Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Simvastatin-Loaded Bilosomes (SMV-BS)
2.3. Optimization of SMV-BS
2.4. Characterization of SMV-Loaded Bilosomes
2.4.1. Particle Size and Zeta Potential
2.4.2. Surface Morphology
2.4.3. Entrapment Efficiency
2.4.4. Differential Scanning Calorimetry (DSC) Analysis
2.5. In Vitro Release
2.6. Physical Stability Study for the Optimized SMV-Loaded Bilosomes
2.7. Formulation of SMV-Loaded Bilosomal Gel (SMV-BS Gel)
2.8. Characterization of SMV-Loaded Bilosomal Gel
2.8.1. Physical Parameters
2.8.2. pH Measurements
2.8.3. Spreadability
2.9. Ex Vivo Permeation Study
2.10. In Vivo Study
2.10.1. Animals
2.10.2. In Vivo Pharmacokinetic Study
2.10.3. Anti-Inflammatory Activity
2.11. Statistical Analysis
3. Results
3.1. Preparation of SMV-Loaded Bilosomes (SMV-BS)
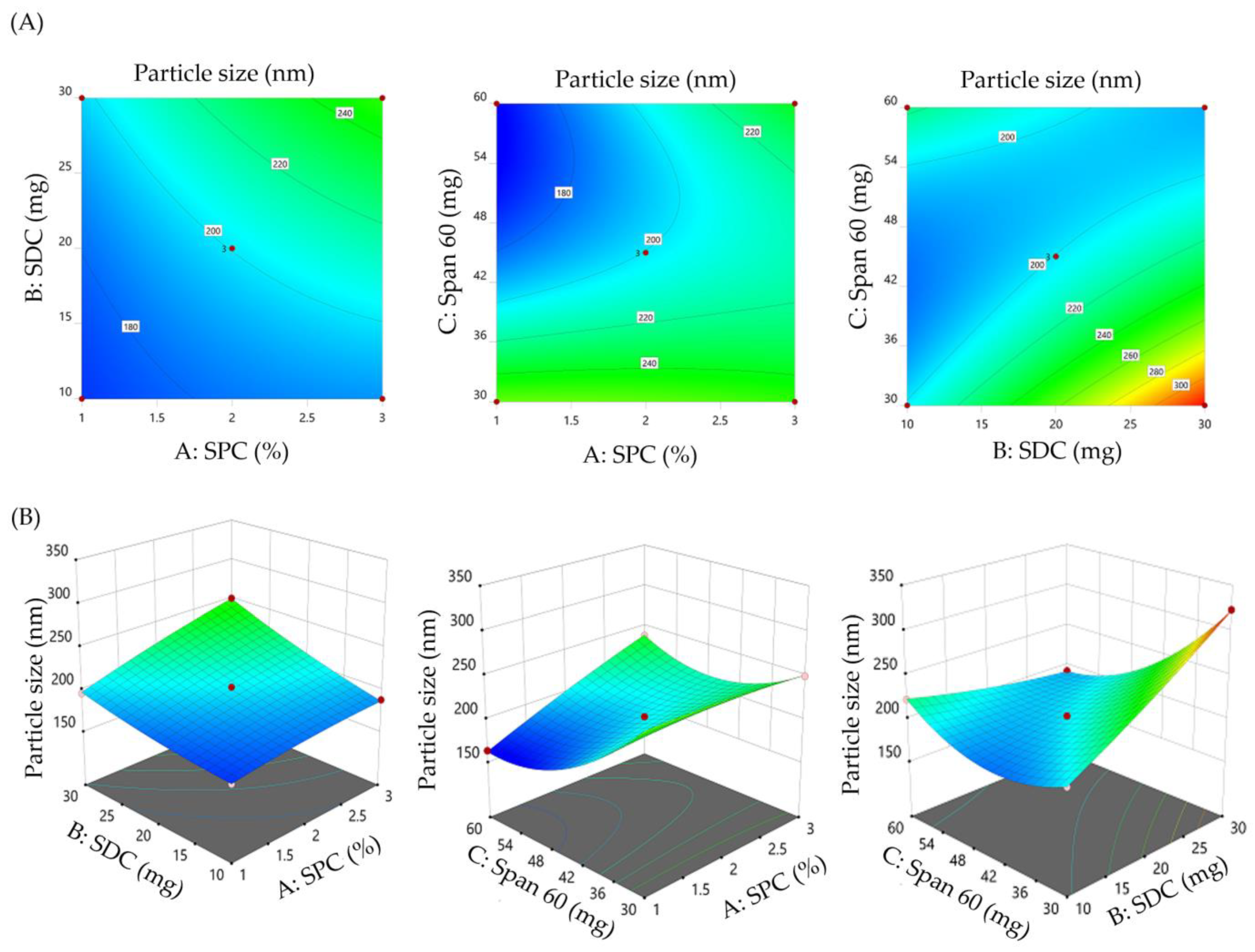
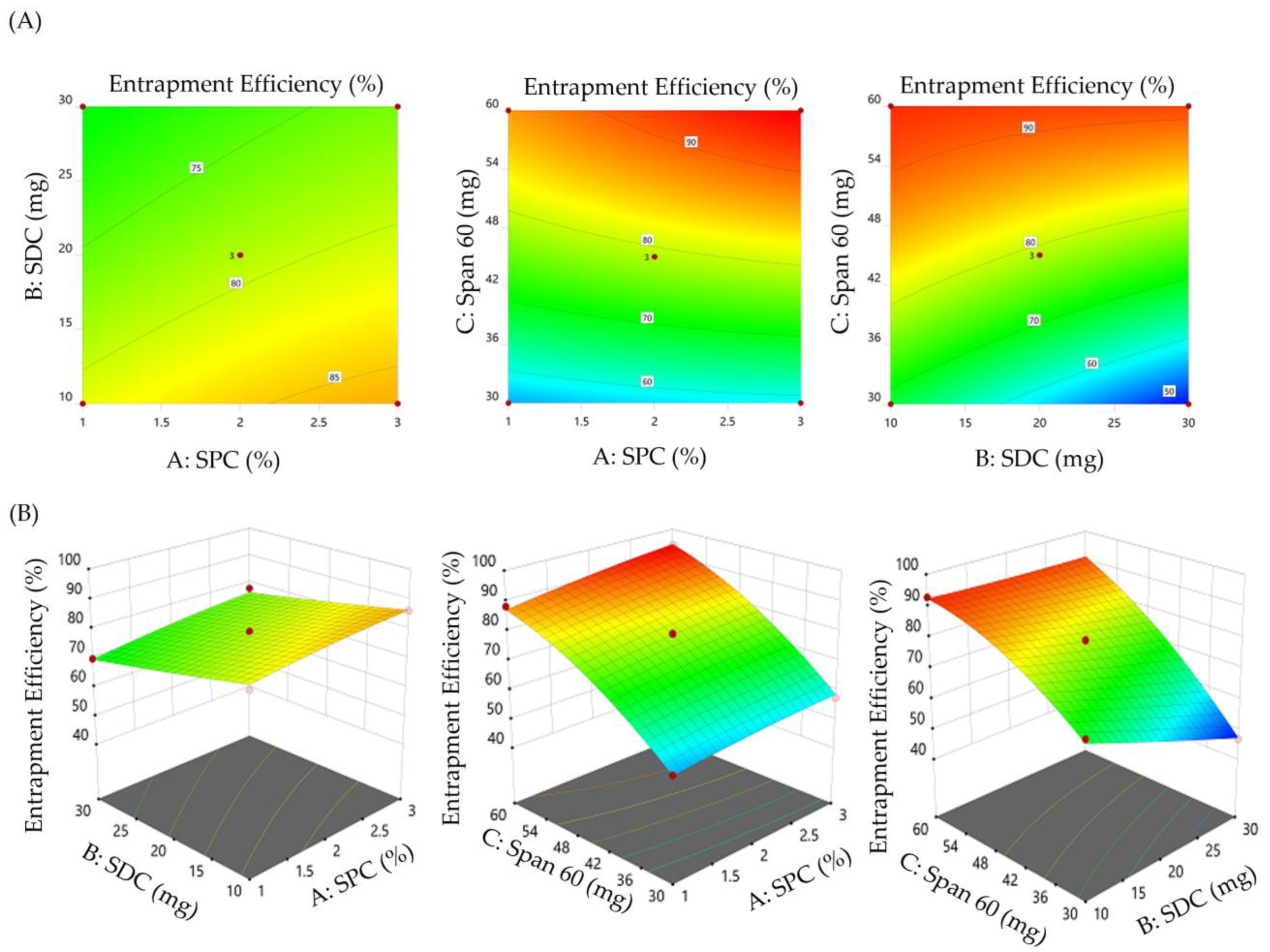
3.2. Influence of Formulation Variables on SMV-BS Characteristics
3.2.1. Impact of Formulation Variables on the Vesicle Size of SMV-BS
3.2.2. Impact of Formulation Variables on the Entrapment Efficiency of SMV-BS
3.2.3. Formulation Optimization
3.3. Characterization of Optimized SMV-BS
3.3.1. Particle Size, Polydispersity Index and Zeta Potential
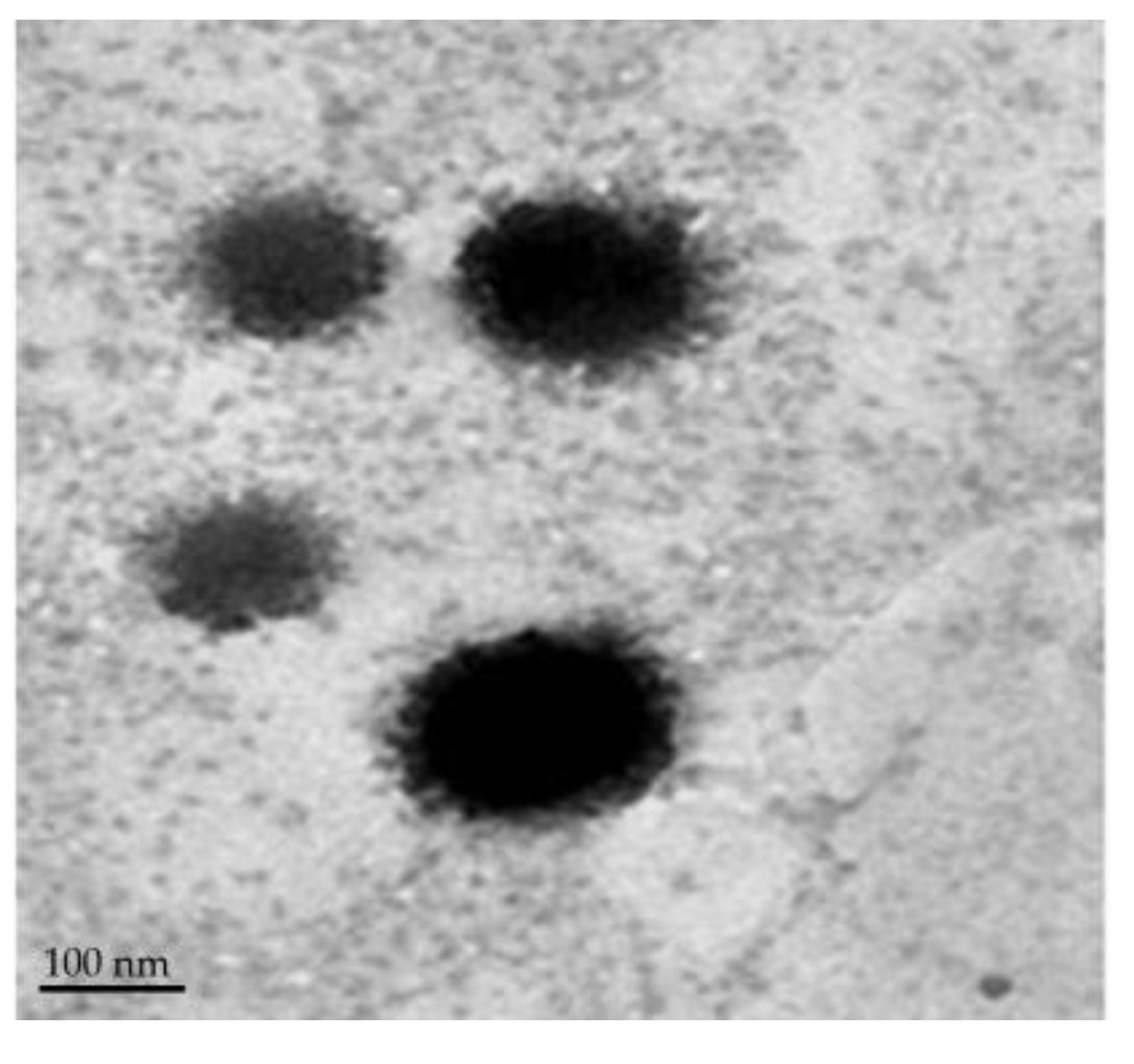
3.3.2. Surface Morphology
3.3.3. Differential Scanning Calorimetry (DSC) Analysis
3.4. In Vitro Release Study
3.5. Stability of SMV-BS
3.6. Characterization of Simvastatin-Loaded Bilosomal Gel (SMV-BS Gel)
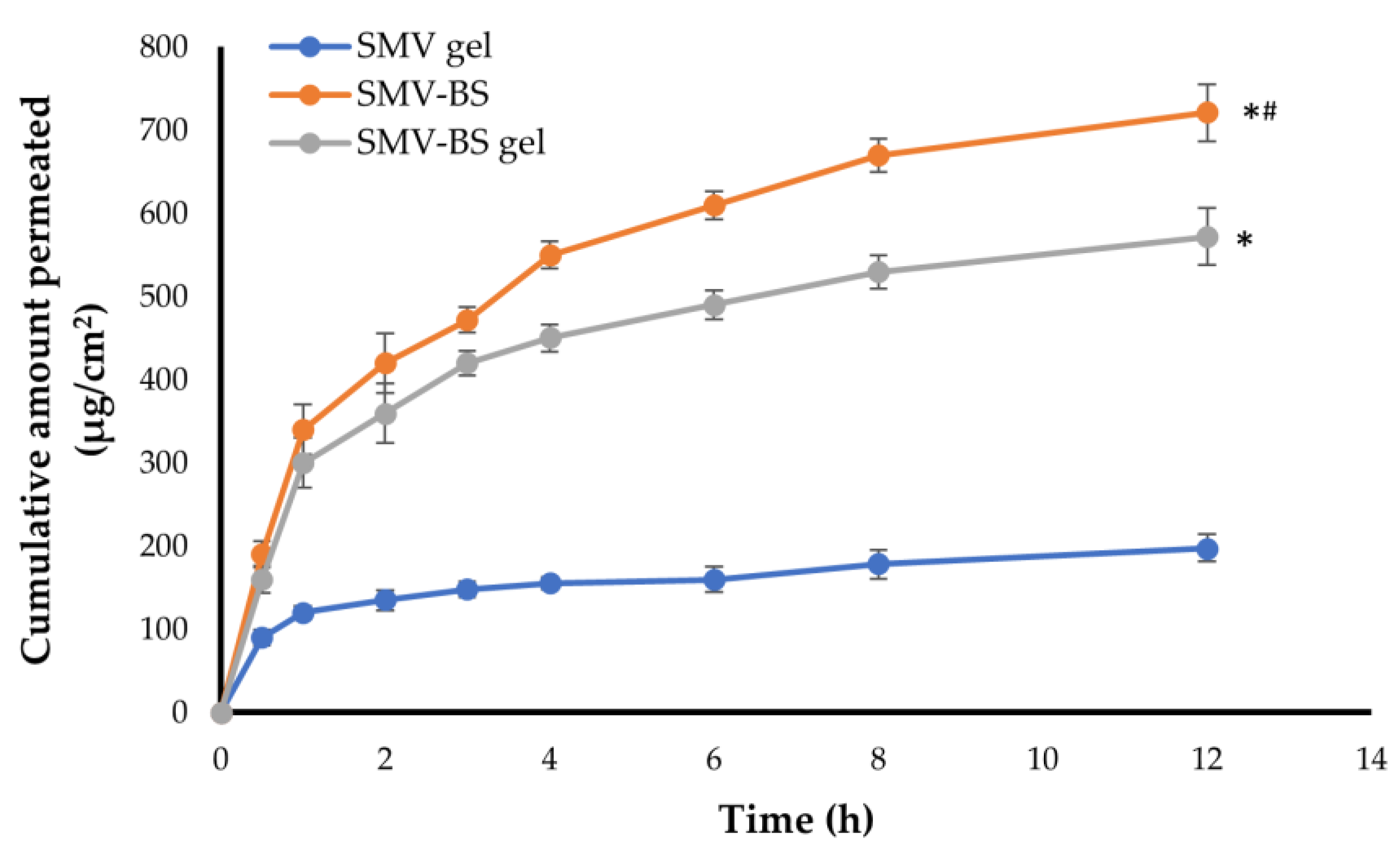
3.7. Ex vivo Permeation of SMV-BS Gel through Abdominal Rat Skin
