An Adhesion Improvement of Low-Density Polyethylene to Aluminum through Modification with Functionalized Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
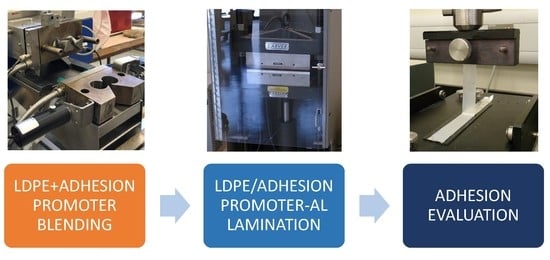
2.2. Samples Preparation
2.3. Wettability Analysis
2.4. Adhesion Investigation
2.5. Mechanical Properties Investigation
2.6. Surface Morphology Analysis
2.7. Chemical Composition Investigation
3. Results
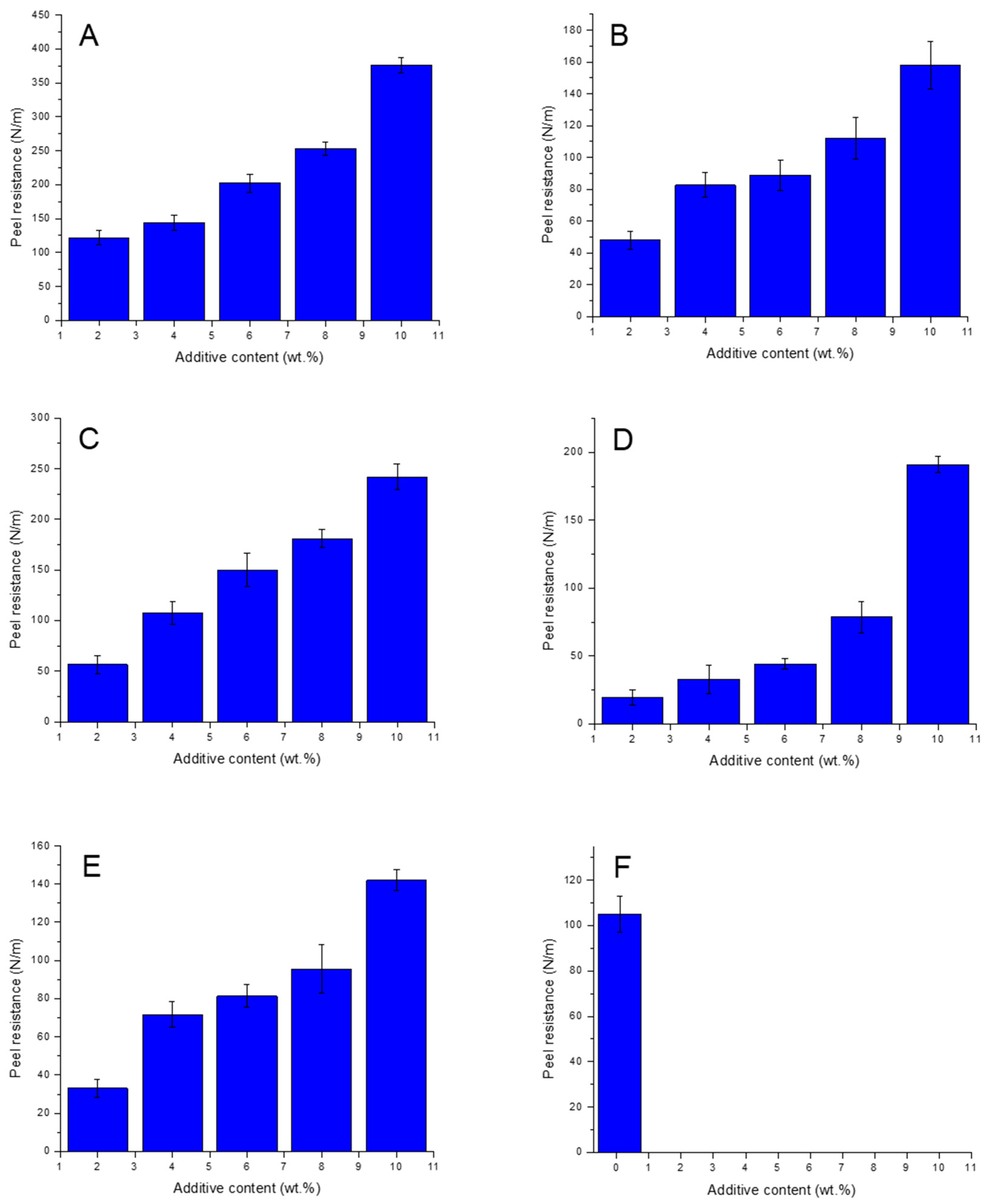
3.1. Adhesive Strength Measurements
3.2. Mechanical Properties Investigation
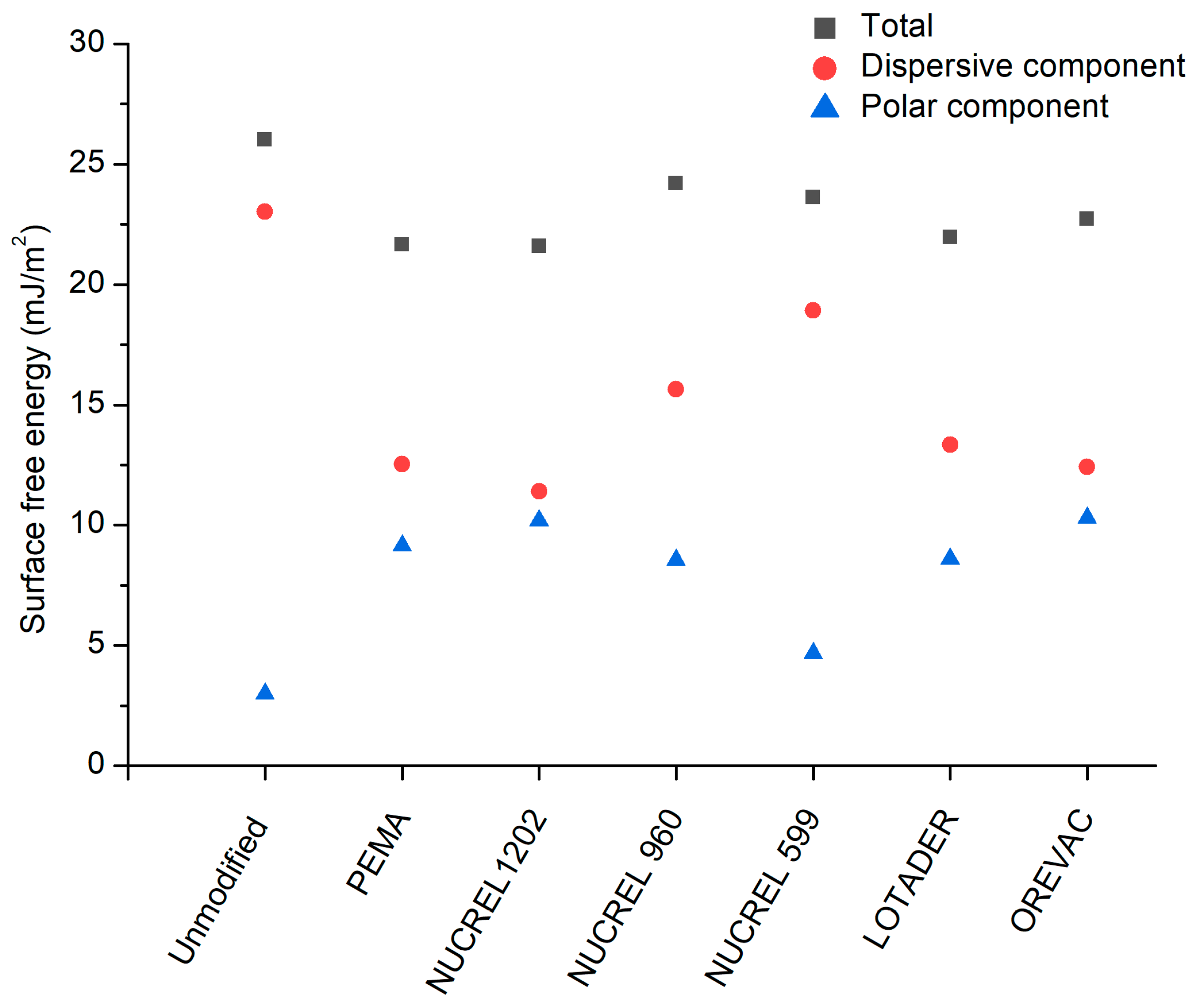
3.3. Surface Wettability Analysis
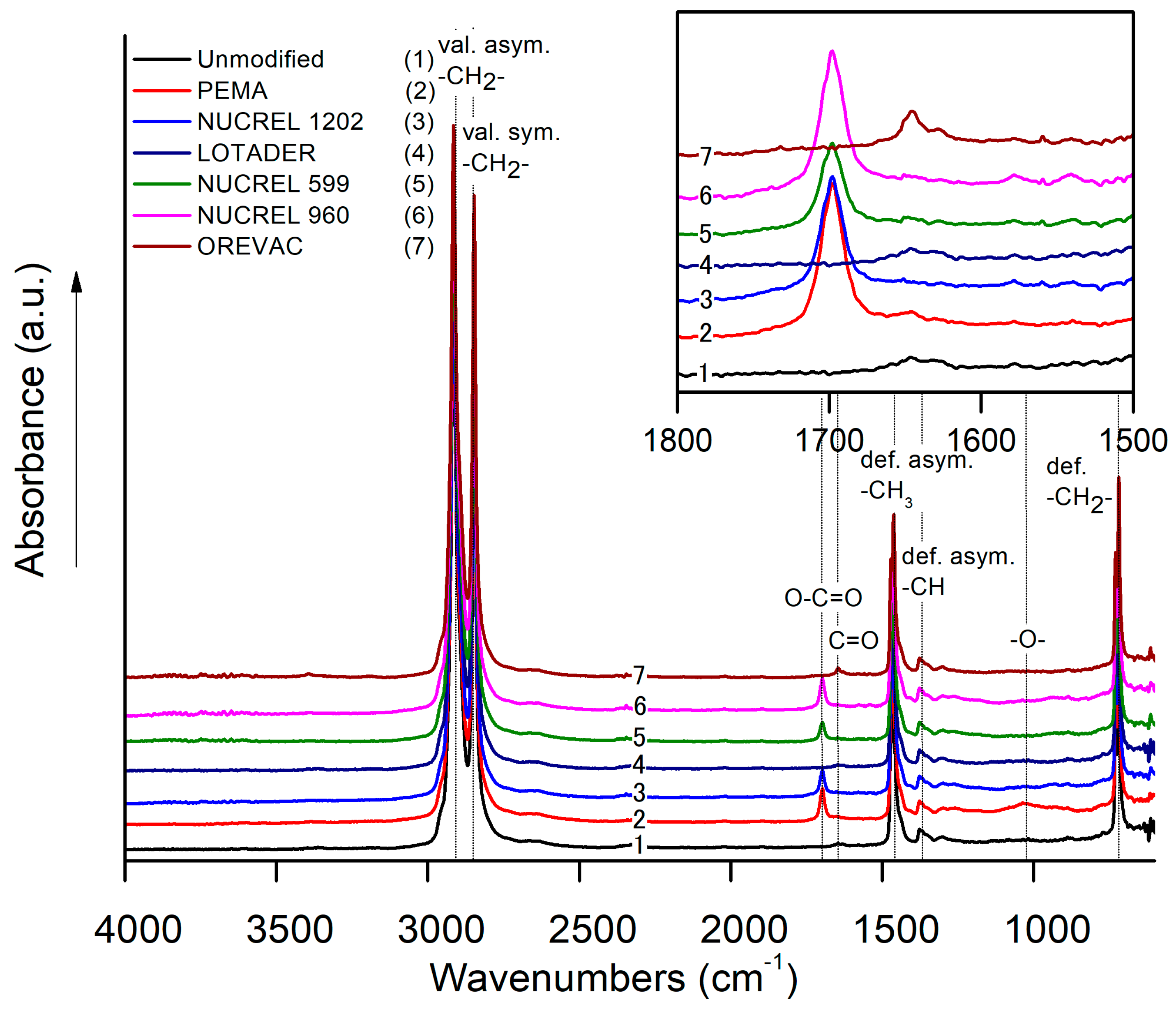
3.4. Chemical Composition Investigation
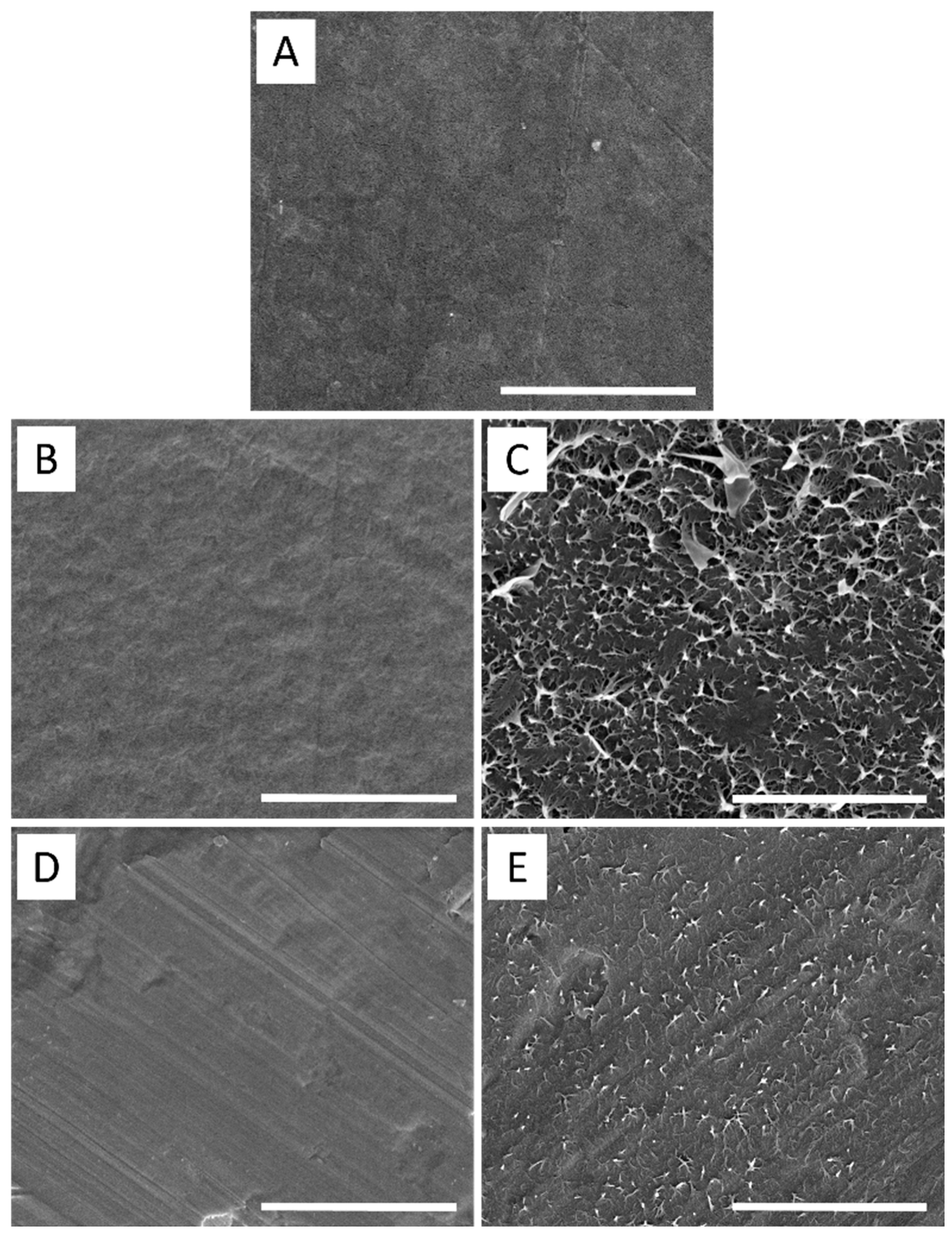
3.5. Surface Morphology/Topography Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahalik, N.P.; Nambiar, A.N. Trends in food packaging and manufacturing systems and technology. Trends Food Sci. Technol. 2010, 21, 117–128. [Google Scholar] [CrossRef]
- Klemchuk, P.P. Degradable plastics: A critical review. Polym. Degrad. Stab. 1990, 27, 183–202. [Google Scholar] [CrossRef]
- Xie, K.; Xu, S.; Hao, W.; Wang, J.; Huang, A.; Zhang, Y. Surface effect of the MgCl2 support in Ziegler–Natta catalyst for ethylene polymerization: A computational study. Appl. Surf. Sci. 2022, 589, 153002. [Google Scholar] [CrossRef]
- Demirors, M. The history of polyethylene. ACS Symp. Ser. 2011, 1080, 115–145. [Google Scholar] [CrossRef]
- Kumar Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Robeson, L.M. Applications of polymer blends: Emphasis on recent advances. Polym. Eng. Sci. 1984, 24, 587–597. [Google Scholar] [CrossRef]
