Regulation of Intersubunit Interactions in Homotetramer of Glyceraldehyde-3-Phosphate Dehydrogenases upon Its Immobilization in Protein—Kappa-Carrageenan Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. ATR-FTIR Spectroscopy
2.3. Molecular Docking
3. Results and Discussion
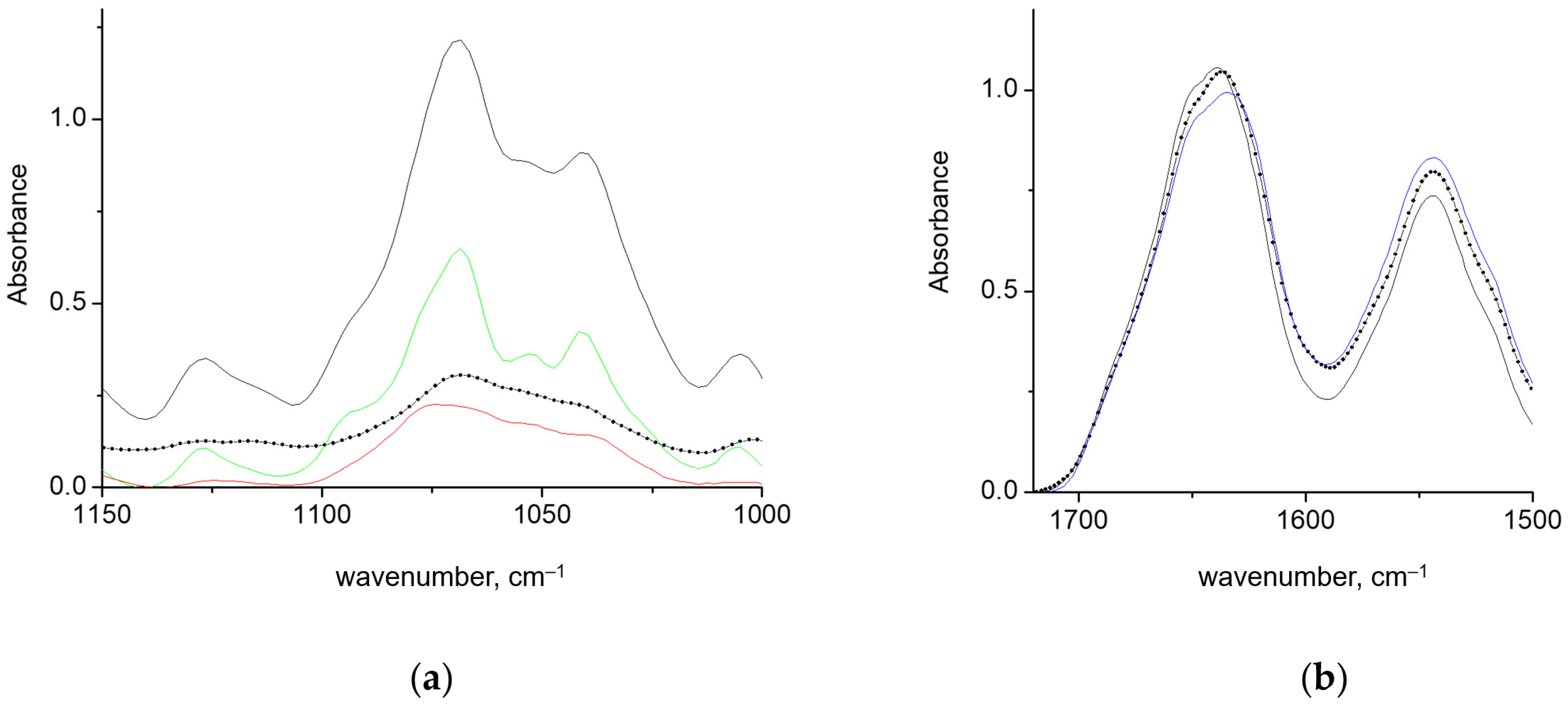
3.1. FTIR Spectroscopy Analysis
3.1.1. Gels of GAPDH and κ-carrageenan in Coil Conformation
3.1.2. Gels of GAPDH and κ-carrageenan in Helical Conformation
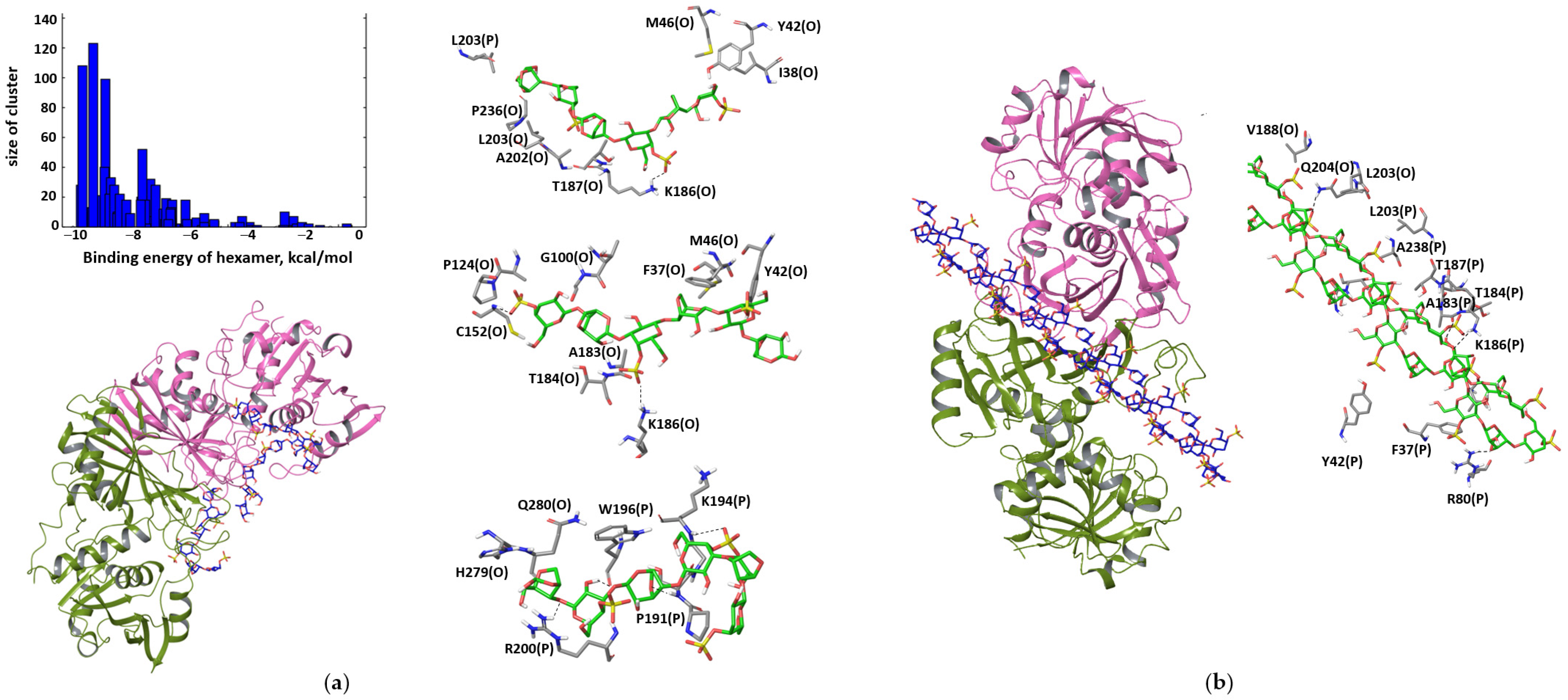
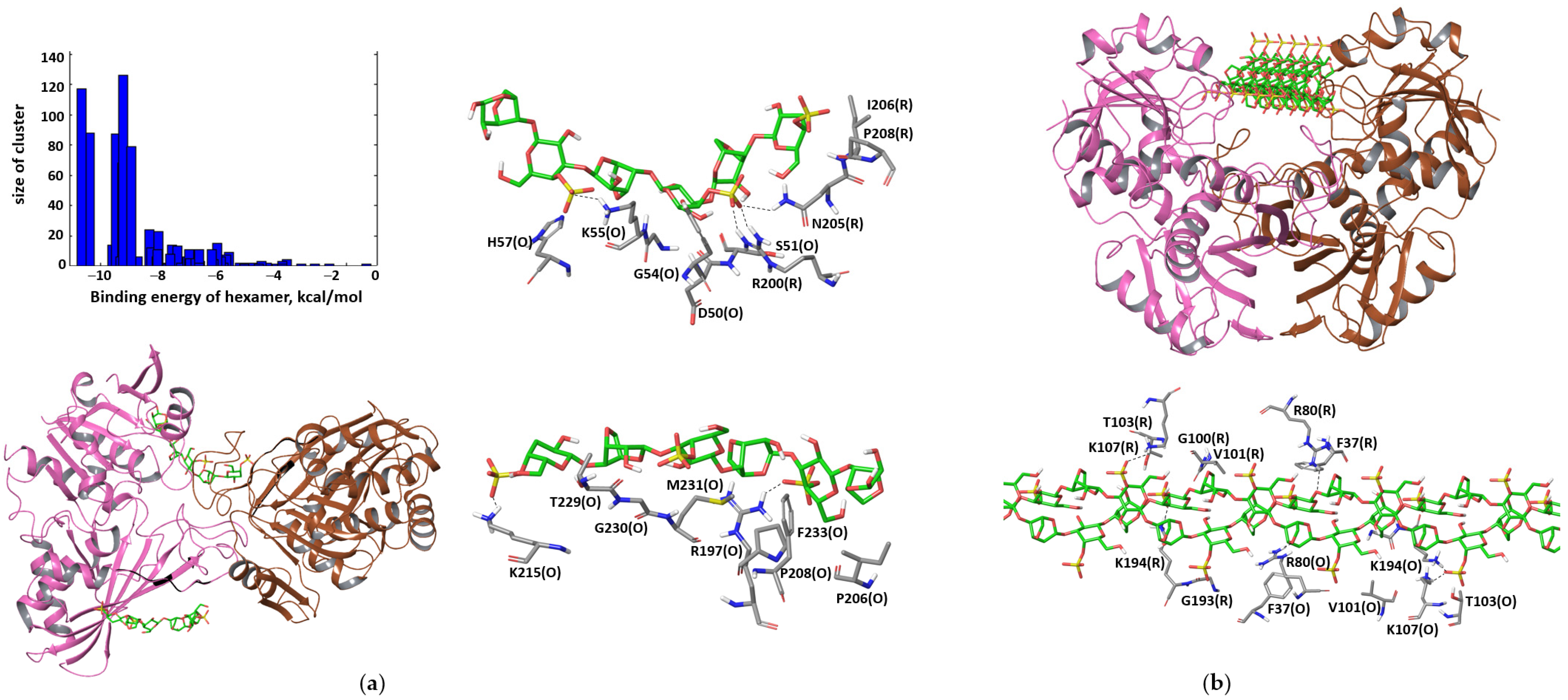
3.2. Computer Modeling of Interactions between GAPDH in Different Oligomeric States and κ-carrageenan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panina, Y.; Germond, A.; Masui, S.; Watanabe, T.M. Validation of Common Housekeeping Genes as Reference for qPCR Gene Expression Analysis During iPS Reprogramming Process. Sci. Rep. 2018, 8, 8716. [Google Scholar] [CrossRef] [PubMed]
- Sirover, M. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH): The Quintessential Moonlighting Protein in Normal Cell Function and in Human Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 1–306. [Google Scholar]
- Zheng, L.; Roeder, R.G.; Luo, Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 2003, 114, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, J.; Kim, J. Regulation of oncogenic transcription factor hTAF(II)68-TEC activity by human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Biochem. J. 2007, 404, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatton, W.G.; Chalmers-Redman, R.M.; Elstner, M.; Leesch, W.; Jagodzinski, F.B.; Stupak, D.P.; Sugrue, M.M.; Tatton, N.A. Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. J. Neural. Transm. Suppl. 2000, 60, 77–100. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Barinova, K.V.; Stroylova, Y.Y.; Semenyuk, P.I.; Schmalhausen, E.V. Glyceraldehyde-3-phosphate dehydrogenase: Aggregation mechanisms and impact on amyloid neurodegenerative diseases. Int. J. Biol. Macromol. 2017, 100, 55–66. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Semenyuk, P.I.; Kuravsky, M.L.; Ermakova, E.A.; Zuev, Y.F.; Muronetz, V.I. Structural basis for regulation of stability and activity in glyceraldehyde-3-phosphate dehydrogenases. Differential scanning calorimetry and molecular dynamics. J. Struct. Biol. 2015, 190, 224–235. [Google Scholar] [CrossRef]
