Influence of Methacrylate and Vinyl Monomers on Radical Bulk Photopolymerization Process and Properties of Epoxy-Acrylate Structural Adhesives
Abstract
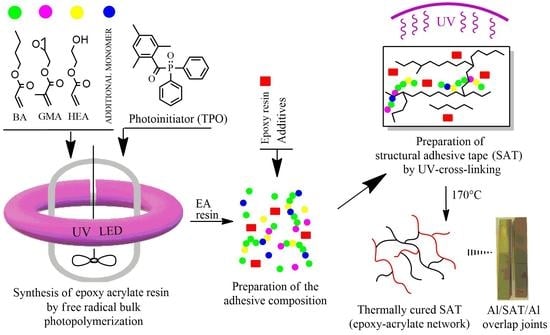
:1. Introduction
2. Materials and Methods
2.1. Materials
- (I)
- base monomers: n-butyl acrylate (BA), glycidyl methacrylate (GMA), 2-hydroxyethyl acrylate (HEA) (Merck Group, Warsaw, Poland);
- (II)
- methacrylic monomers: methyl methacrylate (MMA), ethyl methacrylate (EMA), butyl methacrylate (BMA), lauryl methacrylate (LMA), (2-acetoacetoxy)ethyl methacrylate (AEM) (Merck Group, Warsaw, Poland)
- (III)
- vinyl monomers: N-vinylpyrrolidone (NVP) (BASF, Ludwigshafen, Germany) and styrene (STY) (Merck Group, Warsaw, Poland),
2.2. Synthesis of Epoxy Acrylic Resins
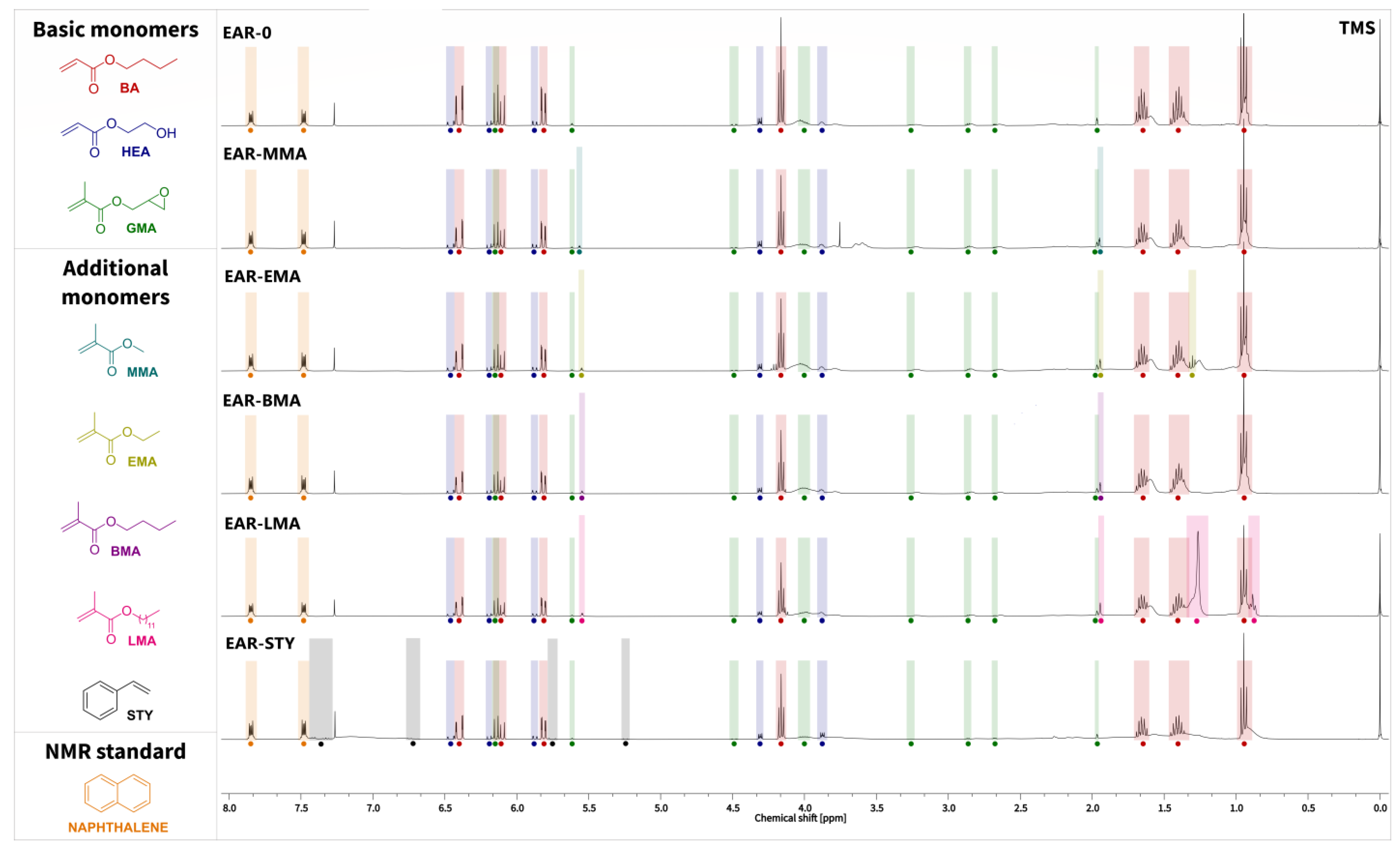
2.3. Characterization of the Epoxy Acrylic Resins
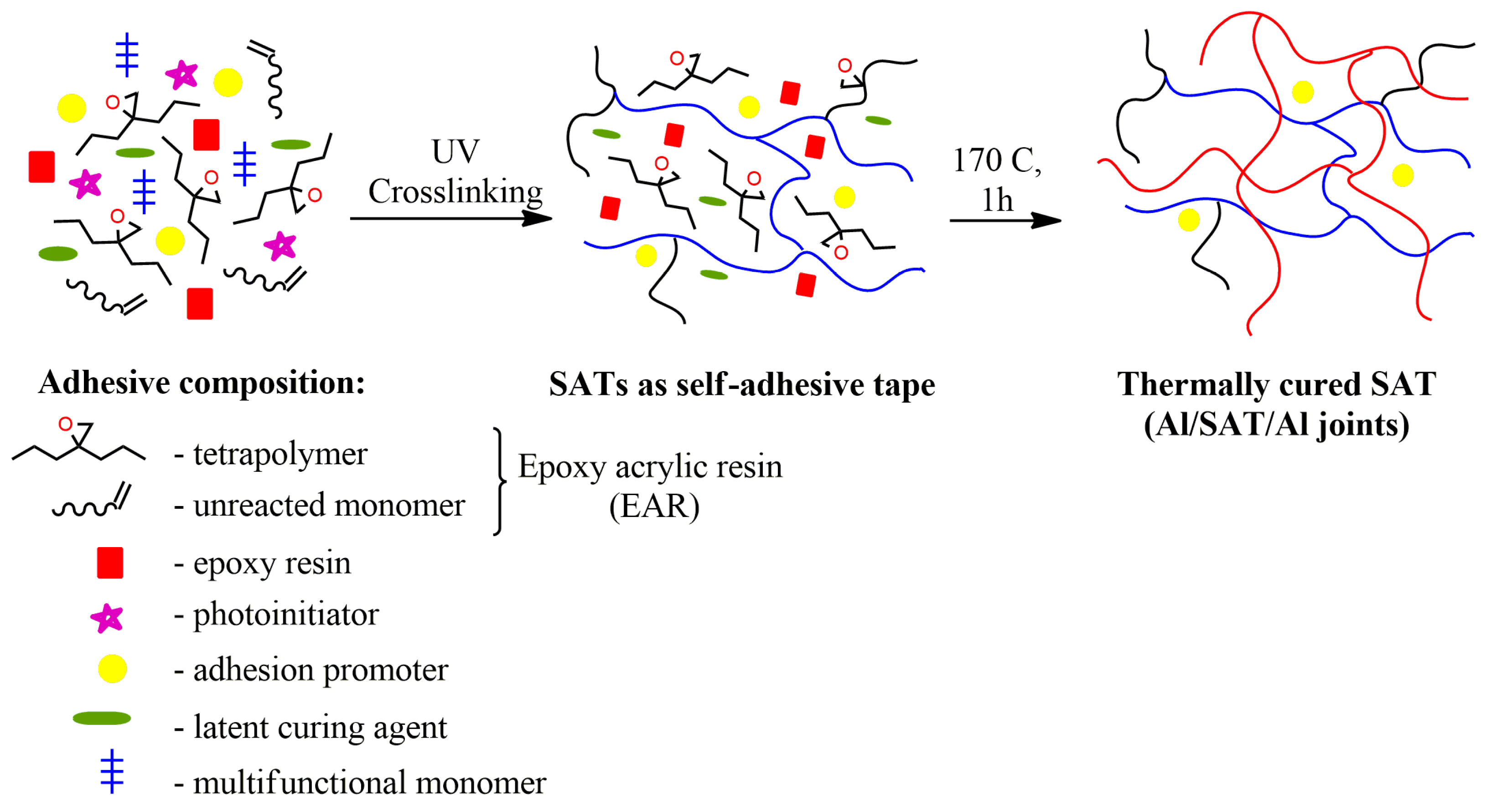