- Van Tienhoven, E.A.E.; Korbee, D.; Schipper, L.; Verharen, H.W.; De Jong, W.H. In vitro and in vivo (cyto)toxicity assays using PVC and LDPE as model materials. J. Biomed. Mater. Res. A 2006, 78, 175–182. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Anal. Health Toxicol. 2020, 35, e2020004. [Google Scholar] [CrossRef]
- Hope, P.S.; Folkes, M.J. Introduction. In Polymer Blends and Alloys; Blackie Academic & Professional: London, UK, 1993; pp. 1–6. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, K.K.; Wang, Y.Z. Properties of Starch Blends with Biodegradable Polymers. J. Macromol. Sci. Part C Polym. Rev. 2007, 43, 385–409. [Google Scholar] [CrossRef]
- Barlow, J.W.; Paul, D.R. Polymer blends and alloys—A review of selected considerations. Polym. Eng. Sci. 1981, 21, 985–996. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1661–1672. [Google Scholar] [CrossRef]
- Roy, P.K.; Hakkarainen, M.; Varma, I.K.; Albertsson, A.C. Degradable polyethylene: Fantasy or reality. Environ. Sci. Technol. 2011, 45, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Brewis, D.M.; Mathieson, I. Adhesion and Bonding to Polyolefins. Rapra Rev. Rep. 2001, 12, 1–132. [Google Scholar]
- Vincent, G.G. Bonding polyethylene to metals. J. Appl. Polym. Sci. 1967, 11, 1553–1562. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Tsao, C.W.; Hromada, L.; Liu, J.; Kumar, P.; DeVoe, D.L. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007, 7, 499–505. [Google Scholar] [CrossRef]