3.8. In Vivo Study
3.8.1. Pharmacokinetic Study
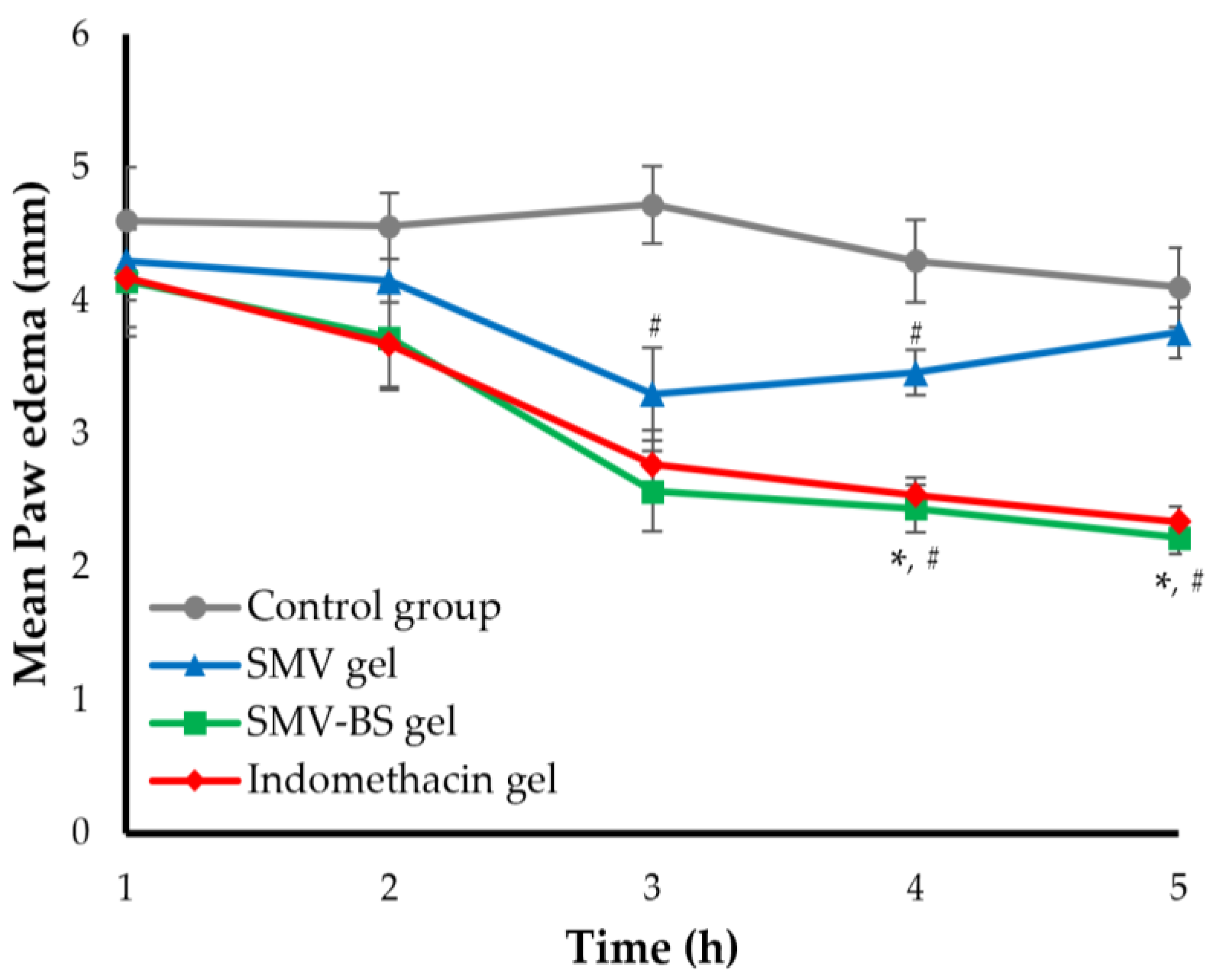
3.8.2. Anti-Inflammatory Activity of SMV-BS Gel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Rinaldi, B.; Mazzon, E.; Donniacuo, M.; Impellizzeri, D.; Paterniti, I.; Capuano, A.; Bramanti, P.; Cuzzocrea, S. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: Involvement of PPAR-α. J. Neuroinflamm. 2012, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Barone, A.; Mendes, M.; Cabral, C.; Mare, R.; Paolino, D.; Vitorino, C. Hybrid Nanostructured Films for Topical Administration of Simvastatin as Coadjuvant Treatment of Melanoma. J. Pharm. Sci. 2019, 108, 3396–3407. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Kang, H.-J.; Jhon, M.; Kim, J.-W.; Lee, J.-Y.; Walker, A.J.; Agustini, B.; Kim, J.-M.; Berk, M. Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front. Psychiatry 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraunberger, P.; Gröne, E.; Gröne, H.J.; Walli, A.K. Simvastatin reduces endotoxin-induced nuclear factor kappaB activation and mortality in guinea pigs despite lowering circulating low-density lipoprotein cholesterol. Shock 2009, 32, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, M.; Owczarek, J.; Orszulak-Michalak, D. Statins: A new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol. Rep. 2007, 59, 483–499. [Google Scholar]