2.4. Preparation and Characterization of Structural Adhesive Tapes (SATs) and Al/SAT/Al Joints
3. Results
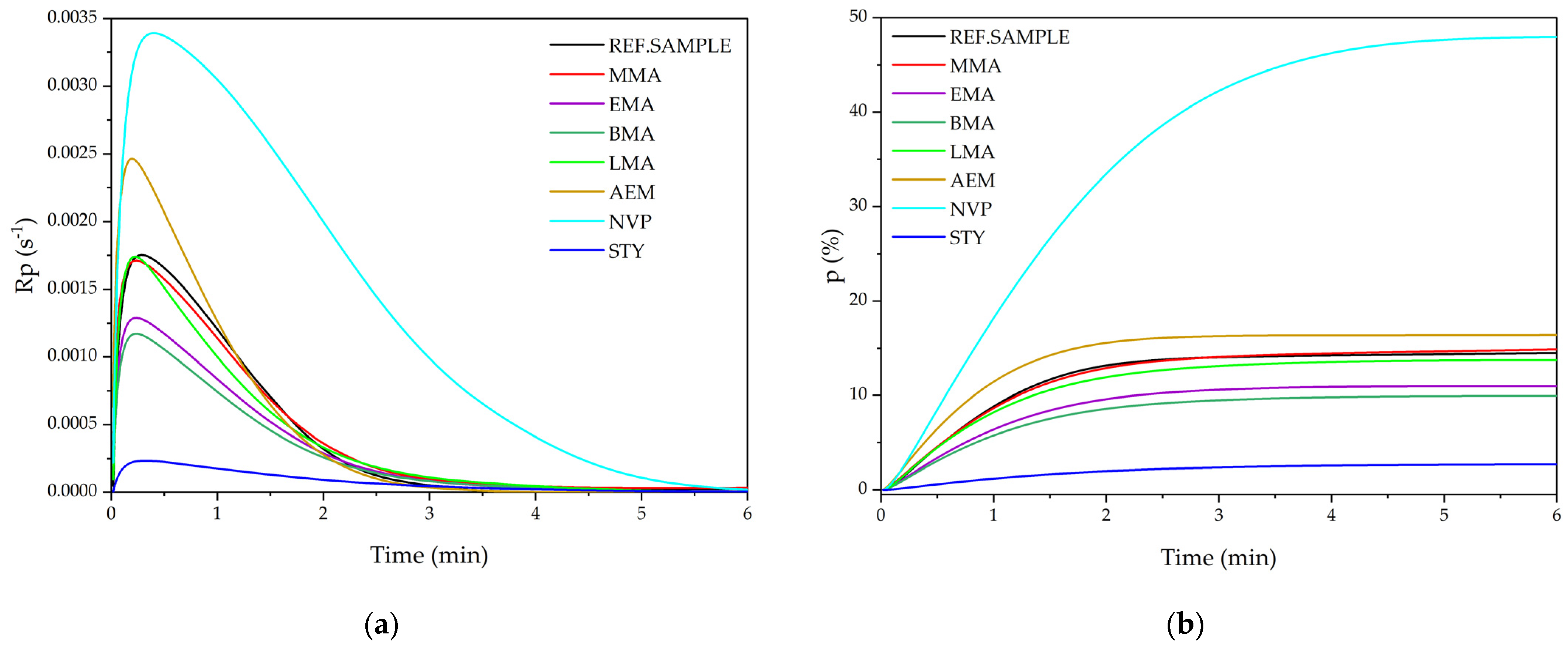
3.1. Kinetic Study of Photopolymerization Process
3.2. The Physicochemical Properties of the Epoxy Acrylic Resins
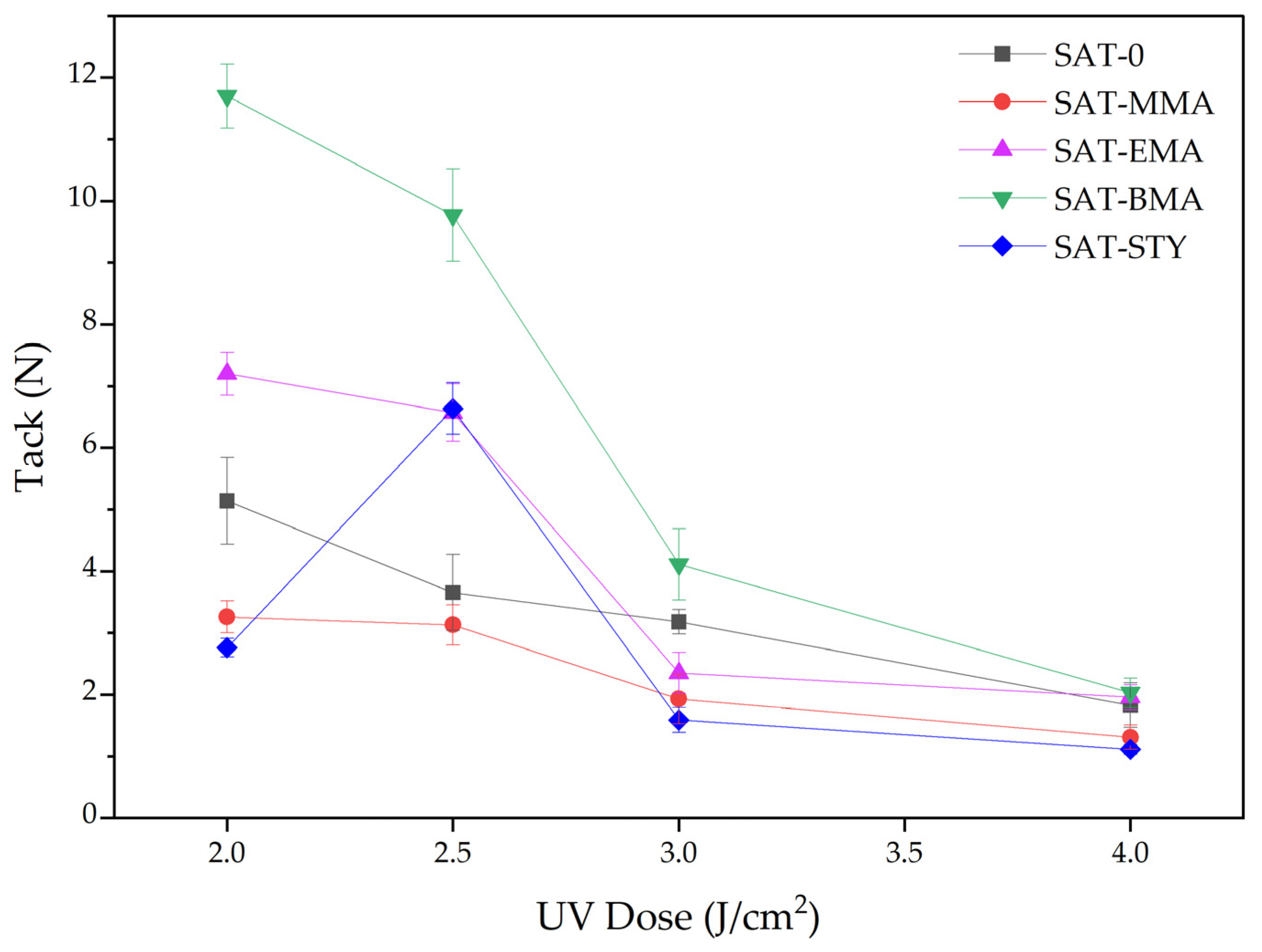
3.3. Properties of UV-Crosslinked SATs Based on EARs
3.4. Mechanical Properties of Thermally Cured SATs Based on Epoxy Acrylic Resins
4. Conclusions
- -
- Heteroatom-containing monomers (like NVP and AEM) are known to increase the rate of reaction and monomer conversion and should therefore not be used in FRBP;
- -
- Methacrylic monomers with short aliphatic chain (C1–C4) allow the reduction of the rate of photopolymerization reaction, extends the time to reach the temperature peak and increases the conversion of monomers (up to ca. 60%). On the other hand, long-chain