Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application
Abstract
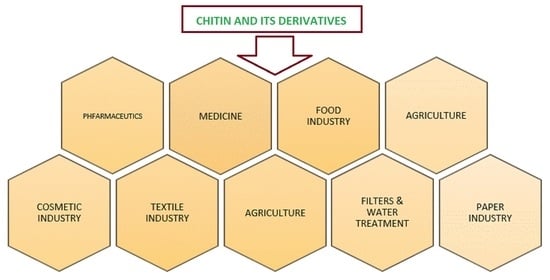
:1. Introduction
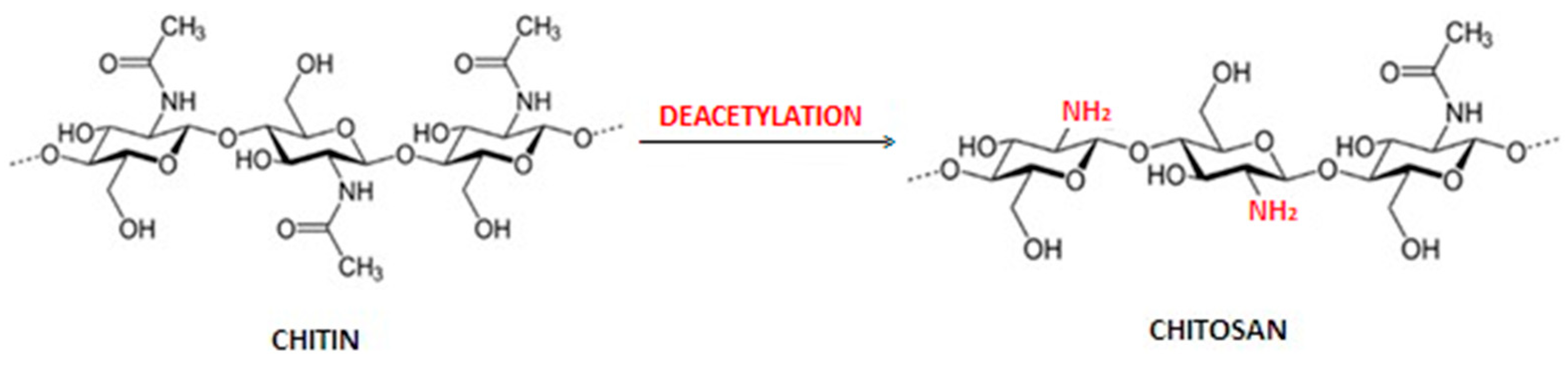
1.1. Obtaining Chitin and Chitosan
1.2. Methods of Chemical Modification of Chitosan
2. Biomimetic Materials as Inspiration for Functional Materials with Chitosan
3. Applications
3.1. Application of Chitosan in Cosmetic Industry
3.2. Application of Chitosan in Packaging and Food Industry
3.3. Application of Chitosan in Medicine
3.3.1. Tissue Engineering
3.3.2. Antibacterial Activity
3.3.3. Chitosan Hemostatic Dressings
3.4. Application of Chitosan in Agriculture
3.5. Other Important Applications
3.5.1. Water and Wastewater Treatments
3.5.2. Textile Industry
3.5.3. Pulp and Paper Industry
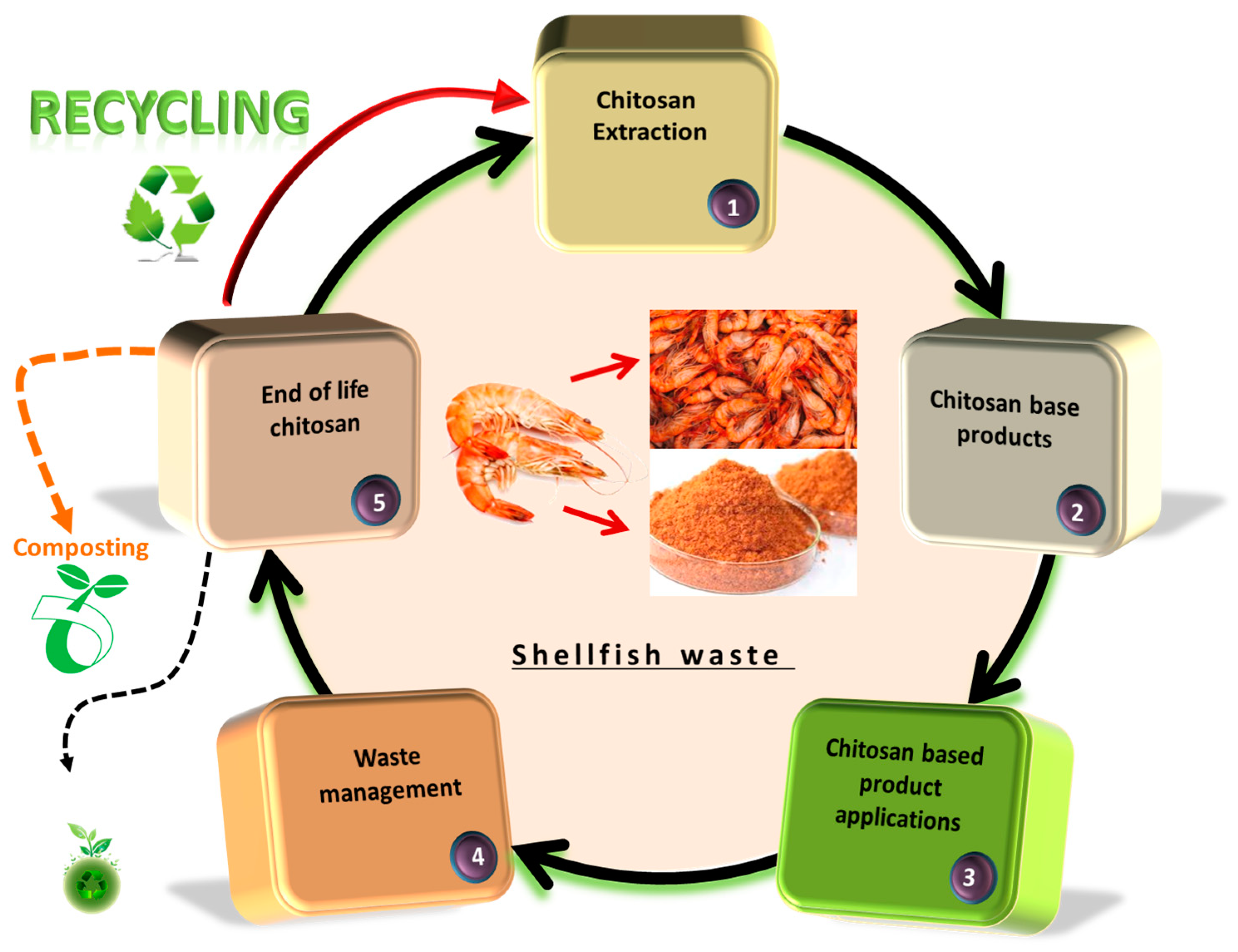
4. The Life Cycle Assessment of Chitin and Chitosan in Relation to Circular Economy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piątkowski, M. Chemiczna modyfikacja chitozanu w polu promieniowania mikrofalowego. Tech. Trans. Chem. 2008, 105, 101–113. [Google Scholar]
- Khoushab, F.; Yamabhai, M. Chitin Research Revised. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shi, X.; Li, X.; Cai, J.; Duan, B.; Du, Y. Homogeneous synthesis and characterization of quaternized chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2012, 87, 422–426. [Google Scholar]
- Struszczyk, M.H. Chitin and Chitosan. Part 1. Properties and production. Polimery 2002, 47, 316–325. [Google Scholar] [CrossRef]
- Kaya, M.; Seyyar, O.; Baran, T.; Erdoğan, S.; Kar, M. A Physicochemical Characterization of the Fully Acetylated Chitin Structure Isolated from Two Spider Species. Int. J. Biol. Macromol. 2014, 65, 553–558. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Juárez-de la Rosa, B.A.; Quintana, P.; Ardisson, P.L.; Yáñez-Limón, J.M.; Alvarado-Gil, J.J. Effects of thermal treatments on the structure of two black coral species chitinous exoskeleton. J. Mater. Sci. 2012, 47, 990–998. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucha, M.; Miśkiewicz, D.; Pawlak, A. Chitosan and its mixtures. Properties and applications. Przemysł Chemiczny 2003, 82, 1138–1142. [Google Scholar]
- Lavall, R.L.; Assis, O.B.; Campana-Filho, S.P. Beta-chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Hackman, R.H.; Goldberg, M. Studies on Chitin VI: Nature of Alpha-and Beta-Chitins. Aust. J. Biol. Sci. 1965, 18, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Einbu, A.; Naess, S.N.; Elgsaeter, A.; Vårum, K.M. Solution properties of chitin in alkali. Biomacromolecules 2004, 5, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Terbojevich, M.; Carraro, C.; Cosani, A.; Marsano, E. Solution studies of the chitin-lithium chloride-N, N-dimethylacetamide system. Carbohydr. Res. 1988, 180, 73–86. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Li, G.; Du, Y.; Tao, Y.; Liu, Y.; Li, S.; Hu, X.; Yang, J. Dilute solution properties of four natural chitin in NaOH/urea aqueous. Carbohydr. Polym. 2010, 80, 970–976. [Google Scholar] [CrossRef]
- Li, G.; Du, Y.; Tao, Y.; Deng, H.; Luo, X.; Yang, J. Iron(II) cross-linked chitin-based gel beads: Preparation, magnetic property and adsorption of methyl orange. Carbohydr. Polym. 2010, 82, 706–713. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cord-Landwehr, S.; Moerschbacher, B.M. Deciphering the ChitoCode: Fungal chitins and chitosans as functional biopolymers. Fungal Biol. Biotechnol. 2021, 8, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan Chemistry: Relevance to the Biomedical Sciences. Polysacch. I Struct. Charact. Use 2005, 186, 151–209. [Google Scholar]
- Vinsova, J.; Vavrikova, E. Recent advances in drugs and prodrugs design of chitosan. Curr. Pharm. Des. 2008, 14, 1311–1326. [Google Scholar] [CrossRef]
- Struszczyk, H.; Allan, G.G. Chitozane modification method. PL Patent No PL 127084 B2, 30 December 1983. [Google Scholar]
- Roberts, G.A.F. Chitin Chemistry, 1st ed.; Macmillan Press Ltd.: London, UK, 1992. [Google Scholar]
- Jaworska, M.M. Growth of Absidia orchidis fungi in submerge cultures. Inz. Chem. I Proces. 2006, 27, 47–58. [Google Scholar]
- Suyatma, N.E.; Copinet, A.; Tighzert, L.; Coma, V. Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J. Polym. Environ. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Leffler, C.C.; Müller, B.W. Influence of the acid type on the physical and drug liberation properties of chitosan–gelatin sponges. Int. J. Pharm. 2000, 194, 229–237. [Google Scholar] [CrossRef]
- Piątkowski, M.; Bogdał, D.; Radomski, P.; Jarosiński, A. Application of chemically modified chitosan in metal ions sorption. Tech. Trans. Chem. 2010, 10, 257–266. [Google Scholar]
- Masuelli, M.A. Mark-Houwink parameters for aqueous-soluble polymers and biopolymers at various temperatures. J. Polym. Biopolym. Phys. Chem. 2014, 2, 37–43. [Google Scholar]
- Meister, J. Polymer Modification: Principles, Techniques and Applications, 1st ed.; Marcel Dekker: New York, NY, USA; CRC Press: Boca Raton, FL, USA, 2000; pp. 5–20. [Google Scholar]
- Braun, D.; Cherdron, H.; Rehahn, M.; Ritter, H.; Voit, B. Polymer Synthesis: Theory and Practice. Fundamentals, Methods, Experiments, 5th ed.; Springer: Heidelberg, Germany, 2001; pp. 33–375. [Google Scholar]
- Ostrowska-Czubenko, J.; Pieróg, M.; Gierszewska, M. Modification of chitosan—A concise overview. Wiadomości Chem. 2016, 70, 9–10. [Google Scholar]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Kana, M.T.H.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Gomez d’Ayala, G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef]
