Designed Synthesis of Three-Dimensional Covalent Organic Frameworks: A Mini Review
Abstract
:1. Introduction
2. Principles of Reticular Chemistry
- (1)
- The desired framework of the topology is selected and the framework is divided by slicing the edges into vertices;
- (2)
- The geometry, accurate angles, and vertices are analysed depending on the number of points required for extension;
- (3)
- Identification of molecular equivalents of vertices is conducted by selecting huge polyaromatic molecules as building blocks as an accurate geometry can be predicted with the rigid linkers;
- (4)
- The building blocks are connected through covalent linkages to create COFs by modulating the reaction kinetics and reversibility at a microscopic scale between the building units;
- (5)
- The reticulated structure should be analytically confirmed. The accurate architecture of the vertices should be clear since topologies depend on the type of connectivity.
3. Growth Mechanism of 3D COFs Using Different Synthetic Methods
3.1. Homogeneous Synthetic Condition
3.2. Purification of the Amorphous State
3.3. Maintenance of Thermodynamic Equilibrium
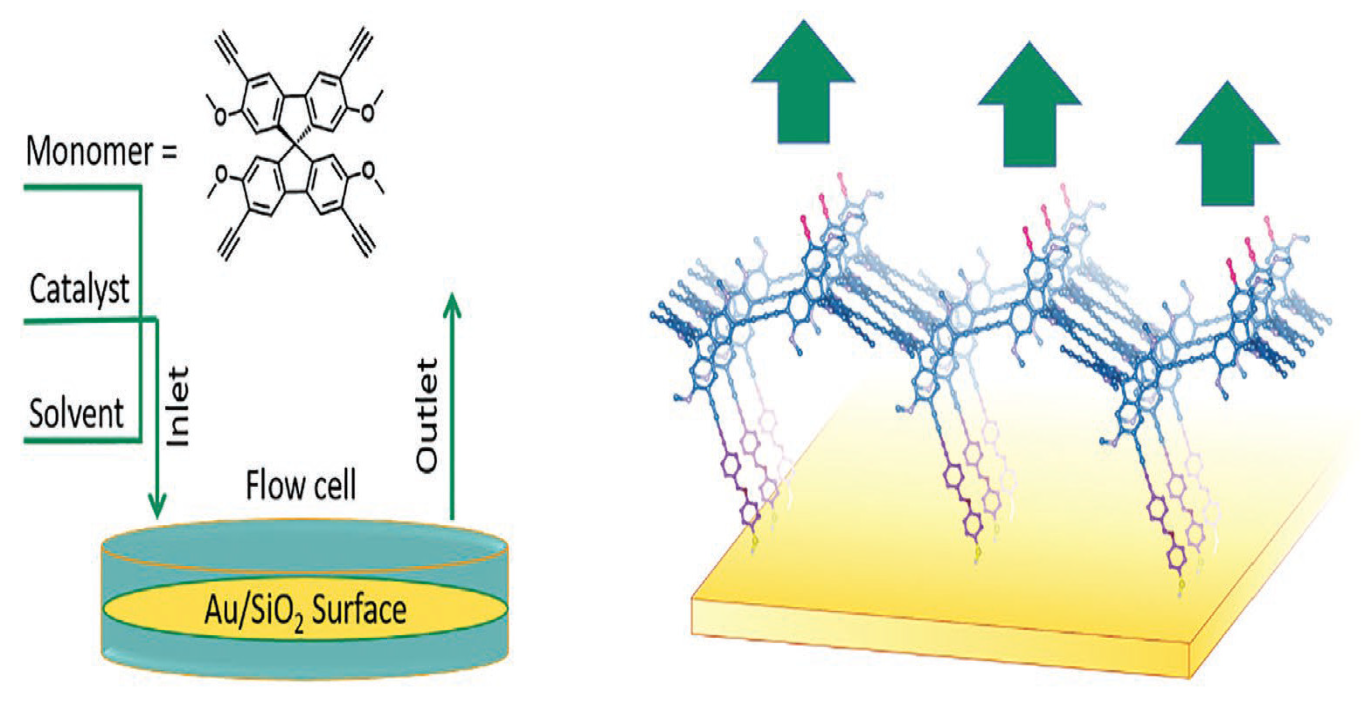
3.4. Continuous Flow Method
3.5. Tuning the Linkers to Obtain a Predefined Topology
3.6. Steric Hindrance Method
3.7. Imine Exchange Strategy
3.8. The Partition Method
4. Topologies of 3D COFs Using Tetrahedral Units
4.1. The pts Topology
4.2. Flexible 3D COFs
4.3. The dia Topology
4.4. The ctn Net or bor Topology
4.5. ljh Topology
4.6. rra Topology
4.7. Topologies of 3D COFs Using Tritopic Building Blocks: srs Topology
5. Application of 3D COFs as Catalyst
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Jhulki, S. Porous flexible frameworks: Origins of flexibility and applications. Mater. Horizons 2020, 8, 700–727. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Lanni, L.M.; Tilford, R.W.; Bharathy, M.; Lavigne, J.J. Enhanced Hydrolytic Stability of Self-Assembling Alkylated Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2011, 133, 13975–13983. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1870229. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X.; et al. Covalent organic framework photocatalysts: Structures and applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef]
- Wang, G.-B.; Li, S.; Yan, C.-X.; Zhu, F.-C.; Lin, Q.-Q.; Xie, K.-H.; Geng, Y.; Dong, Y.-B. Covalent organic frameworks: Emerging high-performance platforms for efficient photocatalytic applications. J. Mater. Chem. A 2020, 8, 6957–6983. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Tri, N.N.; Sreedhar, A.; Noh, J.-S. Highly Surface-Active Si-Doped TiO2/Ti3C2Tx Heterostructure for Gas Sensing and Photodegradation of Toxic Matters. Chem. Eng. J. 2021, 425, 131437. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.; Li, W.; Li, Y.; Dong, Y. Covalent Organic Frameworks (COFs) for Cancer Therapeutics. Chem. A Eur. J. 2019, 26, 5583–5591. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Cho, E.; Sreedhar, A.; Noh, J.-S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Bhunia, S.; Deo, K.A.; Gaharwar, A.K. 2D Covalent Organic Frameworks for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 2002046. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Yang, C.; Cheng, K.; Tan, T.; Lv, Y.; Liu, Y. Hybrid Porous Crystalline Materials from Metal Organic Frameworks and Covalent Organic Frameworks. Adv. Sci. 2021, 8, 2101883. [Google Scholar] [CrossRef]
