Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating LCST Phase Transition and Photochromic Isomerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Monolayer Hydrogels
2.3. Preparation of the Bilayer Hydrogel
2.4. Tensile Measurement
2.5. Equilibrium Swelling Ratio of the Hydrogels
2.6. Relative Swelling Test of the Hydrogels
2.7. Bending Degree of the Hydrogels
2.8. Microscopic Fourier Transform Infrared (Micro-FTIR) Spectroscopy
2.9. Ultraviolet Test
2.10. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Mechanical Properties of Hydrogels
3.2. Thermo-Responsive Bending Behavior
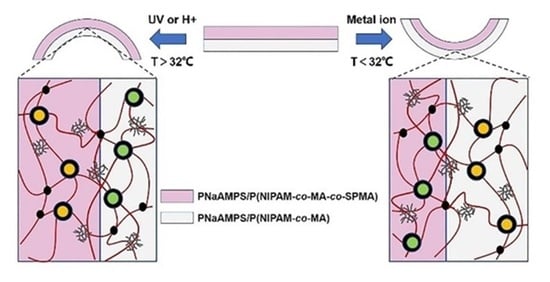
3.3. UV Light and Acid Responsive Bending Behavior
3.4. Metal Ion Responsive Bending Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based Smart Hydrogels: Design, Properties and Applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Wei, S.; Lu, W.; Le, X.; Ma, C.; Lin, H.; Wu, B.; Zhang, J.; Theato, P.; Chen, T. Bioinspired Synergistic Fluorescence-Color-Switchable Polymeric Hydrogel Actuators. Angew. Chem. Int. Ed. 2019, 58, 16243–16251. [Google Scholar] [CrossRef] [PubMed]
- Francis, W.; Dunne, A.; Delaney, C.; Florea, L.; Diamond, D. Spiropyran Based Hydrogels Actuators-Walking in the Light. Sens. Actuators B 2017, 250, 608–616. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Zhang, C.; Liao, L. Robust Near-Infrared-Responsive Composite Hydrogel Actuator Using Fe3+/Tannic Acid as the Photothermal Transducer. ACS Appl. Mater. Interfaces 2021, 13, 18175–18183. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Luo, S.; Wang, Y.; Zhu, X.; Yang, S. A Smart Enzyme Reactor Based on a Photo-responsive Hydrogel for Purifying Water from Phenol Contaminated Sources. Soft Matter 2022, 18, 826–831. [Google Scholar] [CrossRef]
- Osada, Y.; Okuzaki, H.; Hori, H. A Polymer Gel with Electrically Driven Motility. Nature 1992, 355, 242–244. [Google Scholar] [CrossRef]
- Morales, D.; Palleau, E.; Dickey, M.D.; Velev, O.D. Electro-Actuated Hydrogel Walkers with Dual Responsive Legs. Soft Matter 2014, 10, 1337–1348. [Google Scholar] [CrossRef]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A Novel Magnetic Hydrogel with Aligned Magnetic Colloidal Assemblies Showing Controllable Enhancement of Magnetothermal Effect in the Presence of Alternating Magnetic Field. Adv. Matter. 2015, 27, 2507–2514. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Chang, Q.; Darabi, M.A.; Lin, B.; Zhong, W.; Xing, M. Highly Flexible and Resilient Elastin Hybrid Cryogels with Shape Memory, Injectability, Conductivity, and Magnetic Responsive Properties. Adv. Mater. 2016, 28, 7758–7767. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Wang, T.; Sun, W.; Tong, Z. Polyampholyte Hydrogels with pH Modulated Shape Memory and Spontaneous Actuation. Adv. Funct. Mater. 2018, 28, 1707245. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-Responsive Hydrogel Actuator for Lipophilic Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zheng, J.; Wen, X.; Cai, Z.; Zhang, J.; Chen, T. pH- and Sugar-Induced Shape Memory Hydrogel Based on Reversible Phenylboronic Acid-Diol Ester Bonds. Macromol. Rapid. Commun. 2015, 36, 533–537. [Google Scholar] [CrossRef]
- Long, S.; Ye, Z.; Jin, Y.; Huang, J.; Huang, Y.; Liao, Y.; Li, X. High-Performance Photochromic Hydrogels for Rewritable Information Record. Macromol. Rapid. Commun. 2021, 42, 2000701. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cui, L.; Wang, H.; Chen, Q.; Guan, Y.; Zhang, Y. Tough, Resilient, Adhesive, and Anti-Freezing Hydrogels Cross-Linked with a Macromolecular Cross-Linker for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 42052–42062. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Bai, M.; Zhu, Y.; Liu, X.; Tian, S.; Long, Y.; Ma, Y.; Wen, C.; Li, Q.; Yang, J.; et al. Pro-Healing Zwitterionic Skin Sensor Enables Multi-Indicator Distinction and Continuous Real-Time Monitoring. Adv. Funct. Mater. 2021, 31, 2106406. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, D.; Shu, M.; Shang, L.; Xia, M.; Huang, Y. High-Strength, Thermosensitive Double Network Hydrogels with Antibacterial Functionality. Soft Matter 2021, 17, 6688–6696. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Yang, T.; Liu, T.; Yu, X.; Bai, Y.; Zhang, Y.; Chen, X. Nanocomposite Hydrogel with NIR/Magnet/Enzyme Multiple Responsiveness to Accurately Manipulate Local Drugs for On-Demand Tumor Therapy. Biomaterials 2020, 262, 120357. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent Progress in Biomimetic Anisotropic Hydrogel Actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Li, J.; Ma, Q.; Xu, Y.; Yang, M.; Wu, Q.; Wang, F.; Sun, P. Highly Bidirectional Bendable Actuator Engineered by LCST–UCST Bilayer Hydrogel with Enhanced Interface. ACS Appl. Mater. Interfaces 2020, 12, 55290–55298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Chen, L.; Zhang, C.; Liao, L. Multi-Responsive Hydrogel Actuator with Photo-Switchable Color Changing Behaviors. Dyes Pigm. 2020, 174, 108042. [Google Scholar] [CrossRef]