- Kurita, K. Controlled Functionalization of the Polysaccharide Chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Gzyra-Jagieła, K.; Pęczek, B.; Wiśniewska-Wrona, M.; Gutowska, N. Physicochemical Properties of Chitosan and its Degradation Products. In Chitin and Chitosan, 1st ed.; van den Broek, L.A.M., Boeriu, C.G., Eds.; Wiley: Wageningen, The Netherlands, 2019; Volume 3, pp. 61–80. [Google Scholar]
- Sashiwa, H.; Aiba, S.I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Prashanth, K.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Tech. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Sahoo, D.; Nayak, P.L. Chitosan: The Most Valuable Derivative of Chitin. In Biopolymers: Biomedical and Environmental Applications, 1st ed.; Kalia, S., Averous, L., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 129–166. [Google Scholar]
- Anitha, A.; Rejinold, N.S.; Bumgardner, J.D.; Nair, S.V.; Jayakumar, R. Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics, 1st ed.; Sarmento, B., das Neves, J., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 107–124. [Google Scholar]
- Thomas, D.; Thomas, S. Chemical modification of chitosan and its biomedical application. In Biopolymer Nanocomposites: Processing, Properties and Applications, 1st ed.; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 33–51. [Google Scholar]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifications of chitosan: Syntheses and applications. In Critical Reviews™ in Therapeutic Drug Carrier Systems, 1st ed.; PB Begell House: Danbury, CT, USA, 2013; pp. 10–90. [Google Scholar]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosan. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Goszczyńska, A.; Gołda-Cępa, M.; Kotarba, A.; Sobolewski, P.; El Fray, M. Biofunctional catheter coatings based on chitosan-fatty acids derivatives. Carbohydr. Polym. 2019, 225, 115263. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Kaczorowski, P.; El Fray, M. Spin-coated chitosan on copolyester substrates. Prog. Chem. Appl. Chitin Deriv. 2015, 20, 236–245. [Google Scholar] [CrossRef]
- Alkabli, J. Progress in preparation of thiolated, crosslinked, and imino-chitosan derivatives targeting specific applications. Eur. Polym. J. 2022, 165, 110998. [Google Scholar] [CrossRef]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef]
- Yong, S.K.; Bolan, N.S.; Lombi, E.; Skinner, W.; Guibal, E. Sulfur-Containing Chitin and Chitosan Derivatives as Trace Metal Adsorbents: A Review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1741–1794. [Google Scholar] [CrossRef]
- Shastri, D.H. Thiolated chitosan: A boon to ocular delivery of therapeutics. MOJ Bioequiv. Availab 2017, 3, 34–37. [Google Scholar] [CrossRef]
- Ciro, Y.; Rojas, J.; Salamanca, C. Thiolated chitosan: A promising strategy for improving the effectiveness of anticancer drugs. In Analytical and Pharmceutical Chemistry; SMGroup: Dover, DE, USA, 2017; pp. 1–7. [Google Scholar]
- Yong, S.K.; Bolan, N.; Lombi, E.; Skinner, W. Synthesis and characterization of thiolated chitosan beads for removal of Cu(II) and Cd(II) from wastewater. Water Air Soil Pollut. 2013, 224, 1720–1726. [Google Scholar] [CrossRef]
- Radomski, P.; Piątkowski, M.; Bogdał, D.; Jarosiński, A. The use of chitosan and its modified derivatives to remove trace amounts of heavy metals from industrial wastewater. Chemik 2014, 68, 39–46. [Google Scholar]
- Jayakumar, R.; Selvamurugan, N.; Nair, S.V.; Tokura, S.; Tamura, H. Preparative methods of phosphorylated chitin and chitosan—An overview. Int. J. Biol. Macromol. 2008, 43, 221–225. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodriguez, N.M.; Diaz, M.F.; Rodriguez, M.S.; Heras, A.; Agullo, E. N-methylene phosphonic chitosan. Effect of preparation methods on its properties. Carbohydr. Polym. 2003, 52, 39–46. [Google Scholar] [CrossRef]
- Jayakumar, R.; Rajkumar, M.; Freitas, H.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H. Preparation, characterization, bioactive and metal uptake studies of alginate/phosphorylated chitin blend films. Int. J. Biol. Macromol. 2009, 44, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Yaoa, F.; Chen, W. A study on cytocompatible poly(chitosan-g-L-lactic acid). Polymer 2003, 44, 6435–6441. [Google Scholar] [CrossRef]
- Wyrębska, Ł.; Szuster, L.; Stawska, H. Synthesis and application of new derivatives of selected polysaccharides Part I: Literature review. Technol. I Jakość Wyr. 2014, 59, 3–16. [Google Scholar]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohyd. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Mi, F.L.; Sung, H.W.; Shyu, S.S.; Su, C.C.; Peng, C.K. Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer 2003, 44, 6521–6530. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Teng, D.; Deng, L.; Yao, F.; Shen, J.; Zhu, A. Chitosan—Based Hydrogels: Functions and Application; CRC Press: Boca Raton, FL, USA, 2012; pp. 25–179. [Google Scholar]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Sung, H.W. Chitosan: Its applications in drug-eluting devices. Chitosan Biomater. I 2011, 1, 185–230. [Google Scholar]
- Pahuja, S.; Aggarwal, S.; Sarup, P. Formulation and characterization of losartan loaded chitosan microspheres: Effect of crosslinking agents. Drug Res. 2021, 71, 204–212. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohyd. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Peng, C.K. Characterization of ring-opening polymerization of genipin and pH-dependent cross-linking reactions between chitosan and genipin. J. Polym. Sci. A Polym. Chem. 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, J.Y.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Li, C.; Xie, X.; Li, G.; Hu, Z.; Li, S. Research Progress of Chitosan-Based Biomimetic Materials. Mar. Drugs 2021, 19, 372. [Google Scholar] [CrossRef]