- Ma, X.; Scott, T.F. Approaches and challenges in the synthesis of three-dimensional covalent-organic frameworks. Commun. Chem. 2018, 1, 98. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Reticular Design of Covalent Organic Frameworks. In Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; John Wiley & Sons: Weinheim, Germany, 2019; pp. 225–243. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Clowes, R.; Cui, P.; Chen, L.; Little, M.A.; Cooper, A.I. 3D Cage COFs: A Dynamic Three-Dimensional Covalent Organic Framework with High-Connectivity Organic Cage Nodes. J. Am. Chem. Soc. 2020, 142, 16842–16848. [Google Scholar] [CrossRef]
- Kang, X.; Han, X.; Yuan, C.; Cheng, C.; Liu, Y.; Cui, Y. Reticular Synthesis of tbo Topology Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 16346–16356. [Google Scholar] [CrossRef]
- Martínez-Abadía, M.; Strutyński, K.; Lerma-Berlanga, B.; Stoppiello, C.T.; Khlobystov, A.N.; Martí-Gastaldo, C.; Saeki, A.; Melle-Franco, M.; Mateo-Alonso, A. π-Interpenetrated 3D Covalent Organic Frameworks from Distorted Polycyclic Aromatic Hydrocarbons. Angew. Chem. Int. Ed. 2021, 60, 9941–9946. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, J.; Guan, X.; Chen, F.; Li, C.; Zhu, L.; Xue, M.; Yuan, D.; Valtchev, V.; Yan, Y.; et al. Three-Dimensional Large-Pore Covalent Organic Framework with stp Topology. J. Am. Chem. Soc. 2020, 142, 13334–13338. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ding, H.; Yuan, D.; Wang, B.; Wang, C. A Pyrene-Based, Fluorescent Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2016, 138, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klöck, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, L.; Gándara, F.; Ma, Y.; Liu, Z.; Zhu, C.; Lyu, H.; Trickett, C.A.; Kapustin, E.A.; Terasaki, O.; et al. A Synthetic Route for Crystals of Woven Structures, Uniform Nanocrystals, and Thin Films of Imine Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 13166–13172. [Google Scholar] [CrossRef]
- Fischbach, D.M.; Rhoades, G.; Espy, C.; Goldberg, F.; Smith, B.J. Controlling the crystalline structure of imine-linked 3D covalent organic frameworks. Chem. Commun. 2019, 55, 3594–3597. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Z.-L.; Wei, L.; Zhou, B.; Tan, J.; Zhou, H.-L.; Zhang, Y.-B. Guest-Dependent Dynamics in a 3D Covalent Organic Framework. J. Am. Chem. Soc. 2019, 141, 3298–3303. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef]