- Sparrow, C.P.; Burton, C.A.; Hernandez, M.; Mundt, S.; Hassing, H.; Patel, S.; Rosa, R.; Hermanowski-Vosatka, A.; Wang, P.R.; Zhang, D.; et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Han, N.; Zhao, B.; Xie, Y.; Wang, S. Enhanced dissolution rate and oral bioavailability of simvastatin nanocrystal prepared by sonoprecipitation. Drug Dev. Ind. Pharm. 2012, 38, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Garg, A.; Jin, B.; Keshavarz, S.S.; Bieberdorf, F.A.; Chodakewitz, J.; Wagner, J.A. Assessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 2009, 67, 520–526. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, B.-J.; Chae, H.-S.; Han, S.; Doh, K.; Choi, J.; Jun, Y.K.; Lee, Y.W.; Yim, D.-S. A Population Pharmacokinetic-Pharmacodynamic Model for Simvastatin that Predicts Low-Density Lipoprotein-Cholesterol Reduction in Patients with Primary Hyperlipidaemia. Basic Clin. Pharmacol. Toxicol. 2011, 109, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhi, R.; Swain, S. Transdermal Evaporation Drug Delivery System: Concept to Commercial Products. Adv. Pharm. Bull. 2018, 8, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Chapter 5—Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 57–72. [Google Scholar]
- d’Avanzo, N.; Cristiano, M.C.; Di Marzio, L.; Bruno, M.C.; Paolino, D.; Celia, C.; Fresta, M. Multidrug Idebenone/Naproxen Co-loaded Aspasomes for Significant in vivo Anti-inflammatory Activity. ChemMedChem 2022, 17, e202200067. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, R.; Gagliardi, A.; Mancuso, A.; Cosco, D.; Soliman, M.E.; Casettari, L.; Paolino, D. Development and In Vivo Evaluation of Multidrug Ultradeformable Vesicles for the Treatment of Skin Inflammation. Pharmaceutics 2019, 11, 644. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Yadav, R.; Patel, S.; Verma, K.; Tak, K. Flurbiprofen loaded ethosomes—Transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019, 18, 133. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef]