- Naik, R.R.; Singamaneni, S. Introduction: Bioinspired and Biomimetic Materials. Chem. Rev. 2017, 117, 12581–12583. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Wang, Y.L.; Cui, H.Q.; Du, X.M. Bio-inspired sensing and actuating materials. J. Mater. Chem. C 2019, 7, 6493–6511. [Google Scholar] [CrossRef]
- Mano, J.F. Biomimetic Approaches for Biomaterials Development 3; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Kim, E.; Kang, M.; Liu, H.; Cao, C.; Liu, C.; Bentley, W.E.; Qu, X.; Payne, G.F. Pro- and Anti-oxidant Properties of Redox-Active Catechol-Chitosan Films. Front. Chem. 2019, 7, 541. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.P.; Koh, M.Y.; Kim, P.; Lee, J.H.; Shin, M.; Lee, H. Chitosan-catechol: A writable bioink under serum culture media. Biomater. Sci. 2018, 6, 1040–1047. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Zvarec, O.; Purushotham, S.; Masic, A.; Ramanujan, R.V.; Miserez, A. Catechol-functionalized chitosan/iron oxide nanoparticle composite inspired by mussel thread coating and squid beak interfacial chemistry. Langmuir 2013, 29, 10899–10906. [Google Scholar] [CrossRef]
- Machałowski, T.; Jesionowski, T. Hemolymph of molluscan origin: From biochemistry to modern biomaterials science. Appl. Phys. A 2021, 127, 3. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and adhesive properties of multilayered coatings based on catechol-functionalized chitosan/hyaluronic acid and bioactive glass nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.T.; Hunger, P.M.; Kalidindi, S.R.; Wegst, U.G.K. Nacre-like hybrid films: Structure, properties, and the effect of relative humidity. J. Mech. Behav. Biomed. Mater. 2015, 55, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.B.; Fang, H.Y.; Tan, Z.H.; Wu, L.H.; Yu, S.H. Biologically Inspired, Strong, Transparent, and Functional Layered Organic–Inorganic Hybrid Films. Angew. Chem. Int. Ed. 2010, 49, 2140–2145. [Google Scholar] [CrossRef]
- Yao, H.B.; Tan, Z.H.; Fang, H.Y.; Yu, S.H. Artificial Nacre-like Bionanocomposite Films from the Self-Assembly of Chitosan-Montmorillonite Hybrid Building Blocks. Angew. Chem. Int. Ed. 2010, 49, 10127–10131. [Google Scholar] [CrossRef]
- Xie, H.L.; Lai, X.J.; Wang, Y.L.; Li, H.Q.; Zeng, X.R. A green approach to fabricating nacre-inspired nanocoating for super-efficiently fire-safe polymers via one-step self-assembly. J. Hazard. Mater. 2018, 365, 125–136. [Google Scholar] [CrossRef]
- Fang, Y.C.; Liu, X.H.; Zheng, H.L.; Shang, W.C. Bio-inspired fabrication of nacre-mimetic hybrid nanocoating for eco-friendly fire-resistant precious cellulosic chinese xuan paper—Sciencedirect. Carbohydr. Polym. 2020, 235, 115782. [Google Scholar] [CrossRef]
- Saito, A.; Miyazaki, H.; Fujie, T.; Ohtsubo, S.; Kinoshita, M.; Saitoh, D.; Takeoka, S. Therapeutic efficacy of an antibiotic-loaded nanosheet in a murine burn-wound infection model. Acta Biomater. 2012, 8, 2932–2940. [Google Scholar] [CrossRef]
- Huang, G.Y.; Li, F.; Zhao, X.; Ma, Y.F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenviron. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Singh, M.; Berkland, C.; Detamore, M.S. Strategies and applications for incorporating physical and chemical signal gradients in tissue engineering. Tissue Eng. Part B Rev. 2008, 14, 341–366. [Google Scholar] [CrossRef]
- Wenger, M.P.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical properties of collagen fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- María, P.B.; Lorena, B.G.; Fung, S.; Kohn, J.; Blanca, V.; Julio, S.R. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar]
- Ewa, S.Z.; Piotr, J.; Ewa, D.; Małgorzata, K.B.; Łukasz, Z.; Maciej, B.; Alicja, R.K.; Beata, K. Modification of chitosan fibers with short peptides as a model of synthetic extracellular matrix. J. Mol. Struct. 2020, 1211, 128061. [Google Scholar]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Tangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked extracellular matrix of calcified soft tissue based on chitosan/gelatin/compounded calcium phosphate hydrogel to design ex vivo model for heterotopic ossification. Mater. Des. 2017, 134, 486–493. [Google Scholar] [CrossRef]
- Park, K.C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef]
- Liu, M.; Peng, Z.L.; Yao, Y.; Yang, Y.Z.; Chen, S.H. A flexible functional surface for efficient water collection. ACS Appl. Mater. Interfaces 2020, 12, 12256–12263. [Google Scholar] [CrossRef]
- Al-Gharabli, S.; Al-Omari, B.; Kujawski, W.; Kujawa, J. Biomimetic hybrid membranes with covalently anchored chitosan—Material design, transport and separation. Desalination 2020, 491, 114550. [Google Scholar] [CrossRef]
- Zhang, L.W.; Ma, S.J.; Chen, Y.; Wang, Y.; Ou, J.; Uyama, H.; Ye, M.L. Facile Fabrication of Biomimetic Chitosan Membrane with Honeycomb-Like Structure for Enrichment of Glycosylated Peptides. Anal. Chem. 2019, 91, 2985–2993. [Google Scholar] [CrossRef]