- Rotter, J.M.; Weinberger, S.; Kampmann, J.; Sick, T.; Shalom, M.; Bein, T.; Medina, D.D. Covalent Organic Framework Films through Electrophoretic Deposition—Creating Efficient Morphologies for Catalysis. Chem. Mater. 2019, 31, 10008–10016. [Google Scholar] [CrossRef]
- Ratsch, M.; Ye, C.; Yang, Y.; Zhang, A.; Evans, A.M.; Börjesson, K. All-Carbon-Linked Continuous Three-Dimensional Porous Aromatic Framework Films with Nanometer-Precise Controllable Thickness. J. Am. Chem. Soc. 2020, 142, 6548–6553. [Google Scholar] [CrossRef]
- Yang, Y.; Mallick, S.; Izquierdo-Ruiz, F.; Schäfer, C.; Xing, X.; Rahm, M.; Börjesson, K. A Highly Conductive All-Carbon Linked 3D Covalent Organic Framework Film. Small 2021, 17, 2103152. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.; Makal, T.; Li, J.-R.; Zhou, H. A Highly Porous and Robust (3,3,4)-Connected Metal-Organic Framework Assembled with a 90° Bridging-Angle Embedded Octacarboxylate Ligand. Angew. Chem. Int. Ed. 2012, 51, 1580–1584. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Gropp, C.; Ma, Y.; Zhu, C.; Yaghi, O.M. 3D Covalent Organic Frameworks Selectively Crystallized through Conformational Design. J. Am. Chem. Soc. 2020, 142, 20335–20339. [Google Scholar] [CrossRef]
- Bisbey, R.P.; Dichtel, W.R. Covalent Organic Frameworks as a Platform for Multidimensional Polymerization. ACS Central Sci. 2017, 3, 533–543. [Google Scholar] [CrossRef]
- Geng, K.; Arumugam, V.; Xu, H.; Gao, Y.; Jiang, D. Covalent organic frameworks: Polymer chemistry and functional design. Prog. Polym. Sci. 2020, 108, 101288. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, H.; Guan, X.; Xue, M.; Yan, Y.; Valtchev, V.; Qiu, S.; Fang, Q. Three-Dimensional Mesoporous Covalent Organic Frameworks through Steric Hindrance Engineering. J. Am. Chem. Soc. 2020, 142, 3736–3741. [Google Scholar] [CrossRef]
- Ma, T.; Kapustin, E.A.; Yin, S.X.; Liang, L.; Zhou, Z.; Niu, J.; Li, L.-H.; Wang, Y.; Su, J.; Li, J.; et al. Single-crystal X-ray diffraction structures of covalent organic frameworks. Science 2018, 361, 48–52. [Google Scholar] [CrossRef]
- Liang, L.; Qiu, Y.; Wang, W.D.; Han, J.; Luo, Y.; Yu, W.; Yin, G.; Wang, Z.; Zhang, L.; Ni, J.; et al. Non-Interpenetrated Single-Crystal Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 17991–17995. [Google Scholar] [CrossRef]
- Lan, Y.; Han, X.; Tong, M.; Huang, H.; Yang, Q.; Liu, D.; Zhao, X.; Zhong, C. Materials genomics methods for high-throughput construction of COFs and targeted synthesis. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Higashino, T.; Imahori, H. Porphyrins as excellent dyes for dye-sensitized solar cells: Recent developments and insights. Dalton Trans. 2014, 44, 448–463. [Google Scholar] [CrossRef]