- Chaudhary, S.; Dhiman, A.; Patidar, A.; Malhotra, H.; Talukdar, S.; Dilawari, R.; Chaubey, G.K.; Modanwal, R.; Raje, C.I.; Raje, M. Moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) modulates protein aggregation. Biochim. Biophys. Acta. Mol. Basis. Dis. 2021, 1867, 166202. [Google Scholar] [CrossRef]
- Ávila, C.L.; Torres-Bugeau, C.M.; Barbosa, L.R.; Sales, E.M.; Ouidja, M.O.; Socías, S.B.; Celej, M.S.; Raisman-Vozari, R.; Papy-Garcia, D.; Itri, R.; et al. Structural characterization of heparin-induced glyceraldehyde-3-phosphate dehydrogenase protofibrils preventing α-synuclein oligomeric species toxicity. J. Biol. Chem. 2014, 289, 13838–13850. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef]
- Arutyunova, E.I.; Domnina, L.V.; Chudinova, A.A.; Makshakova, O.N.; Arutyunov, D.Y.; Muronetz, V.I. Localization of non-native D-glyceraldehyde-3-phosphate dehydrogenase in growing and apoptotic HeLa cells. Biochemistry 2013, 78, 91–95. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Medvedeva, M.V.; Sevostyanova, I.A.; Schmalhausen, E.V. Modification of Glyceraldehyde-3-Phosphate Dehydrogenase with Nitric Oxide: Role in Signal Transduction and Development of Apoptosis. Biomolecules 2021, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Cortez, L.M.; Avila, C.L.; Bugeau, C.M.; Farías, R.N.; Morero, R.D.; Chehín, R.N. Glyceraldehyde-3-phosphate dehydrogenase tetramer dissociation and amyloid fibril formation induced by negatively charged membranes. FEBS Lett. 2010, 584, 625–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Tuominen, E.K.; Kinnunen, P.K. Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry 2004, 43, 10302–10307. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bugeau, C.M.; Avila, C.L.; Raisman-Vozari, R.; Papy-Garcia, D.; Itri, R.; Barbosa, L.R.; Cortez, L.M.; Sim, V.L.; Chehin, R.N. Characterization of heparin-induced glyceraldehyde-3-phosphate dehydrogenase early amyloid-like oligomers and their implication in alpha-synuclein aggregation. J. Biol. Chem. 2012, 287, 2398–2409. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-F.; Zhou, J.-M. Unfolding and aggregation-associated changes in the secondary structure of D-glyceraldehyde-3-phosphate dehydrogenase during denaturation by guanidine hydrochloride as monitored by FTIR. Biospectroscopy 1997, 3, 121–129. [Google Scholar] [CrossRef]
- Sunaga, K.; Takahashi, H.; Chuang, D.M.; Ishitani, R. Glyceraldehyde-3-phosphate dehydrogenase is over-expressed during apoptotic death of neuronal cultures and is recognized by a monoclonal antibody against amyloid plaques from Alzheimer’s brain. Neurosci. Lett. 1995, 200, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Tamaoka, A.; Endoh, R.; Shoji, S.; Takahashi, H.; Hirokawa, K.; Teplow, D.B.; Selkoe, D.J.; Mori, H. Antibodies to amyloid beta protein (A beta) crossreact with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Neurobiol. Aging 1996, 17, 405–414. [Google Scholar] [CrossRef]
- Maïza, A.; Chantepie, S.; Vera, C.; Fifre, A.; Huynh, M.B.; Stettler, O.; Ouidja, M.O.; Papy-Garcia, D. The role of heparan sulfates in protein aggregation and their potential impact on neurodegeneration. FEBS Lett. 2018, 592, 3806–3818. [Google Scholar] [CrossRef] [Green Version]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front. Chem. 2014, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Rees, D.A.; Robinson, G. Cation-specific aggregation of carrageenan helices: Domain model of polymer gel structure. J. Mol. Biol. 1980, 138, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.S.; Campbell, J.W.; Harding, M.M.; Rees, D.A.; Samuel, J.W. X-ray diffraction studies of polysaccharide sulphates: Double helix models for k- and l-carrageenans. J. Mol. Biol. 1969, 45, 85–99. [Google Scholar] [CrossRef]