- Tej, K.V.M.S.; Moin, A.; Gowda, D.V.; Anjali, P.B.; Karunakar, G.; Patel, N.P.; Kamal, S.S. Nano structured lipid carrier based drug delivery system. J. Chem. Pharm. Res. 2016, 8, 627–643. [Google Scholar]
- Hasan, A.A.; Samir, R.M.; Abu-Zaid, S.S.; Abu Lila, A.S. Revitalizing the local anesthetic effect of Mebeverine hydrochloride via encapsulation within ethosomal vesicular system. Colloids Surf. B Biointerfaces 2020, 194, 111208. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef] [PubMed]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Matloub, A.A.; Salama, A.H.; Aglan, H.A.; AbouSamra, M.M.; ElSouda, S.S.M.; Ahmed, H.H. Exploiting bilosomes for delivering bioactive polysaccharide isolated from Enteromorpha intestinalis for hacking hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2018, 44, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Nafady, M.M.; Kharshoum, R.M.; Abd El-Ghafar, O.A.; Farouk, H.O. Novel Enhanced Therapeutic Efficacy of Dapoxetine HCl by Nano-Vesicle Transdermal Gel for Treatment of Carrageenan-Induced Rat Paw Edema. AAPS PharmSciTech 2020, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Novel Surface-Modified Bilosomes as Functional and Biocompatible Nanocarriers of Hybrid Compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Khafagy, E.S.; Alotaibi, H.F.; Abu Lila, A.S. Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers 2022, 14, 4184. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Rahamathulla, M.; HV, G.; Veerapu, G.; Hani, U.; Alhamhoom, Y.; Alqahtani, A.; Moin, A. Characterization, Optimization, In Vitro and In Vivo Evaluation of Simvastatin Proliposomes, as a Drug Delivery. AAPS PharmSciTech 2020, 21, 129. [Google Scholar] [CrossRef]
- Vishwa, B.; Moin, A.; Gowda, D.V.; Rizvi, S.M.D.; Hegazy, W.A.H.; Abu Lila, A.S.; Khafagy, E.S.; Allam, A.N. Pulmonary Targeting of Inhalable Moxifloxacin Microspheres for Effective Management of Tuberculosis. Pharmaceutics 2021, 13, 79. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.S.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Farouk, H.O.; Zaki, R.M. Fabrication and Appraisal of Simvastatin via Tailored Niosomal Nanovesicles for Transdermal Delivery Enhancement: In Vitro and In Vivo Assessment. Pharmaceutics 2021, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.A.; Khafagy, E.-S.; Lila, A.S.A.; El-Horany, H.E.; El-Housiny, S. Ginger Extract-Loaded Sesame Oil-Based Niosomal Emulgel: Quality by Design to Ameliorate Anti-Inflammatory Activity. Gels 2022, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alsaidan, O.A.; Imam, S.S.; Yasir, M.; Alharbi, K.S.; Khalid, M. Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation. Gels 2022, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Ali, A.A.; Hegazy, A.M.; Sadek, A.A.; Aboud, H.M. Harnessing of Doxylamine Succinate/Pyridoxine Hydrochloride-Dual Laden Bilosomes as a Novel Combinatorial Nanoparadigm for Intranasal Delivery: In Vitro Optimization and In Vivo Pharmacokinetic Appraisal. J. Pharm. Sci. 2022, 111, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Abu Lila, A.S.; Sallam, N.M.; Sanad, R.A.; Ahmed, M.M.; Ghorab, M.M.; Alotaibi, H.F.