- Lin, G.; Ding, H.; Chen, R.; Peng, Z.; Wang, B.; Wang, C. 3D Porphyrin-Based Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, J.; Yin, S.; Lin, G.; Ma, T.; Meng, Y.; Sun, J.; Wang, C. Isostructural Three-Dimensional Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 9770–9775. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, J.; Xie, G.; Lin, G.; Chen, R.; Peng, Z.; Yang, C.; Wang, B.; Sun, J.; Wang, C. An AIEgen-based 3D covalent organic framework for white light-emitting diodes. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Gao, M.; Cai, Y.; Zhan, C.; Zhao, Z.; Zhang, D.; Tang, B.Z. Introductory lecture: Recent research progress on aggregation-induced emission. Faraday Discuss. 2016, 196, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Yuan, S.; Qin, J.; Liu, C.; Lollar, C.; Wu, M.; Yuan, D.; Zhou, H.-C.; Hong, M. Control the Structure of Zr-Tetracarboxylate Frameworks through Steric Tuning. J. Am. Chem. Soc. 2017, 139, 16939–16945. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Yin, S.; Sun, J.; Wang, C. Twist Building Blocks from Planar to Tetrahedral for the Synthesis of Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 3718–3723. [Google Scholar] [CrossRef]
- Liu, Y.; Diercks, C.S.; Ma, Y.; Lyu, H.; Zhu, C.; Alshmimri, S.A.; Alshihri, S.; Yaghi, O.M. 3D Covalent Organic Frameworks of Interlocking 1D Square Ribbons. J. Am. Chem. Soc. 2018, 141, 677–683. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Gui, B.; Lin, G.; Fu, Q.; Yin, S.; Liu, X.; Sun, J.; Wang, C. A Crystalline Three-Dimensional Covalent Organic Framework with Flexible Building Blocks. J. Am. Chem. Soc. 2021, 143, 2123–2129. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, J.; Gu, S.; Kaspar, R.B.; Zhuang, Z.; Zheng, J.; Guo, H.; Qiu, S.; Yan, Y. 3D Porous Crystalline Polyimide Covalent Organic Frameworks for Drug Delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ge, R.; Song, X.; Xing, X.; Jiang, Q.; Lu, H.; Hao, C.; Guo, X.; Gao, Y.; et al. A 3D Covalent Organic Framework with Exceptionally High Iodine Capture Capability. Chem. A Eur. J. 2017, 24, 585–589. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Guan, X.; Tang, J.; Yusran, Y.; Li, Z.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; et al. Three-Dimensional Ionic Covalent Organic Frameworks for Rapid, Reversible, and Selective Ion Exchange. J. Am. Chem. Soc. 2017, 139, 17771–17774. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Li, Z.-J.; Wei, L.; Ding, S.-Y.; Zhang, Y.-B.; Wang, W. A Dynamic Three-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 4995–4998. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Yuan, C.; Liu, Y.; Cui, Y. Chiral 3D Covalent Organic Frameworks for High Performance Liquid Chromatographic Enantioseparation. J. Am. Chem. Soc. 2018, 140, 892–895. [Google Scholar] [CrossRef]
- Guan, X.; Ma, Y.; Li, H.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Fast, Ambient Temperature and Pressure Ionothermal Synthesis of Three-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Liu, H.; Duan, C.; Pan, Q.; Zhu, J.; Hu, F.; Ma, X.; Jiu, T.; Li, Z.; et al. Highly Conjugated Three-Dimensional Covalent Organic Frameworks Based on Spirobifluorene for Perovskite Solar Cell Enhancement. J. Am. Chem. Soc. 2018, 140, 10016–10024. [Google Scholar] [CrossRef]
- Douthwaite, R.E.; Taylor, A.; Whitwood, A.C. A new polymorph and two inclusion compounds of 9,9′-spirobifluorene. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2005, 61, o328–o331. [Google Scholar] [CrossRef]