- Jun, G.; Lee, J.W.; Shin, Y.; Kim, K.; Hwang, W. Solvent-aided direct adhesion of a metal/polymer joint using micro/nano hierarchical structures. J. Mater. Process. Technol. 2020, 285, 116744. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.J.; Sun, Z. Effects of polyethylene-grafted maleic anhydride (PE-g-MA) on thermal properties, morphology, and tensile properties of low-density polyethylene (LDPE) and corn starch blends. J. Appl. Polym. Sci. 2003, 88, 2904–2911. [Google Scholar] [CrossRef]
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2018, 47, 2153–2183. [Google Scholar] [CrossRef]
- Packham, D.E.; Bright, K.; Malpass, B.W. Mechanical factors in the adhesion of polyethylene to aluminium. J. Appl. Polym. Sci. 1974, 18, 3237–3247. [Google Scholar] [CrossRef]
- Fourche, G. An overview of the basic aspects of polymer adhesion. Part I: Fundamentals. Polym. Eng. Sci. 1995, 35, 957–967. [Google Scholar] [CrossRef]
- Yoo, S.I.; Lee, T.Y.; Yoon, J.S.; Lee, I.M.; Kim, M.N.; Lee, H.S. Interfacial adhesion reaction of polyethylene and starch blends using maleated polyethylene reactive compatibilizer. J. Appl. Polym. Sci. 2002, 83, 767–776. [Google Scholar] [CrossRef]
- Hidalgo-Salazar, M.A.; Mina, J.H.; Herrera-Franco, P.J. The effect of interfacial adhesion on the creep behaviour of LDPE–Al–Fique composite materials. Compos. Part B Eng. 2013, 55, 345–351. [Google Scholar] [CrossRef]
- Popelka, A.; Krupa, I.; Novák, I.; Al-Maadeed, M.A.S.A.; Ouederni, M. Improvement of aluminum/polyethylene adhesion through corona discharge. J. Phys. D Appl. Phys. 2016, 50, 035204. [Google Scholar] [CrossRef]