- Le Questel, J.Y.; Cros, S.; Mackie, W.; Perez, S. Computer modelling of sulfated carbohydrates: Applications to carrageenans. Int. J. Biol. Macromol. 1995, 17, 161–175. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between secondary structure and ion binding upon thermoreversible gelation of kappa-carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Zhuravleva, I.L.; Cardinaels, R.; Moldenaers, P. Macromolecular complexes of lysozyme with kappa carrageenan. Food Hydrocoll. 2018, 74, 227–238. [Google Scholar] [CrossRef] [Green Version]
- van de Weert, M.; Andersen, M.B.; Frokjaer, S. Complex coacervation of lysozyme and heparin: Complex characterization and protein stability. Pharm. Res. 2004, 21, 2354–2359. [Google Scholar] [CrossRef]
- Souza, C.J.F.; da Costa, A.R.; Souza, C.F.; Tosin, F.F.S.; Garcia-Rojas, E.E. Complex coacervation between lysozyme and pectin: Effect of pH, salt, and biopolymer ratio. Int. J. Biol. Macromol. 2018, 107, 1253–1260. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Bogdanova, L.R.; Faizullin, D.A.; Ermakova, E.A.; Zuev, Y.F.; Sedov, I.A. Interaction-induced structural transformation of lysozyme and kappa-carrageenan in binary complexes. Carbohydr. Polym. 2021, 252, 117181. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of kappa-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Collins, D.; Brassard, J.; Chakravarti, R.; Vempati, R.; Dorlet, P.; Santolini, J.; Dawson, J.H.; Stuehr, D.J. Heme binding properties of glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 2012, 51, 8514–8529. [Google Scholar] [CrossRef] [Green Version]
- Koshkarov, A.A.; Makshakova, O.N. The Influence of Cofactor Binding on the Intramolecular Dynamics of Glyceraldehyde-3-Phosphate Dehydrogenase. Biophysics 2021, 66, 192–201. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Safarova, E.R.; Zuev, Y.F. Structural insights in interactions between RNase from Bacillus Intermedius and rhamnogalacturonan I from potato. Carbohydr. Polym. 2021, 251, 117038. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, S.A.; Zacharias, M.; Chauvot de Beauchene, I. Modeling large protein–glycosaminoglycan complexes using a fragment-based approach. J. Comput. Chem. 2019, 40, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Gauto, D.F.; Petruk, A.A.; Modenutti, C.P.; Blanco, J.I.; Di Lella, S.; Martí, M.A. Solvent structure improves docking prediction in lectin-carbohydrate complexes. Glycobiology 2013, 23, 241–258. [Google Scholar] [CrossRef] [Green Version]