- Guan, P.; Qiu, J.; Zhao, Y.; Wang, H.; Li, Z.; Shi, Y.; Wang, J. A novel crystalline azine-linked three-dimensional covalent organic framework for CO2 capture and conversion. Chem. Commun. 2019, 55, 12459–12462. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; Thompson, C.M.; Nguyen, A.X.; Occhialini, G.; McCandless, G.T.; Smaldone, R.A. An azine-linked hexaphenylbenzene based covalent organic framework. Chem. Commun. 2016, 52, 2843–2845. [Google Scholar] [CrossRef]
- Yan, S.; Guan, X.; Li, H.; Li, D.; Xue, M.; Yan, Y.; Valtchev, V.; Qiu, S.; Fang, Q. Three-dimensional Salphen-based Covalent–Organic Frameworks as Catalytic Antioxidants. J. Am. Chem. Soc. 2019, 141, 2920–2924. [Google Scholar] [CrossRef]
- Huang, J.; Han, X.; Yang, S.; Cao, Y.; Yuan, C.; Liu, Y.; Wang, J.-G.; Cui, Y. Microporous 3D Covalent Organic Frameworks for Liquid Chromatographic Separation of Xylene Isomers and Ethylbenzene. J. Am. Chem. Soc. 2019, 141, 8996–9003. [Google Scholar] [CrossRef]
- Ruthven, D.; Goddard, M. Sorption and diffusion of C8 aromatic hydrocarbons in faujasite type zeolites. I. Equilibrium isotherms and separation factors. Zeolites 1986, 6, 275–282. [Google Scholar] [CrossRef]
- Peng, Y.; Gong, T.; Zhang, K.; Lin, X.; Liu, Y.; Jiang, J.; Cui, Y. Engineering chiral porous metal-organic frameworks for enantioselective adsorption and separation. Nat. Commun. 2014, 5, 4406. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, C.; Sun, Q.; Hu, F.; Pan, Q.; Sun, J.; Jin, Y.; Li, Z.; Zhang, W.; Zhao, Y. Spirobifluorene-Based Three-Dimensional Covalent Organic Frameworks with Rigid Topological Channels as Efficient Heterogeneous Catalyst. CCS Chem. 2021, 3, 2418–2427. [Google Scholar] [CrossRef]
- Li, H.; Pan, Q.; Ma, Y.; Guan, X.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Three-Dimensional Covalent Organic Frameworks with Dual Linkages for Bifunctional Cascade Catalysis. J. Am. Chem. Soc. 2016, 138, 14783–14788. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Lin, C.; Gui, B.; Ji, C.; Yuan, D.; Sun, J.; Wang, C. Tuning the Topology of Three-Dimensional Covalent Organic Frameworks via Steric Control: From pts to Unprecedented ljh. J. Am. Chem. Soc. 2021, 143, 7279–7284. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, J.; Ma, D.; Li, P.; Li, S.; Li, H.; Zhou, J.; Ma, X.; Feng, X.; Wang, B. Three-Dimensional Anionic Cyclodextrin-Based Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 16313–16317. [Google Scholar] [CrossRef]
- Li, H.; Meng, B.; Chai, S.-H.; Liu, H.; Dai, S. Hyper-crosslinked β-cyclodextrin porous polymer: An adsorption-facilitated molecular catalyst support for transformation of water-soluble aromatic molecules. Chem. Sci. 2015, 7, 905–909. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Price, D.J.; Robson, R. Tetraanionic Organoborate Squares Glued Together by Cations To Generate Nanotubular Stacks. Angew. Chem. Int. Ed. 2006, 45, 806–810. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.; Whiteley, J.M.; Wan, S.; Jin, Y.; Lee, S.; Zhang, W. Ionic Covalent Organic Frameworks with Spiroborate Linkage. Angew. Chem. Int. Ed. 2015, 55, 1737–1741. [Google Scholar] [CrossRef]
