A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-ion Polymer Batteries
Abstract
:1. Introduction
2. Polymer Electrolytes (PEs)
3. Ex Situ Preparation Method for Gel Polymer Electrolytes
4. In Situ Polymerization Method for Gel Polymer Electrolytes
4.1. Gel Polymer Electrolytes Prepared by Thermal Polymerization
4.2. Gel Polymer Electrolytes Prepared by UV Photo-Initiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Pillot, C. Lithium Ion Battery Raw Material Supply & Demand 2016–2025. In Proceedings of the Advanced Automotive Battery Conference, Mainz, Germany, 30 January–2 February 2017; Volume 30. [Google Scholar]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Madhavi, S.; Liu, H.-K. Lithium--ion Conducting Electrolyte Salts for Lithium Batteries. Eur. J. Chem. 2011, 17, 14326–14346. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium--ion Batteries: State of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.J.; Han, J.; Song, S.-W. Fire-Preventing LiPF6 and Ethylene Carbonate-Based Organic Liquid Electrolyte System for Safer and Outperforming Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 42868–42879. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on Gel Polymer Electrolytes for Lithium Batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium Battery Chemistries Enabled by Solid-State Electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.; Kang, F.; Li, B. Challenges and Perspectives of Garnet Solid Electrolytes for All Solid-State Lithium Batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on Solid Electrolytes for All-Solid-State Lithium-Ion Batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- eWees, R.; Wang, H. Synthesis and Properties of NaSICON-Type LATP and LAGP Solid Electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar]
- Deng, Y.; Eames, C.; Chotard, J.-N.; Lalère, F.; Seznec, V.; Emge, S.; Pecher, O.; Grey, C.P.; Masquelier, C.; Islam, M.S. Structural and Mechanistic Insights into Fast Lithium-Ion Conduction in Li4SiO4–Li3PO4 Solid Electrolytes. J. Am. Chem. Soc. 2015, 137, 9136–9145. [Google Scholar] [CrossRef]
- Song, S.; Dong, Z.; Deng, F.; Hu, N. Lithium Superionic Conductors Li10MP2O12 (M = Ge, Si). Funct. Mater. Lett. 2018, 11, 1850039. [Google Scholar] [CrossRef]
- Ban, C.W.; Choi, G.M. The Effect of Sintering on the Grain Boundary Conductivity of Lithium Lanthanum Titanates. Solid State Ion. 2001, 140, 285–292. [Google Scholar] [CrossRef]
- Chen, K.; Huang, M.; Shen, Y.; Lin, Y.; Nan, C.W. Improving Ionic Conductivity of Li0.35La0.55TiO3 Ceramics by Introducing Li7La3Zr2O12 Sol into the Precursor Powder. Solid State Ion. 2013, 235, 8–13. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Kim, J.Y.; Lee, Y.-G.; Kim, K.M. Recent Progress and Perspectives of Solid Electrolytes for Lithium Rechargeable Batteries. J. Korean Electrochem. Soc. 2019, 22, 87–103. [Google Scholar]