- Dai, C.B.; Li, Y.; Pan, W.Z.; Wang, G.Q.; Huang, R.Q.; Bu, Y.Y.; Liao, X.J.; Guo, K.J.; Gao, F.L. Three-Dimensional High Porosity Chitosan/Honeycomb Porous Carbon/Hydroxyapatite Scaffold with Enhanced Osteoinductivity for Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 6, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Xiong, S.W.; Jiang, M.Y.; Chen, L.Y.; Zheng, K.; Fu, P.G.; Gai, J.G. Highly thermally conductive and eco-friendly oh-hbn/chitosan nanocomposites by constructing a honeycomb thermal network. Carbohydr. Polym. 2021, 266, 118127. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Boening, K.W.; Grychowska, N.; Paradowska-Stolarz, A. Clinical Application of Chitosan in Dental Specialities. Mini-Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Lennon, M.A. Effective use of fluorides for the prevention of dental caries in the 21st century: The WHO approach. Community Dent. Oral Epidemiol. 2004, 32, 319–321. [Google Scholar] [CrossRef]
- Karpinski, T.M.; Szkaradkiewicz, A.K. Microbiology of dental caries. J. Biol. Earth Sci. 2013, 3, 21–24. [Google Scholar]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Souza, M.G.M.; Andrade Silva, M.L.; da Silva Filho, A.A.; Martins, C.H.G.; Miller Crotti, A.E.; Pauletti, P.M.; Groppo, M.; et al. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: Relevance of carnosic acid and carnosol. Chem. Biodivers. 2010, 7, 1835–1840. [Google Scholar] [CrossRef]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evalua-tion of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundaments and Application of Chitosan. Sustain. Agric. Rev. 2019, 35, 49–123. [Google Scholar]
- Akıncıbay, H.; Şenel, S.; Yetkin Ay, Z. Application of chitosan gel in the treatment of chronic periodontitis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 290–296. [Google Scholar] [CrossRef]
- Schlüter, N.; Klimek, J.; Ganss, C. Effect of a chitosan additive to a Sn2+-containing toothpaste on its anti-erosive/anti-abrasive efficacy—A controlled randomised in situ trial Clin. Oral Investig. 2014, 18, 107–115. [Google Scholar] [CrossRef]
- Carvalho, T.; Lussi, A. Combined effect of a fluoride-, stannous- and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion–abrasion. J. Dent. 2014, 42, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Silva, S.; Madureira, A.; Cardelle-Cobas, A.; Tavaria, F.; Pintado, M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr. Polym. 2014, 101, 1081–1086. [Google Scholar] [CrossRef]
- Farias, J.M.; Stamford, T.C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing a biosurfactant and chitosan: An eco-sustainable option for the control of cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Moghadamnia, A.A.; Kazemi, S.; Shirzad, A.; Motallebnejad, M. Effect of 0.5% Chitosan mouthwash on recurrent aphthous stomatitis: A randomized double-blind crossover clinical trial. Electron. Physician 2018, 10, 6912–6919. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and characterization of collagen/chitosan/hyaluronic acid thin films for application in hair care cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Berkó, S.; Maroda, M.; Bodnár, M.; Erős, G.; Hartmann, P.; Szentner, K.; Szabó-Révész, P.; Kemény, L.; Borbély, J.; Csányi, E. Advantages of cross-linked versus linear hyaluronic acid for semisolid skin delivery systems. Eur. Polym. J. 2013, 49, 2511–2517. [Google Scholar] [CrossRef]
- Chen, K.; Guo, B.; Luo, J. Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohydr. Polym. 2017, 173, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2013, 40, 155–175. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aleem, A.; El-Aidie, M. A Review on Chitosan: Ecofriendly Multiple Potential Applications in the Food Industry. Int. J. Adv. Life Sci. Res. 2018, 1, 1–14. [Google Scholar]
- Yuan, G.F.; Zhang, X.J.; Tang, W.Y.; Sun, H.Y. Effect of chitosan coating combined with green tea extract on the melanosis and quality of Pacific white shrimp during storage in ice. CYTA-J. Food. 2016, 14, 35–40. [Google Scholar] [CrossRef]
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int. J. Food Microbiol. 2017, 246, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as food preservative. J. Appl. Microbiol. 2018, 126, 1318–1331. [Google Scholar] [CrossRef]
- Alfaifi, M.Y.; Alkabli, J.; Elshaarawy, R.F.M. Suppressing of milk-borne pathogenic using new water-soluble chitosan-azidopropanoic acid conjugate: Targeting milk-preservation quality improvement. Int. J. Biol. Macromol. 2020, 164, 1519–1526. [Google Scholar] [CrossRef]
- Picariello, L.; Rinaldi, A.; Blaiotta, G.; Moio, L.; Pirozzi, P.; Gambuti, A. Effectiveness of chitosan as an alternative to sulfites in red wine production. Eur. Food Res. Technol. 2020, 246, 1795–1804. [Google Scholar] [CrossRef]
- Islam, T.; Arfin, N.; Parvin, S.; Dana, N.H.; Rahman, K.S.; Zzaman, W.; Islam, M.N. The impact of chitosan and guava leaf extract as preservative to extend the shelf-life of fruits. Int. Food Res. J. 2018, 25, 2056–2062. [Google Scholar]
- Rocha, M.; Angélica, M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Assaâd, S.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104 Pt A, 953–962. [Google Scholar] [CrossRef]
- Bindes, M.M.M.; Reis, M.H.M.; Cardoso, V.L.; Boffito, D.C. Ultrasound-assisted extraction of bioactive compounds from green tea leaves and clarification with natural coagulants (chitosan and Moringa oleífera seeds). Ultrason. Sonochem. 2018, 15, 111–119. [Google Scholar]
- Irshad, M.; Murtza, A.; Zafar, M.; Bhatti, K.H.; Rehman, A.; Anwar, Z. Chitosan-immobilized pectinolytics with novel catalytic features and fruit juice clari-fication potentialities. Int. J. Biol. Macromol. 2017, 104, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Gassara, F.; Antzak, C.; Ajila, C.; Sarma, S.; Brar, S.K.; Verma, M. Chitin and chitosan as natural flocculants for beer clarification. J. Food Eng. 2015, 166, 80–85. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.; Yue, X.Y.; Fu, R.; Liang, J.; Gao, X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chem. 2016, 221, 1671–1677. [Google Scholar] [CrossRef]