- Baldan, A. Adhesion phenomena in bonded joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- Qin, R.Y.; Schreiber, H.P. Adhesion at partially restructured polymer surfaces. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 156, 85–93. [Google Scholar] [CrossRef]
- Kinloch, A.J. Adhesives in engineering. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2005, 211, 307–335. [Google Scholar] [CrossRef]
- Xie, K.; Wang, W.; Li, Y.; Xu, M.; Han, Z.; Zhang, Y.; Gao, W. Study on structure-performance relationship of RGO enhanced polypropylene composites with improved atomic oxygen resistance. Compos. Part B Eng. 2022, 239, 109970. [Google Scholar] [CrossRef]
- Cao, S.; Ge, W.; Yang, Y.; Huang, Q.; Wang, X. High strength, flexible, and conductive graphene/polypropylene fiber paper fabricated via papermaking process. Adv. Compos. Hybrid Mater. 2022, 5, 104–112. [Google Scholar] [CrossRef]
- Yang, S.; Gu, L.; Gibson, R.F. Nondestructive detection of weak joints in adhesively bonded composite structures. Compos. Struct. 2001, 51, 63–71. [Google Scholar] [CrossRef]
- Kinloch, A.J. The science of adhesion—Part 1 Surface and interfacial aspects. J. Mater. Sci. 1980, 15, 2141–2166. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Lucchetta, G.; Marinello, F.; Bariani, P.F. Aluminum sheet surface roughness correlation with adhesion in polymer metal hybrid overmolding. CIRP Ann.-Manuf. Technol. 2011, 60, 559–562. [Google Scholar] [CrossRef]
- Žukiene, K.; Jankauskaite, V.; Mickus, K.V. Adhesion Studies on Piperylene-Styrene Copolymer Modified Polychloroprene Adhesive: Phase Morphology and Surface Composition. J. Adhes. 2008, 84, 601–618. [Google Scholar] [CrossRef]