methacrylates (C11) do not significantly reduce the rate of photopolymerization, but slightly increase the conversion of monomers (about 15% compared to the reference sample). However, the resulting tetrapolymers are characterized by higher polydispersity;
- -
- Styrene has the greatest impact on reducing the reaction rate and increasing monomer conversion (up to 67%). Significantly more photoinitiator should be used for the photopolymerization process involving styrene. The resulting tetrapolymers have very low average molecular weights. Additionally, structural adhesives based on epoxy acrylate resin with STY were characterized by high adhesion to steel (9–11.5 N/25 mm) and shear strength (20.8 MPa).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascault, J.P.; Williams, R.J. Epoxy Polymers: New Materials and Innovations; Wiley-VCH VerabH: New York, NY, USA, 2010. [Google Scholar]
- Dhole, G.S.; Gunasekaran, G.; Ghorpade, T.; Vinjamur, M. Smart acrylic coatings for corrosion detection. Prog. Org. Coat. 2017, 110, 140–149. [Google Scholar] [CrossRef]
- Paz, E.; Narbón, J.J.; Abenojar, J.; Cledera, M.; Del Real, J.C. Influence of Acrylic Adhesive Viscosity and Surface Roughness on the Properties of Adhesive Joint. J. Adhes. 2016, 92, 877–891. [Google Scholar]
- Márquez, I.; Paredes, N.; Alarcia, F.; Velasco, J.I. Adhesive Performance of Acrylic Pressure-Sensitive Adhesives from Different Preparation Processes. Polymers 2021, 13, 2627. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sauraj, S.; Kumar, B.; Pandey, A.; Dutt, D.; Negi, S.Y.; Maji, P.K.; Kulshreshtha, A. Synthesis of waterborne acrylic copolymer resin as a binding agent for the development of water-based inks in the printing application. Polym. Eng. 2021, 61, 1569–1580. [Google Scholar] [CrossRef]
- Xu, X.; He, L.; Zhu, B.; Lib, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823. [Google Scholar] [CrossRef]
- Gawdzinska, K.; Paszkiewicz, S.; Piesowicz, E.; Bryll, K.; Irska, I.; Lapis, A.; Sobolewska, E.; Kochmanska, A.; Slaczka, W. Preparation and Characterization of Hybrid Nanocomposites for Dental Applications. Appl. Sci. 2019, 9, 1381. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Simon-Masseron, A.; Lalevee, J. Radical photoinitiation with LEDs and applications in the 3D printing of composites. Chem. Soc. Rev. 2021, 50, 3824–3841. [Google Scholar] [CrossRef]
- Markets and Market, Acrylic Resin Market Research. Available online: https://www.marketsandmarkets.com/Market-Reports/acrylic-resin-market-246195771.html?gclid=Cj0KCQiAk4aOBhCTARIsAFWFP9Eiiix9vL4jXMJNPd4409IMjYsYSdgIKR0vrksxkn10wysa4JrnzggaAvGkEALw_wcB (accessed on 21 December 2021).
