Tondera, C.; Gebauer, T.P.; Neffe, A.T.; Lendlein, A.; Jung, F.; Pietzsch, J. Biocompatibility and inflammatory response in vitro and in vivo to gelatin-based biomaterials with tailorable elastic properties. Biomaterials 2014, 35, 9755–9766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandao-Rangel, M.A.; Oliveira, C.R.; da Silva Olímpio, F.R.; Aimbire, F.; Mateus-Silva, J.R.; Chaluppe, F.A.; Vieira, R.P. Hydrolyzed Collagen Induces an Anti-Inflammatory Response That Induces Proliferation of Skin Fibroblast and Keratinocytes. Nutrients 2022, 14, 4975. [Google Scholar] [CrossRef] [PubMed]
- Dreesmann, L.; Ahlers, M.; Schlosshauer, B. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials 2007, 28, 5536–5543. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Ghorbani, M.; Hashemibeni, B.; Bahramian, H. Evaluation of the proliferation and viability rates of nucleus pulposus cells of human intervertebral disk in fabricated chitosan-gelatin scaffolds by freeze drying and freeze gelation methods. Adv. Biomed. Res. 2015, 4, 251. [Google Scholar]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S. Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F.J. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int. J. Pharm. 2018, 537, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Jiankang, H.; Dichen, L.; Yaxiong, L.; Bo, Y.; Bingheng, L.; Qin, L. Fabrication and characterization of chitosan/gelatin porous scaffolds with predefined internal microstructures. Polymer 2007, 48, 4578–4588. [Google Scholar] [CrossRef]





| Sample Designation | Amount of EEJM (%) |
|---|---|
| CGE0 | 0 |
| CGE1 | 1 |
| CGE2.5 | 2.5 |
| CGE5 | 5 |
| CGE7.5 | 7.5 |
| CGE10 | 10 |
| Sample | TPC (mg GAE/g Dry Extract) | TFC (mg QE/g Dry Extract) | TTC (mg CE/g Dry Extract) |
|---|---|---|---|
| EEJM | 22.91 ± 0.84 | 2.96 ± 0.45 | 431.68 ± 33.43 |
| Scaffold Sample | Porosity (%) | Mean Pore Size (µm) |
|---|---|---|
| CGE0 | 59.27 ± 0.96 (A) | 227.67 ± 72.06 (A) |
| CGE1 | 57.83 ± 1.73 (A) | 185.86 ± 55.08 (B) |
| CGE2.5 | 58.55 ± 0.78 (A) | 168.41 ± 57.74 (B, C) |
| CGE5 | 57.20 ± 1.29 (A) | 165.04 ± 48.22 (B, C, D) |
| CGE7.5 | 57.41 ± 1.38 (A) | 138.44 ± 34.22 (C, D) |
| CGE10 | 57.29 ± 1.89 (A) | 155.20 ± 44.23 (D) |
| Sample | Cytotoxic Degree | Definition |
|---|---|---|
| Negative control | 0 | Noncytotoxic |
| Positive control | 4 | Severely cytotoxic |
| CGE0 | 0 | Noncytotoxic |
| CGE1 | 0 | Noncytotoxic |
| CGE2.5 | 0 | Noncytotoxic |
| CGE5 | 0 | Noncytotoxic |
| CGE7.5 | 0 | Noncytotoxic |
| CGE10 | 0 | Noncytotoxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, M.F.; da Silva, H.N.; Rodrigues, J.F.B.; Macêdo, M.D.M.; de Sousa, W.J.B.; Barbosa, R.C.; Fook, M.V.L. Chitosan/Gelatin Scaffolds Loaded with Jatropha mollissima Extract as Potential Skin Tissue Engineering Materials. Polymers 2023, 15, 603. https://doi.org/10.3390/polym15030603
de Souza MF, da Silva HN, Rodrigues JFB, Macêdo MDM, de Sousa WJB, Barbosa RC, Fook MVL. Chitosan/Gelatin Scaffolds Loaded with Jatropha mollissima Extract as Potential Skin Tissue Engineering Materials. Polymers. 2023; 15(3):603. https://doi.org/10.3390/polym15030603
Chicago/Turabian Stylede Souza, Matheus Ferreira, Henrique Nunes da Silva, José Filipe Bacalhau Rodrigues, Maria Dennise Medeiros Macêdo, Wladymyr Jefferson Bacalhau de Sousa, Rossemberg Cardoso Barbosa, and Marcus Vinícius Lia Fook. 2023. "Chitosan/Gelatin Scaffolds Loaded with Jatropha mollissima Extract as Potential Skin Tissue Engineering Materials" Polymers 15, no. 3: 603. https://doi.org/10.3390/polym15030603