- Flinn, R.A.; Trojan, P.K. Engineering Materials and Their Applications, 4th ed.; Wiley: New York, NY, USA, 1994; ISBN 0471125083. [Google Scholar]
- Bikerman, J.J. The Science of Adhesive Joints; Elsevier Science: Amsterdam, The Netherlands, 1968; ISBN 9781483267739. [Google Scholar]
- Boyd, R.H.; Phillips, P.J. The Science of Polymer Molecules; Cambridge University Press: Cambridge, UK, 1993; ISBN 9780521320764. [Google Scholar]
- Akelah, A.; Sherrington, D.C. Recent developments in the application of functionalized polymers in organic synthesis. Polymer 1983, 24, 1369–1386. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane adhesion promoters in coatings. Prog. Org. Coat. 1983, 11, 297–308. [Google Scholar] [CrossRef]
- Tien-Hua Chou, R. Ionomers based on copolymers of ethylene with both mono-and dicarboxylic acids. US5859137A, 12 January 1999. [Google Scholar]
- Tabor, R.L.; Allen, J.A. Maleic anhydride grafts of olefin polymers. US4684576A, 4 August 1987. [Google Scholar]
- Schneider, B.; Hennemann, O.D.; Possart, W. The adhesion of maleic anhydride on native aluminum oxide: An approach by infrared spectroscopy and quantum mechanical modeling. J. Adhes. 2002, 78, 779–797. [Google Scholar] [CrossRef]
- Iqbal, M.; Chuai, C.; Huang, Y.; Che, C. Modification of low-density polyethylene by graft copolymerization with maleic anhydride and blends with polyamide 6. J. Appl. Polym. Sci. 2010, 116, 1558–1565. [Google Scholar] [CrossRef]