- Poth, U.; Schwalm, R.; Schwartz, M.; Baumstark, R. Acrylic Resins; Vincentz Network: Hanover, Germany, 2011; pp. 279–290. [Google Scholar]
- Grady, C.M.; Simonsick, W.J.; Hutchinson, R.A. Studies of higher temperature polymerization of n-butyl methacrylate and n-butyl acrylate. Macromol. Symp. 2002, 182, 149–168. [Google Scholar] [CrossRef]
- Adamsons, K.; Blackman, G.; Gregorovich, B.; Lin, L.; Matheson, R. Oligomers in the evolution of automotive clearcoats: Mechanical performance testing as a function of exposure. Prog. Org. Coat. 1998, 34, 64–74. [Google Scholar] [CrossRef]
- Pirman, T.; Ocepek, M.; Likozar, B. Radical Polymerization of Acrylates, Methacrylates, and Styrene: Biobased Approaches, Mechanism, Kinetics, Secondary Reactions, and Modeling. Ind. Eng. Chem. Res. 2021, 60, 9347–9367. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Shao, Q.; Song, J.; Hu, Q.; Naik, N.; Guo, Z. Advances in Waterborne Acrylic Resins: Synthesis Principle, Modification Strategies, and Their Applications. ACS Omega 2021, 6, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Gziut, K.; Kowalczyk, A.; Schmidt, B.; Kowalczyk, K.; Weisbrodt, M. Epoxy-Based Structural Self-Adhesive Tapes Modified with Acrylic Syrups Prepared via a Free Radical Photopolymerization Process. Polymers 2021, 3, 189. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Gziut, K.; Kowalczyk, A. Preparation and properties of urethane acrylate varnishes obtained by bulk photopolymerization. Przem. Chem. 2018, 97, 1870–1872. [Google Scholar]
- Gziut, K.; Kowalczyk, A. Influence of radical photoinitiators on features of polyacrylate syrups and self-adhesives. Polimery 2020, 65, 268–274. [Google Scholar] [CrossRef]
- Back, J.; Kwon, Y.; Roldao, J.; Yu, Y.; Kim, H.; Gierschner, J.; Lee, W.; Kwon, M.S. Synthesis of solvent-free acrylic pressure-sensitive adhesives via visible-light-driven photocatalytic radical polymerization without additives. Green Chem. 2020, 22, 8289–8297. [Google Scholar]
- Baek, S.; Hwang, S. Preparation and adhesion performance of transparent acrylic pressure-sensitive adhesives containing menthyl acrylate. Polym. Bull. 2016, 73, 87–701. [Google Scholar] [CrossRef]
- Lena, J.-B.; Deschamps, M.; Sciortino, N.F.; Masters, S.L.; Squire, M.A.; Russell, G.T. Effects of Chain Transfer Agent and Temperature on Branching and β-Scission in Radical Polymerization of 2-Ethylhexyl Acrylate. Macromol. Chem. Phys. 2018, 219, 1700579. [Google Scholar] [CrossRef]