- Piran, F.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Controlling the Antioxidant Activity of Green Tea Extract through Encapsulation in Chitosan-Citrate Nanogel. J. Food Qual. 2020, 2020, 7935420. [Google Scholar] [CrossRef]
- Hashem, G.; Ahmed, G.; Fernández-González, A.; Díaz García, M.E. Nano-encapsulation of grape and apple pomace phenolic extract in chitosan and soy protein via nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar]
- Cazón, P.; Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 2020, 18, 257–267. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nano-particle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life. 2020, 24, 100511. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranaz, I.; Mengíbar, M.; Harris, R. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.-A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, J.; Liu, X.; Hu, Q.; Huang, J. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J. Mater. Chem. B 2013, 28, 3477–3485. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Rezaei Mokarram, R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Cerqueira, M.A.; Texeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Effect of an Edible Nanomultilayer Coating by Electrostatic Self-Assembly on the Shelf Life of Fresh-Cut Mangoes. Food Bioprocess Technol. 2014, 8, 647–654. [Google Scholar] [CrossRef]
- Martiñon, M.E.; Moreira, R.G.; Castell-Perez, M.E.; Gomes, C. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 C. LWT-Food Sci. Technol. 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The effect of different preparation methods on the development of chitosan/thyme oil/montmorillonite nanocomposite active packaging films. J. Food Process. Preserv. 2020, 44, e14327. [Google Scholar] [CrossRef]
- Pal, A.K.; Katiyar, V. Nanoamphiphilic Chitosan Dispersed Poly(lactic acid) Bionanocomposite Films with Improved Thermal, Mechanical, and Gas Barrier Properties. Biomacromolecules 2016, 17, 2603–2618. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, D.; Chuai, C.Z. Mechanical and Barrier Properties of LLDPE/Chitosan Blown Films for Packaging. Packag. Technol. Sci. 2015, 28, 915–923. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life. 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Li, Y.-C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Negi, Y.S. Effect of Varying Filler Concentration on Zinc Oxide Nanoparticle Embedded Chitosan Films as Potential Food Packaging Material. J. Polym. Environ. 2016, 25, 1087–1098. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under com-mercial storage conditions. LWT-Food. Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology: A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Ribeiro-Santos, R.; Rodrigues, P.F.; Otoni, C.G.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Nanomaterial migration from composites into food matrices. In Composite Materials for Food Packaging; Cirillo, G., Kozlowski, M.A., Spizzirri, U.G., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2018; p. 465. ISBN 9781119160205. [Google Scholar]
- Ganesan, S.; Alagarasan, J.K.; Sonaimuthu, M.; Aruchamy, K.; Alkallas, F.H.; Ben Gouider Trabelsi, A.; Kusmartsev, F.V.; Polisetti, V.; Lee, M.; Lo, H.M. Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity. Mar Drugs. 2022, 20, 733. [Google Scholar] [CrossRef]
- Malmquist, J.P.; Clemens, S.C.; Oien, H.J.; Wilson, S.L. Hemostasis of oral surgery wounds with the HemCon Dental Dressing. J. Oral Maxillofac. Surg. 2008, 66, 1177–1183. [Google Scholar] [CrossRef]
- Brown, M.A.; Daya, M.R.; Worley, J.A. Experience with chitosan dressings in a civilian EMS system. J. Emerg. Med. 2009, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Francesko, A.; Tzanov, T. Chitin, chitosan and derivatives for wound healing and tissue engineering. Adv. Biochem. Eng. Biotechnol. 2011, 125, 1–27. [Google Scholar] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.E.; Wang, N.; Liu, X.; An, Q.; Ye, X.-M.; Zhao, Z.-T.; Zhao, M.; Han, Y.; et al. Chemical composition and antioxidant activities of polysaccharides from Yingshan Cloud Mist tea. Oxidative Med. Cell. Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs. 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Feng, T.; Du, Y.M.; Li, J.; Wei, Y.A.; Yao, P.J. Antioxidant activity of half N-acetylated water-soluble chitosan in vitro. Eur. Food Res. Technol. 2007, 225, 133. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chou, C.C. Antioxidative activities of water-soluble disaccharide chitosan derivatives. Food Res. Int. 2004, 37, 883–889. [Google Scholar] [CrossRef]
- Choo, C.K.; Kong, X.Y.; Goh, T.L.; Ngoh, G.C.; Horri, B.A.; Salamatinia, B. Chitosan/halloysite beads fabricated by ultrasonic-assisted extrusion-dripping and a case study application for copper ion removal. Carbohydr. Polym. 2016, 138, 16–26. [Google Scholar] [CrossRef]
- Brunel, F.; Véron, L.; Ladaviere, C.; David, L.; Domard, A.; Delair, T. Synthesis and structural characterization of chitosan nanogels. Langmuir 2009, 25, 8935–8943. [Google Scholar] [CrossRef]
- Porporatto, C.; Bianco, I.D.; Riera, C.M.; Correa, S.G. Chitosan induces different L-arginine metabolic pathways in resting and inflammatory macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhao, L.; Yu, Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.N.; Zhang, C.N.; Xu, R.; Niu, J.F.; Song, H.J.; Zhang, X.Y.; Wang, W.W.; Wang, Y.M.; Li, C.; Wei, X.Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ignacak, J.; Wiśniewska-Wrona, M.; Dulińska-Litewka, J.; Pałka, I.; Kucharska, M. The role of oligochitosans in AKT kinase regulation. Prog. Chem. Appl. Chitin Deriv. 2015, XX, 73–81. [Google Scholar] [CrossRef]