- Do Amaral Montanheiro, T.L.; Passador, F.R.; De Oliveira, M.P.; Duran, N.; Lemes, A.P. Preparation and characterization of maleic anhydride grafted poly (hydroxybutirate-CO-hydroxyvalerate)-PHBV-g-MA. Mater. Res. 2016, 19, 229–235. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, B.H.; Choi, S.W.; Kim, S.; Kim, H.J. The effect of types of maleic anhydride-grafted polypropylene (MAPP) on the interfacial adhesion properties of bio-flour-filled polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1473–1482. [Google Scholar] [CrossRef]
- Ali, S.; Ji, Y.; Zhang, Q.; Zhao, H.; Chen, W.; Wang, D.; Meng, L.; Li, L. Preparation of Polyethylene and Ethylene/Methacrylic Acid Copolymer Blend Films with Tunable Surface Properties through Manipulating Processing Parameters during Film Blowing. Polymers 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Su, R.; Zhang, Q.; Du, R.N.; Fu, Q. Effect of ethylene–acrylate–(maleic anhydride) terpolymer on mechanical properties and morphology of poly(ethylene terephthalate)/polyamide-6 blends. Polym. Int. 2008, 57, 139–148. [Google Scholar] [CrossRef]
- Huang, C.-H.; Huang, C.-C.; Lin, L.-S. Adhesion, Permeability, and Mechanical Properties of Multilayered Blown Films Using Maleated Low-Density Polyethylene Blends as Adhesion-Promoting Layers. Polym. J. 2003, 35, 978–984. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Packham, D.E. Theories of Fundamental Adhesion; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Roesner, A.; Scheik, S.; Olowinsky, A.; Gillner, A.; Reisgen, U.; Schleser, M. Laser Assisted Joining of Plastic Metal Hybrids. Phys. Procedia 2011, 12, 370–377. [Google Scholar] [CrossRef]