- López, E.D.; Arcon, J.P.; Gauto, D.F.; Petruk, A.A.; Modenutti, C.P.; Dumas, V.G.; Marti, M.A.; Turjanski, A.G. WATCLUST: A tool for improving the design of drugs based on protein-water interactions. Bioinformatics 2015, 31, 3697–3699. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Cheatham III, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz Jr., K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.; Darden, T.; Lee, H.; Pedersen, L. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Kačuráková, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Sekkal, M.; Legrand, P. A spectroscopic investigation of the carrageenans and agar in the 1500-100 cm−1 spectral range. Spectrochim. Acta Part A Mol. Spectrosc. 1993, 49, 209–221. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier transform infrared microspectroscopy is a new way to look at plant cell walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belton, P.S.; Goodfellow, B.J.; Wilson, R.H. A variable-temperature Fourier-transform infrared study of gelation in ι- and κ-carrageenans. Macromolecules 1989, 22, 1636–1642. [Google Scholar] [CrossRef]
- Dong, A.; Huang, P.; Caughey, W.S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D.M. Protein structure by Fourier transform infrared spectroscopy: Second derivative spectra. Biochem. Biophys. Res. Commun. 1983, 115, 391–397. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Buehner, M.; Ford, G.C.; Olsen, K.W.; Moras, D.; Rossman, M.G. Three-dimensional structure of D-glyceraldehyde-3-phosphate dehydrogenase. J. Mol. Biol. 1974, 90, 25–49. [Google Scholar] [CrossRef]
- Burova, T.V.; Grinberg, N.V.; Grinberg, V.Y.; Usov, A.I.; Tolstoguzov, V.B.; Kruif, C.G. Conformational changes in iota- and kappa-carrageenans induced by complex formation with bovine beta-casein. Biomacromolecules 2007, 8, 368–375. [Google Scholar] [CrossRef]