- Cioffi, M.; Hoffmann, A.C.; Janssen, L.P.B.M. Reducing the gel effect in free radical polymerization. Chem. Eng. Sci. 2001, 56, 911–915. [Google Scholar]
- Taheri, M.; Jahanfar, M.; Ogino, K. Synthesis of acrylic resins for high-solids traffic marking paint by solution polymerization. Des. Monomers Polym. 2019, 22, 213–225. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A.; Schmidt, B. Free-Radical Bulk-Photopolymerization Process as a Method of Obtaining Thermally Curable Structural Self-Adhesive Tapes and Effect of Used Type I Photoinitiators. Polymers 2020, 12, 2191. [Google Scholar] [CrossRef]
- Buback, M.; Huckestein, B.; Russell, G.T. Modeling of termination in intermediate and high conversion free radical polymerizations. Macromol. Chem. Phys. 1994, 195, 539–554. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 618. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Xue, X. Morphology and properties of UV-curing epoxy acrylate coatings modified with methacryl-POSS. Prog. Org. Coat. 2015, 78, 404–410. [Google Scholar] [CrossRef]
- White, T.J.; Liechty, W.B.; Guymon, C.A. The influence of N-vinyl pyrrolidone on polymerization kinetics and thermo-mechanical properties of crosslinked acrylate polymers. J. Polym. Sci. 2007, 45, 4062–4073. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Zych-Tomkowiak, D.; Andrzejewski, M.; Hug, G.; Marciniak, B. Heteroaromatic thiols as co-initiators for type II photoinitiating systems based on camphorquinone and isopropylthioxanthone. Macromolecules 2006, 11, 3777–3785. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley-Interscienc: New York, NY, USA, 1999. [Google Scholar]
- Do, H.-S.; Park, Y.-J.; Kim, H.-J. Preparation and adhesion performance of UV-crosslinkable acrylic pressure sensitive adhesives. J. Adhes. Sci. Technol. 2006, 20, 1529–1545. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, Y.; Ye, L.; Zeng, S.; Yu, P.; Xu, W.A. novel UV-crosslinked pressure-sensitive adhesive based on photoinitiator-grafted SBS. Int. J. Adhes. Adhes. 2010, 30, 43–46. [Google Scholar] [CrossRef]
- Khan, I.; Poh, B.T. Effect of molecular weight and testing rate on adhesion property of pressure-sensitive adhesives prepared from epoxidized natural rubber. Mater. Des. 2011, 32, 2513–2519. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Meredith, H.J.; Wilker, J.J. Molecular Weight Effects upon the Adhesive Bonding of a Mussel Mimetic Polymer. ACS Appl. Mater. Inter. 2013, 5, 5091–5096. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, T.-H.; Shim, K.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y.; Jung, S. Effect of crosslinking density on adhesion performance and flexibility properties of acrylic pressure sensitive adhesives for flexible display applications. Int. J. Adhes. Adhes. 2017, 74, 137–143. [Google Scholar] [CrossRef]