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide Solid Electrolytes for All-Solid-State Lithium Batteries: Structure, Conductivity, Stability and Application. Energy Storage Mater. 2018, 14, 58–74. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Kanno, R. Li10GeP2S12-Type Superionic Conductors: Synthesis, Structure, and Ionic Transportation. Adv. Energy Mater. 2020, 10, 2002153. [Google Scholar] [CrossRef]
- Kudu, Ö.U.; Famprikis, T.; Fleutot, B.; Braida, M.-D.; Le Mercier, T.; Islam, M.S.; Masquelier, C. A Review of Structural Properties and Synthesis Methods of Solid Electrolyte Materials in the Li2S−P2S5 Binary System. J. Power Sources 2018, 407, 31–43. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, F.; Luo, J.; Zhang, L.; Sun, X. Recent Development of Lithium Argyrodite Solid-State Electrolytes for Solid-State Batteries: Synthesis, Structure, Stability and Dynamics. Nano Energy 2021, 83, 105858. [Google Scholar] [CrossRef]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the Current Status and Development of Polymer Electrolytes for Solid-State Lithium Batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Review of Composite Polymer-Ceramic Electrolytes for Lithium Batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium–Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Luntz, A.C.; McCloskey, B.D. Nonaqueous Li–Air Batteries: A Status Report. Chem. Rev. 2014, 114, 11721–11750. [Google Scholar] [CrossRef]
- Choi, K.-H.; Cho, S.-J.; Kim, S.-H.; Kwon, Y.H.; Kim, J.Y.; Lee, S.-Y. Thin, Deformable, and Safety-Reinforced Plastic Crystal Polymer Electrolytes for High-Performance Flexible Lithium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 44–52. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, J. Development of Flexible Li-Ion Batteries for Flexible Electronics. InfoMat 2020, 2, 866–878. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Shen, W.; Xu, T.; Yang, L.; Xu, X.; Yang, F.; Zhang, L.; Wang, Y.; Zhou, Y.; Zhong, M.; et al. Fibrous Gel Polymer Electrolyte for an Ultrastable and Highly Safe Flexible Lithium-Ion Battery in a Wide Temperature Range. Carbon Energy 2021, 3, 916–928. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An Overview. J. Phys. Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, K.; Liu, Y.; Gao, H.; Bai, Y.; Wang, X.; Wu, C. Polymer Electrolytes and Interfaces toward Solid-State Batteries: Recent Advances and Prospects. Energy Storage Mater. 2020, 33, 26–54. [Google Scholar] [CrossRef]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring Inorganic–Polymer Composites for the Mass Production of Solid-State Batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Fenton, D.E. Complexes of Alkali Metal Ions with Poly (Ethylene Oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical Conductivity in Ionic Complexes of Poly (Ethylene Oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Shim, J.; Lee, J.W.; Bae, K.Y.; Kim, H.J.; Yoon, W.Y.; Lee, J.-C. Dendrite Suppression by Synergistic Combination of Solid Polymer Electrolyte Crosslinked with Natural Terpenes and Lithium--Powder Anode for Lithium--Metal Batteries. ChemSusChem 2017, 10, 2274–2283. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.S.; Lim, Y.T.; Choi, Y.J.; Cho, G.B.; Kim, K.W.; Ahn, H.J.; Cho, K.K. Electrochemical Properties of Lithium Sulfur Cells Using PEO Polymer Electrolytes Prepared under Three Different Mixing Conditions. J. Power Sources 2007, 174, 745–750. [Google Scholar] [CrossRef]