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; et al. Preparation and Characterization of a Novel Mucoadhesive Carvedilol Nanosponge: A Promising Platform for Buccal Anti-Hypertensive Delivery. Gels 2022, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Tan, Y.; Guan, P.; Hovgaard, L.; Lu, Y.; Qi, J.; Lian, R.; Li, X.; Wu, W. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: A mechanistic study. Int. J. Pharm. 2014, 460, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Khafagy, E.S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.K.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A.H. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Rizvi, S.M.D.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.S.; Alshammari, F.; Khafagy, E.S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef]
- Mahmoud, T.M.; Nafady, M.M.; Farouk, H.O.; Mahmoud, D.M.; Ahmed, Y.M.; Zaki, R.M.; Hamad, D.S. Novel Bile Salt Stabilized Vesicles-Mediated Effective Topical Delivery of Diclofenac Sodium: A New Therapeutic Approach for Pain and Inflammation. Pharmaceuticals 2022, 15, 1106. [Google Scholar] [CrossRef] [PubMed]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Duodenum-triggered delivery of pravastatin sodium via enteric surface-coated nanovesicular spanlastic dispersions: Development, characterization and pharmacokinetic assessments. Int. J. Pharm. 2015, 483, 77–88. [Google Scholar] [CrossRef]
- Zidan, A.S.; Hosny, K.M.; Ahmed, O.A.; Fahmy, U.A. Assessment of simvastatin niosomes for pediatric transdermal drug delivery. Drug Deliv. 2016, 23, 1536–1549. [Google Scholar] [CrossRef]
- Foco, A.; Hadziabdić, J.; Becić, F. Transdermal drug delivery systems. Med. Arch. 2004, 58, 230–234. [Google Scholar]




| Independent Variables | Code Value | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| A: SPC molar concentration | 1 | 2 | 3 |
| B: SDC amount (mg) | 10 | 20 | 30 |
| C: Span 60 amount (mg) | 30 | 45 | 60 |
| Dependent variables | Constrains | ||
| Y1: Vesicle size (nm) | Minimize | ||
| Y2: Entrapment efficiency (%) | Maximize | ||
| Formula | Independent Variables | Responses | |||
|---|---|---|---|---|---|
| A: SPC Molar Concentration | B: SDC (mg) | C: Span 60 (mg) | Y1: Vesicle Size (nm) | Y2: Entrapment Efficiency (%) | |
| F1 | 2 | 20 | 45 | 199.7 ± 17.3 | 79.1 ± 2.8 |
| F2 | 1 | 20 | 30 | 260.1 ± 12.1 | 54.9 ± 1.9 |
| F3 | 2 | 10 | 30 | 201.3 ± 11.2 | 69.2 ± 2.3 |
| F4 | 2 | 10 | 60 | 222.5 ± 19.5 | 92.8 ± 3.1 |
| F5 | 2 | 20 | 45 | 203.4 ± 13.4 | 79.3 ± 2.9 |
| F6 | 3 | 20 | 60 | 238.1 ± 11.7 | 94.4 ± 4.1 |
| F7 | 3 | 20 | 30 | 249.9 ± 17.6 | 57.5 ± 1.8 |
| F8 | 2 | 30 | 30 | 323.1 ± 12.9 | 46.8 ± 0.9 |
| F9 | 2 | 30 | 60 | 192.1 ± 10.8 | 89.9 ± 2.4 |
| F10 | 1 | 20 | 60 | 163.4 ± 8.5 | 88.2 ± 3.2 |
| F11 | 1 | 10 | 45 | 171.3 ± 11.4 | 79.9 ± 3.1 |
| F12 | 3 | 30 | 45 | 250.8 ± 13.5 | 77.8 ± 3.6 |
| F13 | 1 | 30 | 45 | 195.8 ± 18.6 | 69.9 ± 2.5 |
| F14 | 2 | 20 | 45 | 198.2 ± 16.4 | 78.1 ± 1.8 |
| F15 | 3 | 10 | 45 | 188.1 ± 15.3 | 86.2 ± 2.2 |