- Ignacak, J.; Wiśniewska-Wrona, M.; Dulińska-Litewka, J.; Pałka, I.; Kucharska, M.; Kazimierski, J. The role of chitosan in AKT kinase regulation activity. Prog. Chem. Appl. Chitin Deriv. 2016, XXI, 73–82. [Google Scholar] [CrossRef]
- Huang, R.; Mendisa, E.; Rajapaksea, N.; Kim, S.-K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2005, 78, 2399–2408. [Google Scholar] [CrossRef]
- Liu, L.; Yi, X.; Liu, J.; Zhang, E.; Weiling, L. Inhibitory effect of chitosan oligosaccharide on human hepatoma cells in vitro. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 272–277. [Google Scholar]
- Nam, K.S.; Kim, M.K.; Shon, Y.H. Chemopreventive effect of chitosan oligosaccharide against colon carcinogenesis. J. Microbiol. Biotechnol. 2007, 17, 1546–1549. [Google Scholar]
- Mattaveewonga, T.; Wongkrasanta, P.; Chanchaib, S.; Pichyangkurac, R.; Chatsudthipongade, V.; Muanprasatade, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-κB and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef]
- Sikora, M.; Wisniewska-Wrona, M.; Arabski, M. Biomedical properties of chitosan: Use in tissue engineering. Adv. Hyg. Exp. Med. 2021, 75, 1020–1037. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan-based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Sułtankulow, B.; Berillo, D.; Sułtankulowa, K.; Tokaj, T.; Saparow, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Nosrati, H.; Pourmotabed, S.; Sharifi, E. A review on some natural biopolymers and their applications in angiogenesis and tissue engineering. J. Appl. Biotechnol. Rep. 2018, 5, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Raafat, D.; Bargen, K.; Haas, A.; Sahl., H.G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Park, K.; Seo, J. Regeneration Approach to Enhance the Antimicrobial and Antioxidant Activities of Chitosan for Biomedical Applications. Polymers 2022, 15, 132. [Google Scholar] [CrossRef]
- Basseri, H.; Bakhtijari, R.; Haszemi, S.J.; Baniardelani, M.; Shahraki, H.; Hosainpour, L. Antibacterial/Antifungal Activity of Extracted Chitosan from American Cockroach (Dictyoptera: Blattidae) and German Cockroach (Blattodea: Blattellidae). J. Med. Entomol. 2019, 56, 1208–1214. [Google Scholar] [CrossRef]
- Kucharska, M.; Sikora, M.; Brzoza-Malczewska, K.; Owczarek, M. Antimicrobial Properties of Chitin and Chitosan. In Chitin and Chitosan: Properties and Applications; Lambertus van den Broek, A.M., Boeriu, C.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; Chapter 7. [Google Scholar]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015, 10, 015014. [Google Scholar] [CrossRef] [PubMed]
- Santosh, S.; Biranje Pallavi, V.; Madiwale Kaustubh Patankar, C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Ravindra Adivarekar, V. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohyd. Polym. 2020, 239, 116106. [Google Scholar]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.; Piekarska, K.; Wiśniewska-Wrona, M. The Use of Carbohydrate Biopolymers in Plant Protection against Pathogenic Fungi. Polymers 2022, 14, 2854. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Chien, P.J.; Chou, C.C. Antifungal activity of chitosan and its application to control post-harvest quality and fungal rotting of Tankan citrus fruit (Citrus tankan Hayata). J. Sci. Food Agric. 2006, 86, 1964–1969. [Google Scholar] [CrossRef]
- Attjioui, M.; Gillet, D.; El Gueddari, N.E.; Moerschbacher, B.M. Synergistic antimicrobial effect of chitosan polymers and oligomers. Mol. Plant Microbe Interact. 2021, 34, 770–778. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Kaur, P.; Duhan, J.S.; Thakur, R. Comparative pot studies of chitosan and chitosan-metal nanocom-posites as nano-agrochemicals against fusarium wilt of chickpea (Cicer arietinum L.). Biocatal. Agric. Biotechnol. 2018, 14, 466–471. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Ghule, M.R.; Ramteke, P.K.; Ramteke, S.D.; Kodre, P.S.; Langote, A.; Gaikwad, A.V.; Holkar, S.K.; Jambhekar, H. Impact of chitosan seed treatment of fenugreek for management of root rot disease caused by Fusarium solani under in vitro and in vivo conditions. 3 Biotech 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Samarah, N.H.; Al-Quraan, N.A.; Massad, R.S.; Welbaum, G.E. Treatment of bell pepper (Capsicum annuum L.) seeds with chitosan increases chitinase and glucanase activities and enhances emergence in a standard cold test. Sci. Hortic. 2020, 269, 109393. [Google Scholar] [CrossRef]
- Madanipour, S.; Alimohammadi, M.; Rezaie, S.; Nabizadeh, R.; Khaniki, G.J.; Hadi, M.; Yousefi, M.; Bidgoli, S.M.; Yousefzadeh, S. Influence of postharvest application of chitosan combined with ethanolic extract of liquorice on shelflife of apple fruit. J. Environ. Health Sci. Eng. 2019, 17, 331–336. [Google Scholar] [CrossRef]
- Gonzalez-Saucedo, A.; Barrera-Necha, L.L.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Banos, S.; Hernandez-Lopez, M. Extension of the postharvest quality of bell pepper by applying nanostruc-tured coatings of chitosan with Byrsonima crassifolia extract (L.) Kunth. Postharvest Biol. Technol. 2019, 149, 74–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Ding, Q.; Yu, D.; Li, R. Novel dual modified alkali lignin based adsorbent for the removal of Pb2+ in water. Ind. Crops Prod. 2021, 173, 114100. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.; Zhang, W.; Zhao, R.; Ru, Y.; Liu, T. One-pot method to prepare lignin-based magnetic biosorbents for bioadsorption of heavy metal ions. Ind. Crops Prod. 2022, 176, 114387. [Google Scholar] [CrossRef]