- Le, H.T.; Ngo, D.T.; Kalubarme, R.S.; Cao, G.; Park, C.-N.; Park, C.-J. Composite Gel Polymer Electrolyte Based on Poly (Vinylidene Fluoride-Hexafluoropropylene)(PVDF-HFP) with Modified Aluminum-Doped Lithium Lanthanum Titanate (A-LLTO) for High-Performance Lithium Rechargeable Batteries. ACS Appl. Mater. Interfaces 2016, 8, 20710–20719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chuang, Y.C.; Su, J.H.; Yu, H.C.; Chen-Yang, Y.W. High Discharge Capacity Solid Composite Polymer Electrolyte Lithium Battery. J. Power Sources 2011, 196, 2802–2809. [Google Scholar] [CrossRef]
- Shukla, N.; Thakur, A.K. Role of Salt Concentration on Conductivity Optimization and Structural Phase Separation in a Solid Polymer Electrolyte Based on PMMA-LiClO 4. Ionics 2009, 15, 357–367. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Yue, L.; Wang, Q.; Chai, J.; Liu, Z.; Zhou, X.; Li, H.; Guo, Y.; Cui, G. Safety--reinforced Poly (Propylene Carbonate)--based All--solid--state Polymer Electrolyte for Ambient--temperature Solid Polymer Lithium Batteries. Adv. Energy Mater. 2015, 5, 1501082. [Google Scholar] [CrossRef]
- Subban, R.H.Y.; Arof, A.K. Charge–Discharge Characteristics of LiCoO2/Mesocarbon Microbeads Battery with Poly (Vinyl Chloride)-Based Composite Polymer Electrolyte. J. Power Sources 2004, 134, 211–221. [Google Scholar] [CrossRef]
- Jiang, Z.; Carroll, B.; Abraham, K.M. Studies of Some Poly(Vinylidene Fluoride) Electrolytes. Electrochimica Acta 1997, 42, 2667–2677. [Google Scholar] [CrossRef]
- Francis, S.; Varshney, L.; Sabharwal, S. Thermal Degradation Behavior of Radiation Synthesized Polydiallyldimethylammonium Chloride. Eur. Polym. J. 2007, 43, 2525–2531. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M.; Jaschik, M.; Warmuzinski, K.; Jansen, J.C.; Vankelecom, I.F.J. Poly(Vinylbenzyl Chloride)-Based Poly(Ionic Liquids) as Membranes for CO2 Capture from Flue Gas. J. Mater. Chem. A 2017, 5, 19808–19818. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A Review of Polymer Electrolytes: Fundamental, Approaches and Applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Xia, Y.; Fujieda, T.; Tatsumi, K.; Prosini, P.P.; Sakai, T. Thermal and Electrochemical Stability of Cathode Materials in Solid Polymer Electrolyte. J. Power Sources 2001, 92, 234–243. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, H.; Duan, H.; Lü, X.; Xin, S.; Zhou, W.; Xue, L.; Fu, G.; Manthiram, A. Hybrid Polymer/Garnet Electrolyte with a Small Interfacial Resistance for Lithium--ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 753–756. [Google Scholar] [CrossRef]