| Days Post Storage | Vesicle Size (nm) | Entrapment Efficiency (%) |
|---|---|---|
| Day 0 | 172.1 ± 8.1 | 89.2 ± 1.8 |
| Day 30 | 175.3 ± 11.2 | 87.5 ± 2.5 |
| Day 60 | 184.9 ± 16.7 | 86.9 ± 2.2 |
| Day 90 | 190.1 ± 17.5 | 84.9 ± 3.1 |
| Pharmacokinetic Parameter | Oral SMV Suspension | SMV-Gel | SMV-BS Gel |
|---|---|---|---|
| Cmax (μg/mL) | 13.20 ± 0.22 | 17.18 ± 2.1 | 26.23 ± 1.5 |
| Tmax (h) | 4 | 3 | 3 |
| T1/2 (h) | 15.28 ± 1.1 | 12.08 ± 0.9 | 18.42 ± 1.4 |
| Ke (h−1) | 0.045 ± 0.001 | 0.057 ± 0.001 | 0.037 ± 0.001 |
| AUC0–24 (μg/mL·h) | 60.70 ± 5.9 | 85.01 ± 7.8 | 177.30 ± 21.2 |
| MRT (h) | 14.78 ± 1.3 | 12.64 ± 1.0 | 23.58 ± 1.7 |
| Formula | 1 h | 2 h | 3 h | 4 h | 5 h |
|---|---|---|---|---|---|
| SMV gel | 6.52 ± 0.32 | 8.90 ± 0.47 | 30.08 ± 2.9 | 19.53 ± 0.9 | 8.29 ± 0.81 |
| SMV-BS gel | 9.78 ± 0.41 | 18.42 ± 1.1 | 45.55 ± 3.1 | 43.26 ± 3.5 | 45.85 ± 4.5 |
| Indomethacin gel | 9.35 ± 0.69 | 19.52 ± 1.7 | 41.31 ± 2.6 | 40.93 ± 1.8 | 42.93 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khafagy, E.-S.; Almutairy, B.K.; Abu Lila, A.S. Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation. Polymers 2023, 15, 677. https://doi.org/10.3390/polym15030677
Khafagy E-S, Almutairy BK, Abu Lila AS. Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation. Polymers. 2023; 15(3):677. https://doi.org/10.3390/polym15030677
Chicago/Turabian StyleKhafagy, El-Sayed, Bjad K. Almutairy, and Amr Selim Abu Lila. 2023. "Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation" Polymers 15, no. 3: 677. https://doi.org/10.3390/polym15030677