- Ji, C.; Su, K.; Wang, W.; Chang, J.; El-Sayed, E.-S.M.; Zhang, L.; Yuan, D. Tunable Cage-Based Three-Dimensional Covalent Organic Frameworks. CCS Chem. 2022, 4, 3095–3105. [Google Scholar] [CrossRef]
- Chen, X.; Addicoat, M.; Irle, S.; Nagai, A.; Jiang, D. Control of Crystallinity and Porosity of Covalent Organic Frameworks by Managing Interlayer Interactions Based on Self-Complementary π-Electronic Force. J. Am. Chem. Soc. 2012, 135, 546–549. [Google Scholar] [CrossRef]
- Carrington, E.J.; McAnally, C.A.; Fletcher, A.J.; Thompson, S.P.; Warren, M.; Brammer, L. Solvent-switchable continuous-breathing behaviour in a diamondoid metal–organic framework and its influence on CO2 versus CH4 selectivity. Nat. Chem. 2017, 9, 882–889. [Google Scholar] [CrossRef] [Green Version]























| Type of 3D COFs | Topology | Pore Size | BET Surface Area m2 g−1 | Pore Volume cm3 g−1 | Ref |
|---|---|---|---|---|---|
| LZU-301 | dia-C9 | 5.8 × 10.4 Å2 | 654 | [53] | |
| 3D Por-COF | pts | 0.60 nm | 1398 | [42] | |
| 3D CuPor-COF | pts | 0.63 nm | 1335 | ||
| 3D TPB-COF-H | pts | 1050 | 0.49 | [43] | |
| 3DTPB-COF-Me | pts | 950 | 0.45 | ||
| 3D TPB-COF-F | pts | 850 | 0.42 | ||
| Chiral-COF | dia | 0.62 nm | 655 | 0.51 | [54] |
| 3D IL-COF-PDA | dia | 8.3 Å | 517 | 0.36 | [55] |
| 3D IL-COF-DABP | dia | 10.7 Å | 653 | 0.53 | |
| 3D IL-COF-DATP | dia | 12.4 Å | 870 | 0.56 | |
| SP-3D-COF-2 | dia | 641 | 0.45 | [56] | |
| SP-3D-COF-1 | dia | 1582 | 0.97 | ||
| 3D-TPE-COF | pts | 1084 | 0.55 | [44] | |
| 3D-HNU5 | dia | 864 | 0.89 | [58] | |
| JUC-508 | dia | 1.3 nm | 1513 | [60] | |
| JUC-509 | dia | 1.2 nm | 1443 | ||
| 3D COF-salen-Si | dia | 7.8 Å | 666 | 0.43 | [61] |
| 3D COF-salen-Me | dia | 7.8 Å | 701 | 0.38 | |
| 3D COF-SP-BPY | dia | 1.36 nm | 1945 | 1.10 | [64] |
| 3Dchiral-COF LZU-111 | lon-b-c3 | 10.9 Å | 2120 | 0.92 | [50] |
| 3D COF-500-Cu | pts | 12.6 Å | 352 | [60] | |
| 3D LZU-306 | pts | 10.9 Å | 2059 | [51] | |
| 3D cage-COF | acs | 8.8 Å, 5.6 Å | 1040 | [32] | |
| 3D cage-COF-H | acs | 10.5 Å, 11.4 Å | 1143 | [71] | |
| 3D cage-COF-OH, | acs | 6.5 Å | 923 | ||
| 3D cage-COF-Cl | acs | 5.3 Å | 660 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, P.T.; Ta, Q.T.H.; Nguyen, P.K.T. Designed Synthesis of Three-Dimensional Covalent Organic Frameworks: A Mini Review. Polymers 2023, 15, 887. https://doi.org/10.3390/polym15040887
Phan PT, Ta QTH, Nguyen PKT. Designed Synthesis of Three-Dimensional Covalent Organic Frameworks: A Mini Review. Polymers. 2023; 15(4):887. https://doi.org/10.3390/polym15040887
Chicago/Turabian StylePhan, Pham Thi, Qui Thanh Hoai Ta, and Phan Khanh Thinh Nguyen. 2023. "Designed Synthesis of Three-Dimensional Covalent Organic Frameworks: A Mini Review" Polymers 15, no. 4: 887. https://doi.org/10.3390/polym15040887