- Dong, D.; Zhou, B.; Sun, Y.; Zhang, H.; Zhong, G.; Dong, Q.; Fu, F.; Qian, H.; Lin, Z.; Lu, D. Polymer Electrolyte Glue: A Universal Interfacial Modification Strategy for All-Solid-State Li Batteries. Nano Lett. 2019, 19, 2343–2349. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-Conductive Macromolecular Gels and Membranes for Solid Lithium Cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Isken, P.; Winter, M.; Passerini, S.; Lex-Balducci, A. Methacrylate Based Gel Polymer Electrolyte for Lithium-Ion Batteries. J. Power Sources 2013, 225, 157–162. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, L.; Shi, L.; Wang, Z.; Zhu, J.; Zhao, Y.; Yuan, S. Gel Polymer Electrolyte with High Li Transference Number Enhancing the Cycling Stability of Lithium Anodes. ACS Appl. Mater. Interfaces 2019, 11, 5168–5175. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for Solid-State Lithium Rechargeable Batteries: Recent Advances and Perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S. Review on Composite Polymer Electrolytes for Lithium Batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer Electrolytes for Lithium Polymer Batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H.; Cui, Y. A Silica--aerogel--reinforced Composite Polymer Electrolyte with High Ionic Conductivity and High Modulus. Adv. Mater. 2018, 30, 1802661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, K.-H.; Cho, S.-J.; Park, J.-S.; Cho, K.Y.; Lee, C.K.; Lee, S.B.; Shim, J.K.; Lee, S.-Y. A Shape-Deformable and Thermally Stable Solid-State Electrolyte Based on a Plastic Crystal Composite Polymer Electrolyte for Flexible/Safer Lithium-Ion Batteries. J. Mater. Chem. A 2014, 2, 10854–10861. [Google Scholar] [CrossRef]
- Liang, X.; Han, D.; Wang, Y.; Lan, L.; Mao, J. Preparation and Performance Study of a PVDF–LATP Ceramic Composite Polymer Electrolyte Membrane for Solid-State Batteries. RSC Adv. 2018, 8, 40498–40504. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Zhang, Z.; Li, X.; Yang, L.; Tachibana, K.; Hirano, S. Polymer Electrolytes Based on Dicationic Polymeric Ionic Liquids: Application in Lithium Metal Batteries. J. Mater. Chem. A 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Mecerreyes, D.; Forsyth, M.; Zhang, H.; Armand, M. Polymeric Ionic Liquids for Lithium-Based Rechargeable Batteries. Mol. Syst. Des. Eng. 2019, 4, 294–309. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The Design of Polymeric Ionic Liquids for the Preparation of Functional Materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Nishimura, N.; Ohno, H. 15th Anniversary of Polymerised Ionic Liquids. Polymer 2014, 55, 3289–3297. [Google Scholar] [CrossRef]
- Fong, K.D.; Self, J.; McCloskey, B.D.; Persson, K.A. Onsager Transport Coefficients and Transference Numbers in Polyelectrolyte Solutions and Polymerized Ionic Liquids. Macromolecules 2020, 53, 9503–9512. [Google Scholar] [CrossRef]