- Harmoudi, H.E.; Gaini, L.E.; Daoudi, E.; Rhazi, M.; Boughaleb, Y.; Mhammedi, M.A.E.; Migalska-Zalas, A.; Bakasse, M. Removal of 2,4-D from aquaeous solution by adsorption processing using two biopolymers: Chitin and chitosan and their optical properties. Opt. Mater. 2014, 36, 1471–1477. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Chemical modification of chitin with polypyrrole for theuptake of Pb(II) and Cd(II) ions. Int. J. Biol. Macromol. 2015, 78, 157–164. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New ulva lactuca algae based chitosan bio-composites for bioremediation of Cd (II) ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, M.; Wang, J.X. Removal of Cu2+ and turbidity from wastewater by mercaptoacetyl chitosan. J. Hazard. Mater. 2009, 169, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.A.B.; Valle, R.C.S.C.; Bierhalz, A.C.K.; Bezerra, F.M.; Hernandez, A.L.; Lis Arias, M.J. Chitosan microcapsules: Methods of the production and use in the textile finishing. J. Appl. Polym. Sci. 2021, 138, 50482. [Google Scholar] [CrossRef]
- Benita, S. Microencapsulation Techniques for Parenteral Depot Systems and Their Application in the Pharmaceutical Industry. In Microencapsulation, 2nd ed.; Benita, S., Ed.; Taylor & Francis: New York, NY, USA, 2016; pp. 102–122. [Google Scholar]
- Lopes, S.; Afonso, C.; Fernandes, I.; Barreiro, M.F.; Costa, P.; Rodrigues, A.E. Chitosan-cellulose particles as delivery vehicles for limonene fragrance. Ind. Crops Prod. 2019, 139, 111407. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.B.; Zhang, Q.; Li, Y.C.; Li, J.J.; Liu, D.Y.; Peng, L.X. Konjac glucomannan octenyl succinate as a novel encapsulation wall material to improve curcumin stability and bioavailability. Carbohydr. Polym. 2020, 238, 116193. [Google Scholar] [CrossRef]
- Santos, S.S.; Rodrigues, L.M.; Costa, S.C.; Madrona, G.S. Antioxidant compounds from blackberry (Rubus fruticosus) pomace: Microencapsulation by spray-dryer and pH stability evaluation. Food Packag. Shelf Life 2019, 20, 100177. [Google Scholar] [CrossRef]
- Chen, S.; Zong, J.; Jiang, L.; Ma, C.; Li, H.; Zhang, D. Improvement of resveratrol release performance and stability in extruded microparticle by the α-amylase incorporation. J. Food Eng. 2020, 274, 109842. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chang, Y.H. Microencapsulation of a maca leaf polyphenol extract in mixture of maltodextrin and neutral polysaccharides extracted from maca roots. Int. J. Biol. Macromol. 2020, 150, 546–558. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.Y. Use of hybrid microcapsules, chitosan-methyl esterified sericite-tannin, for the removal of harmful lake algae and nutrient. Environ. Technol. 2020, 41, 822–831. [Google Scholar] [CrossRef]
- Chen, B.Y.; Kuo, H.W.; Sharma, V.K.; Den, W. Chitosan encapsulation of ferrateVi for controlled Release to Water: Mechanistic insights and Degradation of organic contaminant. Sci. Rep. 2019, 9, 100177. [Google Scholar]
- Du, W.; Yu, J.; Gu, S.; Wang, R.; Li, J.; Han, X.; Liu, Q. Effect of temperatures on self-healing capabilities of concrete with different shell composition microcapsules containing toluene-di-isocyanate. Constr. Build. Mater. 2020, 247, 118575. [Google Scholar] [CrossRef]
- Tözüm, M.S.; Alkan, C.; Alay Aksoy, S. Preparation of poly (methyl methacrylate-co-ethylene glycol dimethacrylate-co-glycidyl methacrylate) walled thermochromic microcapsules and their application to cotton fabrics. J. Appl. Polym. Sci. 2020, 137, 48815. [Google Scholar] [CrossRef]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of chitin/chitosan and their derivatives in the papermaking industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hassan, M.L.; Abou-Zeid, R.E.; El-Wakil, N.A. Novel nanofibrillated cellulose/chitosan nanoparticles nanocomposites films and their use for paper coating. Ind. Crops Prod. 2016, 93, 219–226. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, L.; He, B. Chitosan/Montmorillonite Coatings for the Fabrication of Food-Safe Greaseproof Paper. Polymers 2021, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wu, L.; Luo, Y.; Yu, F.; Li, H. A facile method to prepare cellulose fiber-based food packaging papers with improved mechanical strength, enhanced barrier, and antibacterial properties. Food Biosci. 2022, 48, 101729. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agricul-ture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Tulska, E. Możliwości zagospodarowania surowców odpadowych do produkcji opakowań. In Co w Przyrodzie Piszczy, 1st ed.; Perek-Długosz, A., Ed.; Wydawnictwo Fundacji Promovendi: Łódź, Poland, 2018; pp. 5–13. [Google Scholar]
- Communication from the Commission to the European Parliament; The Council; The European Economic and Social Committee; The Committee of the Regions. Closing the Loop—An EU Action Plan for the Circular Economy, COM(2015) 614 Final; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Communication from the Commission to the European Parliament; The Council; The European Economic and Social Committee; The Committee Regions. European Strategy for Plastics in a Circular Economy, COM(2018) 28 Final; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Eurostat Statistics Explained. Waste Statistics in 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 19 November 2022).