- Jiao, D.; Zhu, Q.; Li, C.; Zheng, Q.; Wu, Z. Programmable Morphing Hydrogels for Soft Actuators and Robots: From Structure Designs to Active Functions. Acc. Chem. Res. 2022, 55, 1533–1545. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, P.; Le, X.; Lu, W.; Theato, P.; Ma, C.; Du, B.; Zhang, J.; Huang, Y.; Chen, T. Mimosa Inspired Bilayer Hydrogel Actuator Functioning in Multi-Environments. J. Mater. Chem. C 2018, 6, 1320–1327. [Google Scholar] [CrossRef]
- Li, C.; Xue, Y.; Han, M.; Palmer, L.C.; Rogers, J.A.; Huang, Y.; Stupp, S.I. Synergistic Photoactuation of Bilayered Spiropyran Hydrogels for Predictable Origami-Like Shape Change. Matter 2021, 4, 1377–1390. [Google Scholar] [CrossRef]
- Shang, H.; Le, X.; Si, M.; Wu, S.; Peng, Y.; Shan, F.; Wu, S.; Chen, T. Biomimetic Organohydrogel Actuator with High Response Speed and Synergistic Fluorescent Variation. Chem. Eng. J. 2022, 429, 132290. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Zhang, K.; Wang, X. Temporary Actuation of Bilayer Polymer Hydrogels Mediated by the Enzymatic Reaction. Langmuir 2022, 38, 15433–15441. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Q.; Huo, Z.; Zhang, N.; Ma, M. Programmable Multistimuli-Responsive and Multimodal Polymer Actuator Based on a Designed Energy Transduction Network. ACS Appl. Mater. Interfaces 2022, 14, 13768–13777. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based Dynamic Materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef]
- Zou, Q.; Li, X.; Zhou, J.; Bai, K.; Agren, H. Synthesis and Photochromism of a Spirooxazine Derivative Featuring a Carbazole Moiety: Fast Thermal Bleaching and Excellent Fatigue Resistance. Dyes Pigm. 2014, 107, 174–181. [Google Scholar] [CrossRef]
- Gong, J.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, H.; Cheng, Z.; Yan, J.; Xie, X. Biomimetic Gradient Hydrogel Actuators with Ultrafast Thermo-Responsiveness and High Strength. ACS Appl. Mater. Interfaces 2022, 14, 32541–32550. [Google Scholar] [CrossRef]
- Li, C.; Iscen, A.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Light-Driven Expansion of Spiropyran Hydrogels. J. Am. Chem. Soc. 2020, 142, 8447–8453. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, C.; Chen, L.; Liao, L. Re-Usable Colorimetric Polymeric Gel for Visual and Facile Detection of Multiple Metal Ions. React. Funct. Polym. 2021, 160, 104824. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, G.; Chen, A.; Zheng, Z.; Zhang, C.; Zhang, Y.; Liao, L. Multi-responsive Chromatic Hydrogel Exhibiting Reversible Shape Deformations. Dyes Pigm. 2022, 204, 110364. [Google Scholar] [CrossRef]





| Sample | σ (kPa) | ε (%) | E (kPa) | W (kJ m−3) |
|---|---|---|---|---|
| P(NIPAM-co-MA-co-SPMA) | 54 ± 9 | 817 ± 208 | 9 ± 4 | 250 ± 27 |
| PNaAMPS/P(NIPAM-co-MA-co-SPMA) | 335 ± 26 | 398 ± 35 | 82 ± 17 | 752 ± 49 |
| PNaAMPS/P(NIPAM-co-MA) | 289 ± 31 | 352 ± 51 | 69 ± 18 | 556 ± 30 |
| Bilayer hydrogel | 318 ± 22 | 395 ± 44 | 97 ± 21 | 692 ± 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, S.; Huang, J.; Xiong, J.; Liu, C.; Chen, F.; Shen, J.; Huang, Y.; Li, X. Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating LCST Phase Transition and Photochromic Isomerization. Polymers 2023, 15, 786. https://doi.org/10.3390/polym15030786
Long S, Huang J, Xiong J, Liu C, Chen F, Shen J, Huang Y, Li X. Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating LCST Phase Transition and Photochromic Isomerization. Polymers. 2023; 15(3):786. https://doi.org/10.3390/polym15030786
Chicago/Turabian StyleLong, Shijun, Jiacheng Huang, Jiaqiang Xiong, Chang Liu, Fan Chen, Jie Shen, Yiwan Huang, and Xuefeng Li. 2023. "Designing Multistimuli-Responsive Anisotropic Bilayer Hydrogel Actuators by Integrating LCST Phase Transition and Photochromic Isomerization" Polymers 15, no. 3: 786. https://doi.org/10.3390/polym15030786