- Nulwala, H.; Mirjafari, A.; Zhou, X. Ionic Liquids and Poly(Ionic Liquid)s for 3D Printing—A Focused Mini-Review. Eur. Polym. J. 2018, 108, 390–398. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(Ionic Liquid)s: An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Xiao, M.; Wang, S.-J.; Zhao, Q.-Q.; Meng, Y.-Z. Fabrication and Characterization of PEO/PPC Polymer Electrolyte for Lithium-Ion Battery. J. Appl. Polym. Sci. 2010, 115, 2718–2722. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Warren, P.C.; Culver, D.; Gozdz, A.S.; Amatucci, G.G.; Tarascon, J.-M. Plastic PVDF-HFP Electrolyte Laminates Prepared by a Phase-Inversion Process. Solid State Ion. 2000, 135, 249–257. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Kim, J.I.; Choi, Y.; Chung, K.Y.; Park, J.H. A Structurable Gel--polymer Electrolyte for Sodium Ion Batteries. Adv. Funct. Mater. 2017, 27, 1701768. [Google Scholar] [CrossRef]
- Ha, H.-J.; Kwon, Y.H.; Kim, J.Y.; Lee, S.-Y. A Self-Standing, UV-Cured Polymer Networks-Reinforced Plastic Crystal Composite Electrolyte for a Lithium-Ion Battery. Electrochim. Acta 2011, 57, 40–45. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Song, H.-K. Gel/Solid Polymer Electrolytes Characterized by in Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, H.-X.; Zhang, X.-Y.; Guo, X. In Situ Thermally Polymerized Solid Composite Electrolytes with a Broad Electrochemical Window for All-Solid-State Lithium Metal Batteries. J. Mater. Chem. A 2020, 8, 3892–3900. [Google Scholar] [CrossRef]
- Cui, Y.; Chai, J.; Du, H.; Duan, Y.; Xie, G.; Liu, Z.; Cui, G. Facile and Reliable in Situ Polymerization of Poly(Ethyl Cyanoacrylate)-Based Polymer Electrolytes toward Flexible Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 8737–8741. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, J.; Rim, J.; Hong, D.; Kang, J.; Park, S. Vinyl-Integrated In Situ Cross-Linked Composite Gel Electrolytes for Stable Lithium Metal Anodes. ACS Appl. Energy Mater. 2021, 4, 2922–2931. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Li, M.; Li, G.; Li, B.; Zhang, S.; Ming, H.; Qiu, J.; Chen, J.; Zhao, P. A New Composite Gel Polymer Electrolyte Based on Matrix of PEGDA with High Ionic Conductivity for Lithium-Ion Batteries. Electrochimica Acta 2020, 354, 136622. [Google Scholar] [CrossRef]
- Choi, K.-H.; Kim, S.-H.; Ha, H.-J.; Kil, E.-H.; Kee Lee, C.; Bong Lee, S.; Kie Shim, J.; Lee, S.-Y. Compliant Polymer Network-Mediated Fabrication of a Bendable Plastic Crystal Polymer Electrolyte for Flexible Lithium-Ion Batteries. J. Mater. Chem. A 2013, 1, 5224–5231. [Google Scholar] [CrossRef]
- Kim, H.-S.; Moon, S.-I. Synthesis and Electrochemical Performances of Di (Trimethylolpropane) Tetraacrylate-Based Gel Polymer Electrolyte. J. Power Sources 2005, 146, 584–588. [Google Scholar] [CrossRef]
- Zhou, D.; Fan, L.-Z.; Fan, H.; Shi, Q. Electrochemical Performance of Trimethylolpropane Trimethylacrylate-Based Gel Polymer Electrolyte Prepared by in Situ Thermal Polymerization. Electrochim. Acta 2013, 89, 334–338. [Google Scholar] [CrossRef]