- Grand View Research, Market Analysis Report, Chitosan Market Size, Share and Trends Analysis Report by Application (Pharmaceutical & Biomedical, Water Treatment, Cosmetics, Food & Beverage), by Region (APAC, North America, Europe, MEA), And Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/global-chitosan-market (accessed on 19 November 2022).
- Muñoz, I.; Rodríguez, C.; Gillet, D.; Moerschbacher, B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Kijeńska, M.; Gworek, B.; Barański, A.; Tokarz, L. Life Cycle Assessment—LCA in LCAgri Project. In Proceedings of the PODR, Szepietowo, Poland, 2 June 2016. [Google Scholar]
- Ghosh, T.; Katiyar, V. Life Cycle Assessment of Chitosan. In Advances in Sustainable Polymers, 1st ed.; Katiyar, V., Kumar, A., Mulchandani, N., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 363–388. [Google Scholar]
- Beach, E.; Eckelman, M.J.; Cui, Z.; Brentner, L.; Zimmerman, J.B. Preferential technological and life cycle environmental performance of chitosan flocculation for harvesting of the green algae Neochloris oleoabundans. Bioresour. Technol. 2012, 121, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, P.G.; Mani, S. Life cycle assessment of manufacturing cellulose nanofibril-reinforced chitosan composite films for packaging applications. Int. J. Life Cycle Assess. 2022, 27, 380–394. [Google Scholar] [CrossRef]
- FAO; IFA. Global Estimates of Gaseous Emissions of NH3, NO and N2O from Agricultural Land; Food and Agriculture Organization of the United Nations (FAO) and International Fertilizer Industry Association (IFA): Brussels, Belgium, 2001. [Google Scholar]
- Bristow, J. Chitosan Manufacturing Process. U.S. Patent No. 8,318,913 B2, 27 November 2012. [Google Scholar]
- Boldrin, A.; Hartling, K.R.; Laugen, A.; Christensen, T.H. Environmental inventory modelling of the use of compost and peat in growthmedia preparation, 2009, Submitted to Resource, Conservation and Recycling. Resour. Conserv. Recycl. 2010, 54, 1250–1260. [Google Scholar] [CrossRef]
- Gulf Aquarium and Marine Station Cooperative (GAMS). Feasibility of Producing Value Added Products from Snow Crab Processing Waste in Cape Breton; C.P.697: Cheticamp, NS, Canada, 2010. [Google Scholar]
- Muñoz, I.; Milài Canals, L.; Clift, R. Consider a spherical man: A simple model to include human excretion in life cycle assessment of food products. J. Ind. Ecol. 2008, 12, 521–538. [Google Scholar] [CrossRef]





| Additive | Form | Result | Ref. | |
|---|---|---|---|---|
| Polysaccharide | Starch | Films | Improvement in water barrier properties along with enhanced antioxidant and antimicrobial activity | [145,147] |
| Cellulose | Films | Improvement in mechanical properties due to electrostatic interactions between the polymers | [148,149] | |
| Alginate | Films | Very good gas exchange and water vapor transmission | [150] | |
| Pectin | Films | Helps to maintain physicochemical and sensory values | [151] | |
| Synthetic polymers | Polyvinyl alcohol (PVA) | Films | Improvement in mechanical properties and enhanced barrier performances towards water and oxygen | [152] |
| PLA/starch | Films | Improvement in flexibility and thermal properties | [153] | |
| Low-density polyethylene | Films | Improvement in moisture barrier properties | [154] | |
| Other | Silver | Films | Improved antibacterial properties | [155,156] |
| Zinc oxide | Films | Improvement in moisture barrier, mechanical strength and antimicrobial activity | [157] | |
| Extracts from bee secretions (beeswax and propolis) | Films | Maintains food quality, both visual appearance and taste | [158] | |
| p-Coumaric acid (p-CA) | Not given | Partially enhanced water solubility and antioxidant property | [159] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. https://doi.org/10.3390/polym15040793
Piekarska K, Sikora M, Owczarek M, Jóźwik-Pruska J, Wiśniewska-Wrona M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers. 2023; 15(4):793. https://doi.org/10.3390/polym15040793
Chicago/Turabian StylePiekarska, Klaudia, Monika Sikora, Monika Owczarek, Jagoda Jóźwik-Pruska, and Maria Wiśniewska-Wrona. 2023. "Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application" Polymers 15, no. 4: 793. https://doi.org/10.3390/polym15040793
APA StylePiekarska, K., Sikora, M., Owczarek, M., Jóźwik-Pruska, J., & Wiśniewska-Wrona, M. (2023). Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers, 15(4), 793. https://doi.org/10.3390/polym15040793