| Polymer Properties | EC02 |
|---|---|
| Density @ 23 °C (ASTM D-1505) | 0.923 g/cm3 |
| Melt flow index 190 °C/2.16 kg (ASTM D-1238) | 4.0 g/10 min |
| Melting Point (ASTM E-794) | 108 °C |
| Recommended uses | Extrusion of very high-clarity blown and cast films |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|
| Unmodified | 12.2 ± 0.9 | 288.6 ± 18.6 |
| Modified with 0.1 wt.% OREVAC | 11.3 ± 1.4 | 269.5 ± 21.5 |
| Modified with 0.5 wt.% OREVAC | 10.6 ± 0.5 | 253.0 ± 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassr, M.; Krupa, I.; Ouederni, M.; Krishnamoorthy, S.K.; Popelka, A. An Adhesion Improvement of Low-Density Polyethylene to Aluminum through Modification with Functionalized Polymers. Polymers 2023, 15, 916. https://doi.org/10.3390/polym15040916
Nassr M, Krupa I, Ouederni M, Krishnamoorthy SK, Popelka A. An Adhesion Improvement of Low-Density Polyethylene to Aluminum through Modification with Functionalized Polymers. Polymers. 2023; 15(4):916. https://doi.org/10.3390/polym15040916
Chicago/Turabian StyleNassr, Mohamed, Igor Krupa, Mabrouk Ouederni, Senthil Kumar Krishnamoorthy, and Anton Popelka. 2023. "An Adhesion Improvement of Low-Density Polyethylene to Aluminum through Modification with Functionalized Polymers" Polymers 15, no. 4: 916. https://doi.org/10.3390/polym15040916
APA StyleNassr, M., Krupa, I., Ouederni, M., Krishnamoorthy, S. K., & Popelka, A. (2023). An Adhesion Improvement of Low-Density Polyethylene to Aluminum through Modification with Functionalized Polymers. Polymers, 15(4), 916. https://doi.org/10.3390/polym15040916