- Kim, I.; Jang, S.; Lee, K.H.; Tak, Y.; Lee, G. In Situ Polymerized Solid Electrolytes for Superior Safety and Stability of Flexible Solid-State Al-Ion Batteries. Energy Storage Mater. 2021, 40, 229–238. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.; Hong, B.; Bai, M.; Li, J.; Zhang, Z.; Lai, Y. Molecular Engineering of a Gel Polymer Electrolyte via In-Situ Polymerization for High Performance Lithium Metal Batteries. Chem. Eng. J. 2022, 428, 131331. [Google Scholar] [CrossRef]
- Duan, H.; Yin, Y.-X.; Zeng, X.-X.; Li, J.-Y.; Shi, J.-L.; Shi, Y.; Wen, R.; Guo, Y.-G.; Wan, L.-J. In-Situ Plasticized Polymer Electrolyte with Double-Network for Flexible Solid-State Lithium-Metal Batteries. Energy Storage Mater. 2018, 10, 85–91. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, D.; He, Y.-B.; Fu, Y.; Qin, X.; Miao, C.; Du, H.; Li, B.; Yang, Q.-H.; Lin, Z. Novel Gel Polymer Electrolyte for High-Performance Lithium–Sulfur Batteries. Nano Energy 2016, 22, 278–289. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, M.; Chen, G.; Cao, B.; Shi, J.; Du, J.; Chen, Y. An Effectively Inhibiting Lithium Dendrite Growth In-Situ-Polymerized Gel Polymer Electrolyte. Electrochim. Acta 2018, 283, 349–356. [Google Scholar] [CrossRef]
- Guan, H.; Lian, F.; Xi, K.; Ren, Y.; Sun, J.; Kumar, R.V. Polyvinyl Formal Based Gel Polymer Electrolyte Prepared Using Initiator Free In-Situ Thermal Polymerization Method. J. Power Sources 2014, 245, 95–100. [Google Scholar] [CrossRef]
- Wang, Q.-J.; Zhang, P.; Wang, B.; Fan, L.-Z. A Novel Gel Polymer Electrolyte Based on Trimethylolpropane Trimethylacrylate/Ionic Liquid via in Situ Thermal Polymerization for Lithium-Ion Batteries. Electrochim. Acta 2021, 370, 137706. [Google Scholar] [CrossRef]
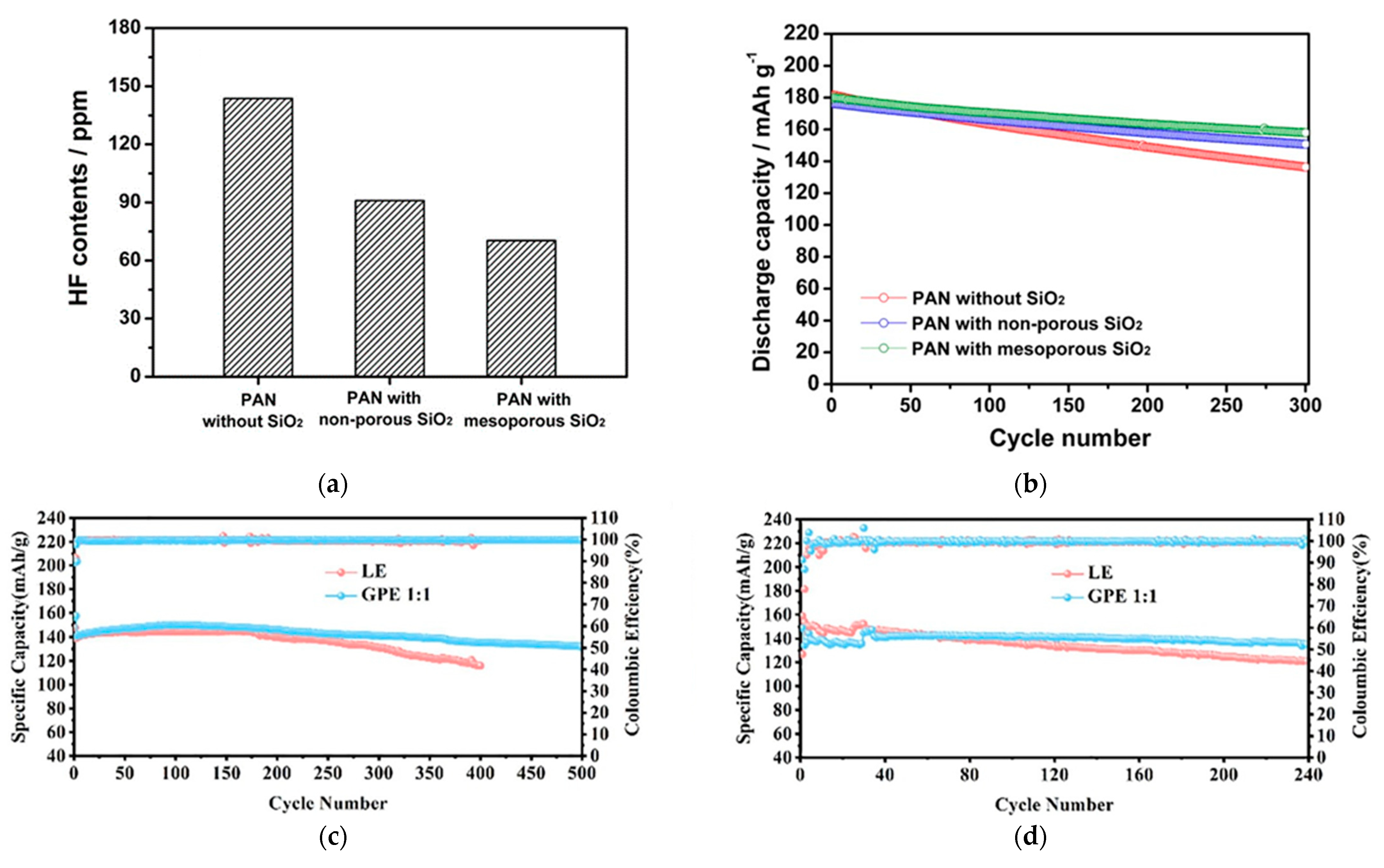
- Shin, W.-K.; Cho, J.; Kannan, A.G.; Lee, Y.-S.; Kim, D.-W. Cross-Linked Composite Gel Polymer Electrolyte Using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Han, J.; Lee, K.; Lee, Y.J.; Kim, B.G.; Jung, K.-N.; Kim, B.J.; Lee, S.W. Elastomeric Electrolytes for High-Energy Solid-State Lithium Batteries. Nature 2022, 601, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cai, B.; Li, S.; Yu, Q.; Lv, F.; Kang, F.; Wang, Q.; Li, B. Long-Cycling and Safe Lithium Metal Batteries Enabled by the Synergetic Strategy of Ex Situ Anodic Pretreatment and an in-Built Gel Polymer Electrolyte. J. Mater. Chem. A 2020, 8, 7197–7204. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, C.; Xin, J.; Wang, Q.; Wu, J.; Wang, W.; Zhou, J.; Liu, Y.; Guo, B.; Shi, S. High Conductive Composite Polymer Electrolyte via in Situ UV-Curing for All-Solid-State Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 9875–9880. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, T.; Wang, M.; Wu, W.; Wang, J.; Li, S.; Zhao, Y.; Wang, C.; Yao, X.; Xu, X. Formation of Excellent Cathode/Electrolyte Interface with UV-Cured Polymer Electrolyte through In Situ Strategy. J. Electrochem. Soc. 2021, 168, 020511. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Kim, S.; Lee, M.; Lee, W.B.; Park, J.H. Polymer--Clay Nanocomposite Solid--State Electrolyte with Selective Cation Transport Boosting and Retarded Lithium Dendrite Formation. Adv. Energy Mater. 2020, 10, 2003114. [Google Scholar] [CrossRef]