- White, M.R.; Garcin, E.D. The Sweet Side of Rna Regulation: Glyceraldehyde-3-Phosphate Dehydrogenase as a Noncanonical Rna-Binding Protein. Wiley Interdiscip. Rev. RNA 2016, 7, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.R.; Khan, M.M.; Deredge, D.; Ross, C.R.; Quintyn, R.; Zucconi, B.E.; Wysocki, V.H.; Wintrode, P.L.; Wilson, G.M.; Garcin, E.D. A Dimer Interface Mutation in Glyceraldehyde-3-Phosphate Dehydrogenase Regulates Its Binding to Au-Rich Rna. J. Biol. Chem. 2015, 290, 1770–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Tightly Bound Polyelectrolytes Enhance Enzyme Proteolysis and Destroy Amyloid Aggregates. Soft. Matter. 2018, 14, 3768–3773. [Google Scholar] [CrossRef] [PubMed]
- Muronetz, V.I.; Pozdyshev, D.V.; Semenyuk, P.I. Polyelectrolytes for Enzyme Immobilization and the Regulation of Their Properties. Polymers 2022, 14, 4204. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makshakova, O.; Antonova, M.; Bogdanova, L.; Faizullin, D.; Zuev, Y. Regulation of Intersubunit Interactions in Homotetramer of Glyceraldehyde-3-Phosphate Dehydrogenases upon Its Immobilization in Protein—Kappa-Carrageenan Gels. Polymers 2023, 15, 676. https://doi.org/10.3390/polym15030676
Makshakova O, Antonova M, Bogdanova L, Faizullin D, Zuev Y. Regulation of Intersubunit Interactions in Homotetramer of Glyceraldehyde-3-Phosphate Dehydrogenases upon Its Immobilization in Protein—Kappa-Carrageenan Gels. Polymers. 2023; 15(3):676. https://doi.org/10.3390/polym15030676
Chicago/Turabian StyleMakshakova, Olga, Maria Antonova, Liliya Bogdanova, Dzhigangir Faizullin, and Yuriy Zuev. 2023. "Regulation of Intersubunit Interactions in Homotetramer of Glyceraldehyde-3-Phosphate Dehydrogenases upon Its Immobilization in Protein—Kappa-Carrageenan Gels" Polymers 15, no. 3: 676. https://doi.org/10.3390/polym15030676
APA StyleMakshakova, O., Antonova, M., Bogdanova, L., Faizullin, D., & Zuev, Y. (2023). Regulation of Intersubunit Interactions in Homotetramer of Glyceraldehyde-3-Phosphate Dehydrogenases upon Its Immobilization in Protein—Kappa-Carrageenan Gels. Polymers, 15(3), 676. https://doi.org/10.3390/polym15030676