- Xu, H.; Ye, W.; Wang, Q.; Han, B.; Wang, J.; Wang, C.; Deng, Y. An in Situ Photopolymerized Composite Solid Electrolyte from Halloysite Nanotubes and Comb-like Polycaprolactone for High Voltage Lithium Metal Batteries. J. Mater. Chem. A 2021, 9, 9826–9836. [Google Scholar] [CrossRef]
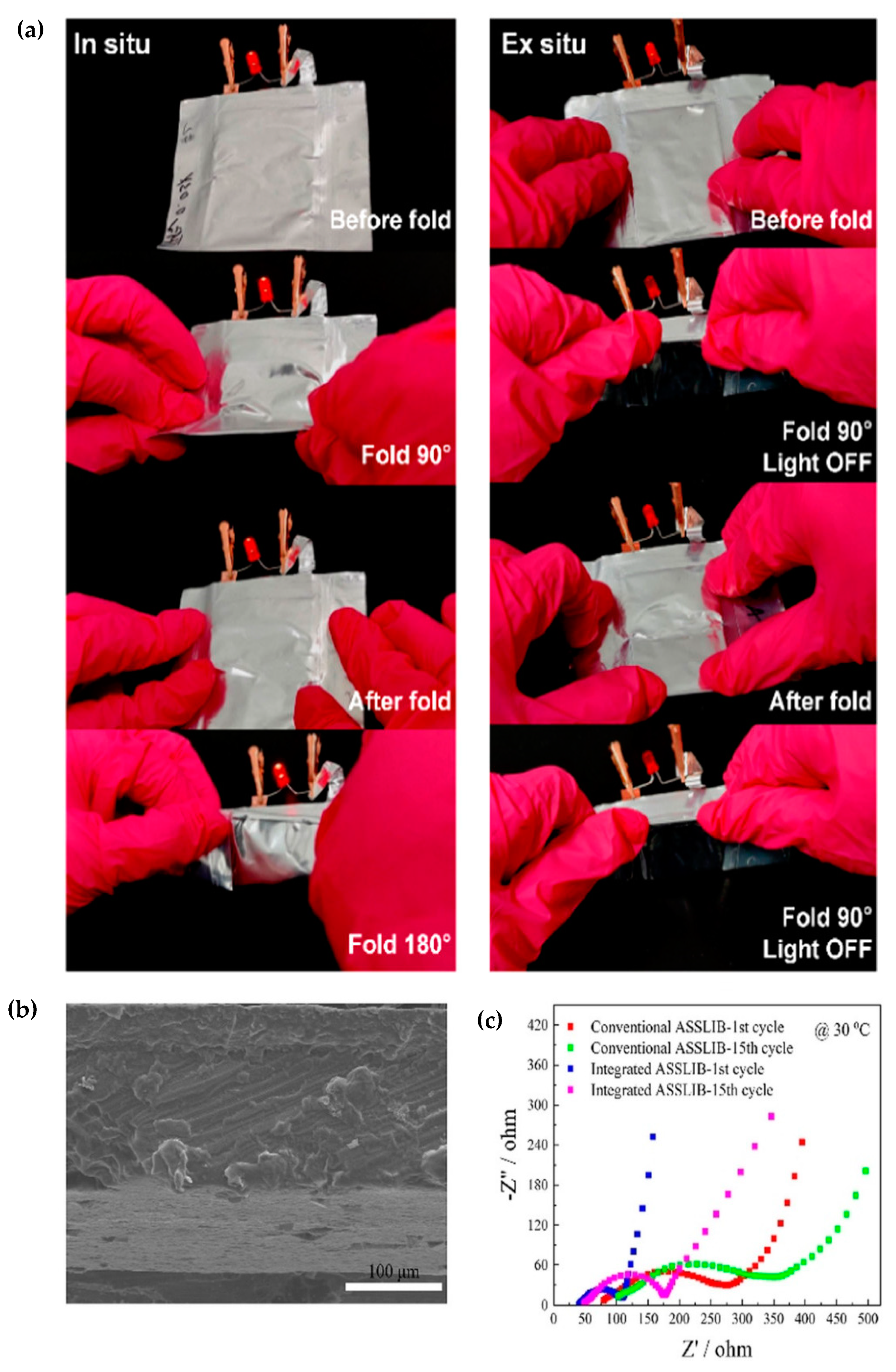
- Liu, C.; Zhu, F.; Huang, Z.; Liao, W.; Guan, X.; Li, Y.; Chen, D.; Lu, Z. An Integrate and Ultra-Flexible Solid-State Lithium Battery Enabled by in Situ Polymerized Solid Electrolyte. Chem. Eng. J. 2022, 434, 134644. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, W.; Yang, Y.; Wu, Y.; Wang, C.; Wu, X.; Zhang, X.; Yuan, Y.; Tang, Y.; Chen, Y.; et al. High-Performance and Highly Safe Solvate Ionic Liquid-Based Gel Polymer Electrolyte by Rapid UV-Curing for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 43397–43406. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, Y.; Gao, X.; Wang, R. High-Safety All-Solid-State Lithium-Ion Battery Working at Ambient Temperature with In Situ UV-Curing Polymer Electrolyte on the Electrode. ChemElectroChem 2020, 7, 2599–2607. [Google Scholar] [CrossRef]
- Bediako, J.K.; Kang, J.-H.; Yun, Y.-S.; Choi, S.-H. Facile Processing of Polyelectrolyte Complexes for Immobilization of Heavy Metal Ions in Wastewater. ACS Appl. Polym. Mater. 2022, 4, 2346–2354. [Google Scholar] [CrossRef]
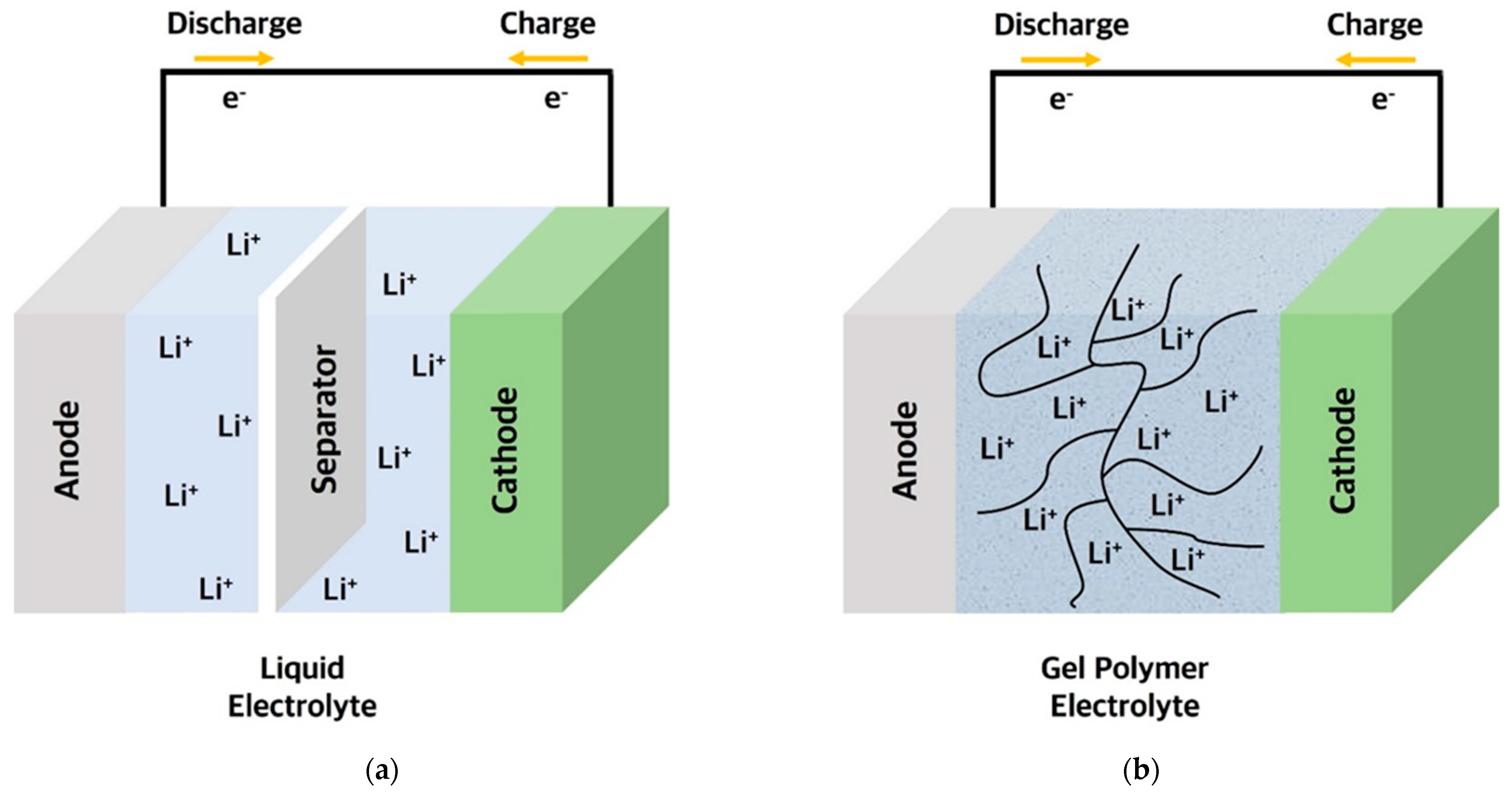
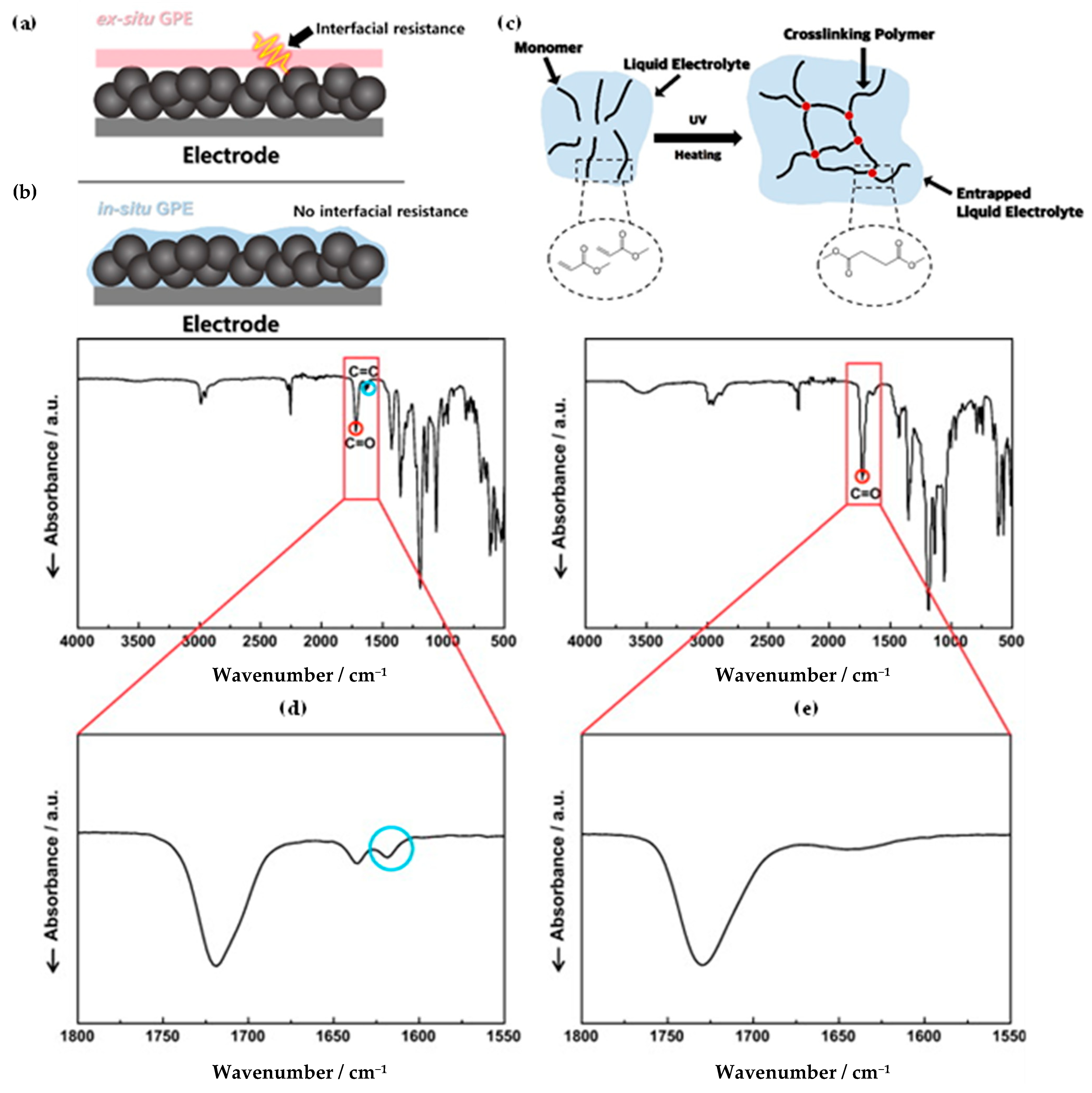
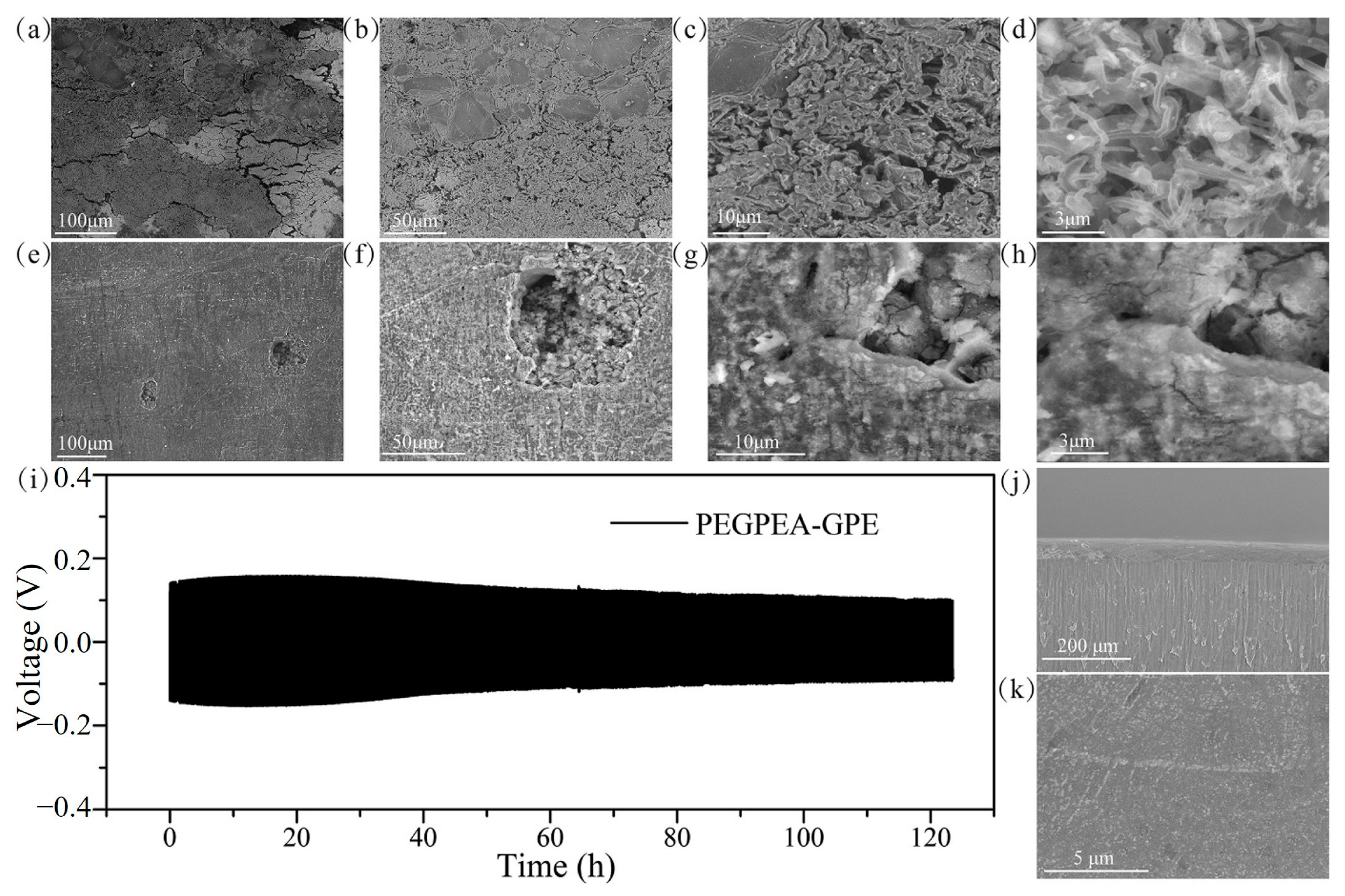
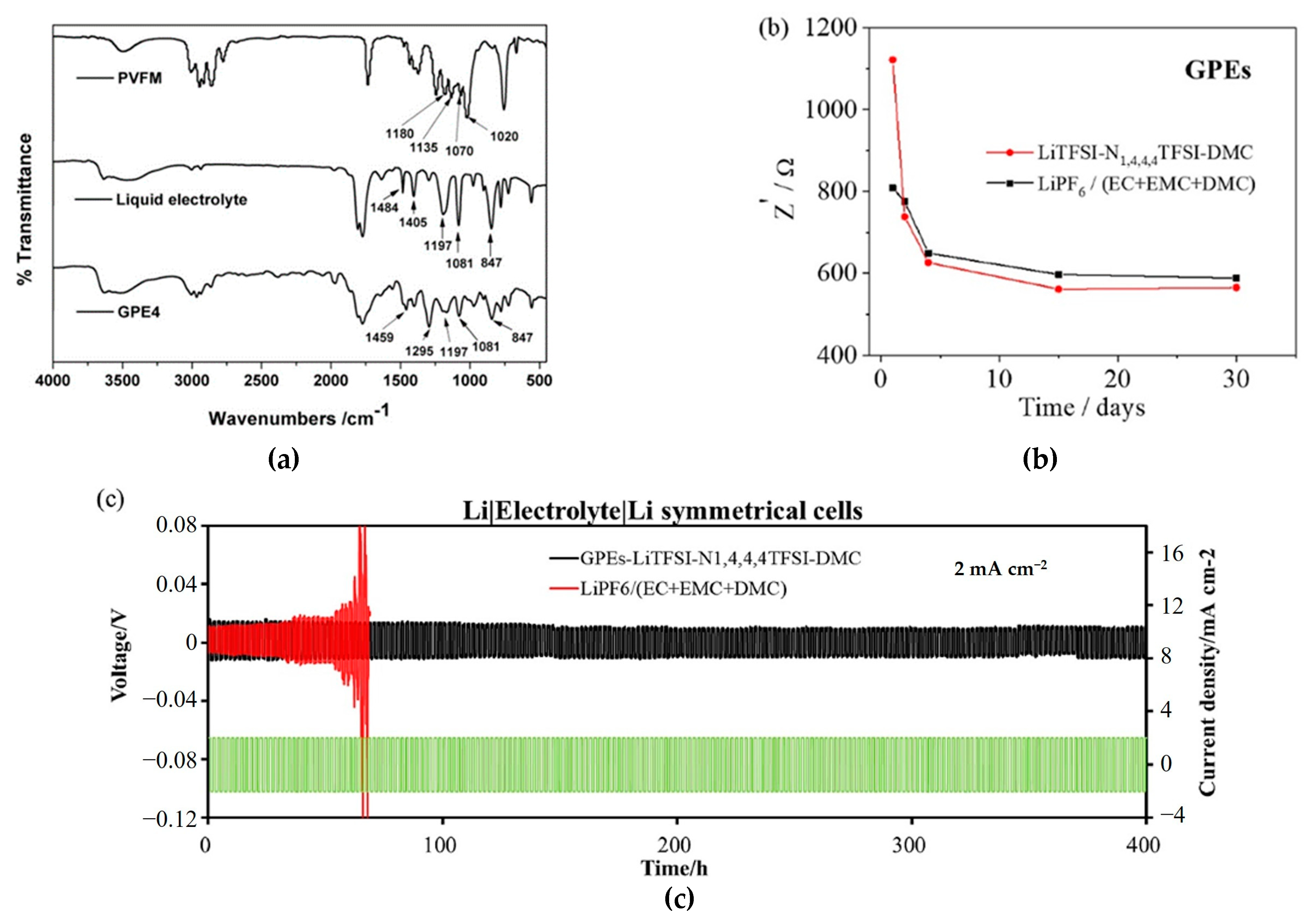


| Type of Solid Electrolyte | Advantages | Disadvantages |
|---|---|---|
| Oxide | Good thermal stability Good mechanical strength Air stability | Poor processability High interfacial and particle resistance |
| Sulfide | High ionic conductivity | Sensitive to moisture (H2S formation) Poor chemical stability |
| Polymer | Flexibility Processability Good interfacial properties | Low thermal and mechanical stability |
| Polymer Electrolyte | Advantages | Disadvantages |
|---|---|---|
| Solid Polymer Electrolytes (SPEs) | Good mechanical properties High thermal stability Solvent-free | Low ionic conductivity at room temperature Non-conformal interface with electrodes. |
| Gel Polymer Electrolytes (GPEs) | Good ionic conductivity Low volatility and reactivity | Poor mechanical strength |
| Composite Polymer Electrolytes (CPEs) | Good ionic conductivity High thermal stability Good mechanical properties | Difficult to disperse filler particles |
| Polymeric Ionic Liquid Electrolytes (PILEs) | High thermal stability Wide electrochemical windows Non-flammability Low vapor pressure | Uncertain ion transport mechanism |
| Polymeric Host | Repeating Unit | Glass Transition Temperature (°C) | Melting Temperature (°C) |
|---|---|---|---|
| PEO | -(CH2CH2O)n- | −64 | 65 |
| PVDF | -(CH2-CF2)n- | −40 | 171 |
| PVDF-HFP | -[(CH2-CF2)-(CF2-CF-(CF3)]n- | −90 | 135 |
| PAN | -(CH2-CH(-CN))n- | 125 | 317 |
| PMMA | -(CH2C(-CH3)(-COOCH3))n- | 105 | Amorphous |
| PVC | -(CH2-CHCl)n- | 80 | 220 |
| PPC | -[CH(CH3)CH2OCO2]n- | 35 | Amorphous |
| PDADMACl | -(C8H16ClN)n- | 150 | - |
| PVBTMATFSI | -[CH2CH(C6H4CH2(CF3SO2)2N)]n- | 74 | - |
| Polymer Electrolytes | Monomer | Initiator | Ionic Conductivity (S cm−1) | Reference |
|---|---|---|---|---|
| 1.3M LiPF6 in EC/DEC = 3/7 (v/v) with 10 wt.% FEC, ETPTA, BPO, SiO2 | 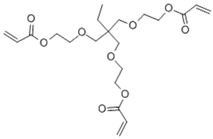 |  | 5.20 × 10−3 | [82] |
| 1.0M LiPF6 in EC/DEC = 1/1 (v/v), PEGDA, AIBN | 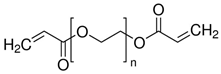 | 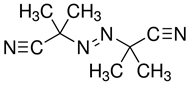 | 8.81 × 10−3 | [83] |
| 1.0M LiTFSI in SN, TPPTA, HMPP | 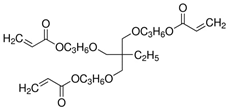 | 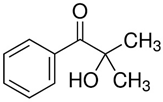 | >10−3 | [84] |
| 1.1M LiPF6 in EC/PC/EMC/ DEC = 3/2/3/2 (v/v/v/v), DTPTA, BBP | 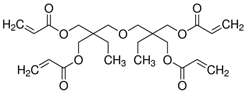 | 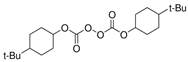 | 6.20 × 10−3 | [85] |
| Type of PE | Monomers | Ionic Conductivity (S cm−1) | Cell Electrodes | Discharge Capacity (mAh g−1) | C-Rate | Reference |
|---|---|---|---|---|---|---|
| GPE | EGPEA | 3.35 × 10−3 | NCM 523/Li | 155.0 | 0.2 C | [91] |
| GPE | PVFM | 8.82 × 10−3 | LFP/Li | 145.0 | 0.1 C | [92] |
| GPE | TMPTMA | 6.15 × 10−3 | NCM 811/Li | 183.1 | 0.1 C | [93] |
| CPE | TEGDA | 1.80 × 10−3 | NCM 111/Li | 179.5 | 0.5 C | [94] |
| SPE | PEGDA, BA | 1.10 × 10−3 | LFP/Li | 93.0 | 1 C | [95] |
| GPE | DOL | ~10−4 | LFP/Li | 126.5 | 1 C | [96] |
| GPE | PEGDMA, PETEA | 7.60 × 10−3 | LFP/Li | ~145.0 | 0.1 C | [88] |
| Type of PE | Monomers | Ionic Conductivity (S cm−1) | Cell Electrodes | Discharge Capacity (mAh g−1) | C-Rate | Reference |
|---|---|---|---|---|---|---|
| SPE | ETPTA | 2.21 × 10−5 | LFP/Li | 147.0 | 0.1 C | [97] |
| SPE | PEGMEMA | 2.95 × 10−5 | LMFP/Li | 164.7 | 0.1 C | [98] |
| CPE | ETPTA | 1.60 × 10−3 | LCO/Li | 152.0 | 0.2 C | [99] |
| CPE | PCLA | 3.31 × 10−5 | LFP/Li | 155.0 | 1 C | [100] |
| GPE | PEGDA, BA | 1.19 × 10−4 | LFP/Li | 160.0 | 0.1 C | [101] |
| GPE | ETPTA | 6.30 × 10−4 | LFP/Li | 141.9 | 0.5 C | [102] |
| SPE | ETPTA | 4.60 × 10−4 | LEP/LTO | 155.9 | 0.2 C | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, W.; Kim, B.; Ryoo, W.S.; Earmme, T. A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-ion Polymer Batteries. Polymers 2023, 15, 803. https://doi.org/10.3390/polym15040803
Chae W, Kim B, Ryoo WS, Earmme T. A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-ion Polymer Batteries. Polymers. 2023; 15(4):803. https://doi.org/10.3390/polym15040803
Chicago/Turabian StyleChae, Wookil, Bumsang Kim, Won Sun Ryoo, and Taeshik Earmme. 2023. "A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-ion Polymer Batteries" Polymers 15, no. 4: 803. https://doi.org/10.3390/polym15040803
APA StyleChae, W., Kim, B., Ryoo, W. S., & Earmme, T. (2023). A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-ion Polymer Batteries. Polymers, 15(4), 803. https://doi.org/10.3390/polym15040803