| Base Monomers | |||
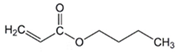 Butyl acrylate (BA) |  2-Hydroxyethyl acrylate (HEA) | 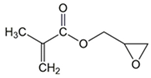 Glycidyl methacrylate (GMA) | |
| Methacrylic comonomers | |||
 Methyl methacrylate (MMA) |  Ethyl methacrylate (EMA) |  Butyl methacrylate (BMA) | |
 Lauryl methacrylate (LMA) | 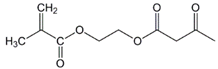 (2-Acetoacetoxy)ethyl methacrylate (AEM) | ||
| Vinyl comonomers | |||
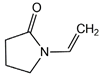 N-vinylpyrrolidone (NVP) |  Styrene (STY) | ||
| EAR Symbol | Monomers (mol %) | TPO * | |||
|---|---|---|---|---|---|
| Base Monomers | Additional Monomer (20 mol %) | (mol) | |||
| BA | HEA | GMA | |||
| EAR-0 | 80 | 10 | 10 | - | 0.01 |
| EAR-MMA | 60 | 10 | 10 | MMA | 0.04 |
| EAR-EMA | EMA | 0.035 | |||
| EAR-BMA | BMA | 0.032 | |||
| EAR-LMA | LMA | 0.022 | |||
| EAR-AEM | AEM | — | |||
| EAR-NVP | NVP | — | |||
| EAR-STY | STY | 0.24 | |||
| EAR Symbol | Monomers Conversion (%) | SC (%) | η (Pa∙s) | Mn (g/mol) | Mw (g/mol) | PDI |
|---|---|---|---|---|---|---|
| EAR-0 | 40 | 37 | 3.5 | 21,410 | 82,120 | 3.83 |
| EAR-MMA | 60 | 56 | 8 | 10,550 | 32,120 | 3.04 |
| EAR-EMA | 59 | 58 | 10 | 11,490 | 38,290 | 3.33 |
| EAR-BMA | 62 | 59 | 11 | 11,790 | 30,570 | 3.27 |
| EAR-LMA | 56 | 54 | 6 | 21,770 | 85,090 | 3.9 |
| EAR-STY | 67 | 64 | 3 | 2780 | 6110 | 2.2 |
| EAR Symbol | Monomers Conversion (%) | Conversion of Individual Comonomers (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BA | GMA | HEA | MMA | EMA | BMA | LMA | STY | ||
| EAR-0 | 40 | 36 | 73 | 37 | - | - | - | - | - |
| EAR-MMA | 60 | 50 | 84 | 48 | 87 | - | - | - | - |
| EAR-EMA | 59 | 48 | 84 | 49 | - | 85 | - | - | - |
| EAR-BMA | 62 | 51 | 86 | 52 | - | - | 84 | - | - |
| EAR-LMA | 56 | 42 | 79 | 45 | - | - | - | 74 | - |
| EAR-STY | 67 | 58 | 87 | 57 | - | - | - | - | 95 |
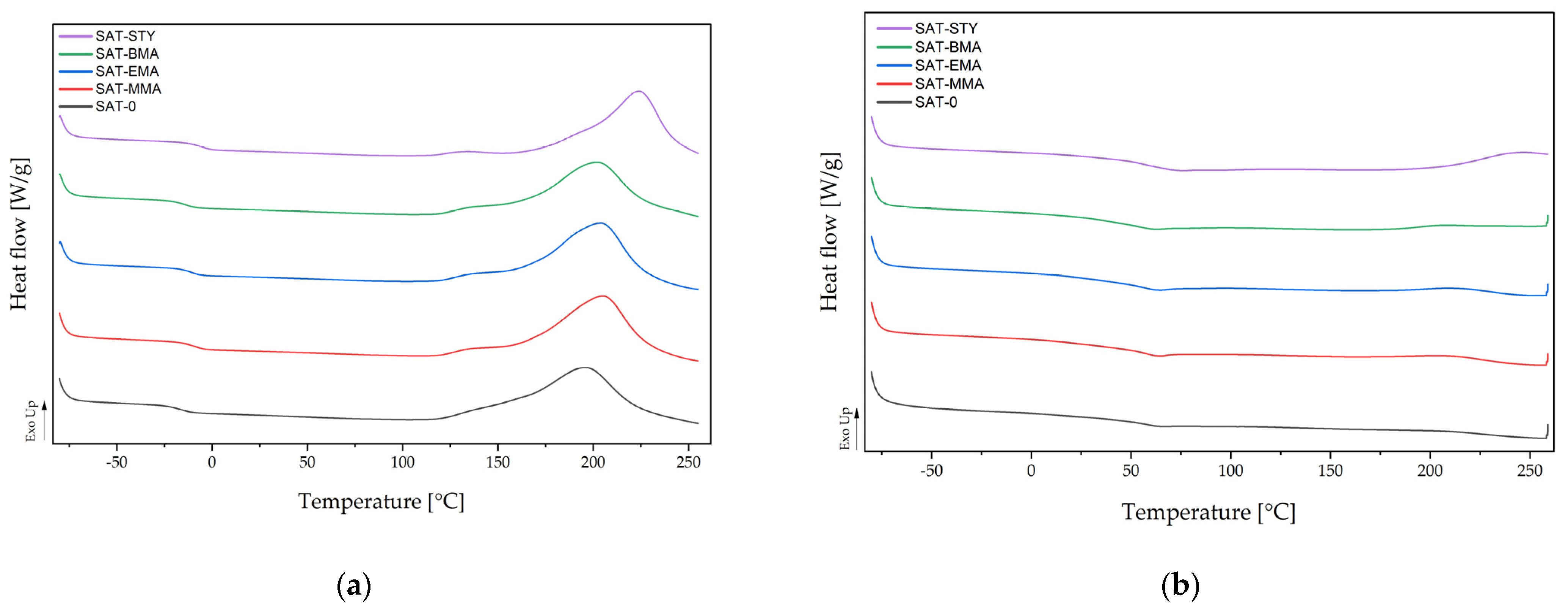
| SAT Acronym | Tg (°C) | Ti (°C) | Tp (°C) | ΔH (J/g) | α (a.u.) |
|---|---|---|---|---|---|
| SAT-0 | −17 | 147 | 196 | 247 | 0.97 |
| SAT-MMA | −8 | 160 | 206 | 214 | 0.96 |
| SAT-EMA | −11 | 161 | 205 | 215 | 0.96 |
| SAT-BMA | −14 | 160 | 202 | 194 | 0.97 |
| SAT-STY | −5 | 189 | 225 | 183 | 0.92 |
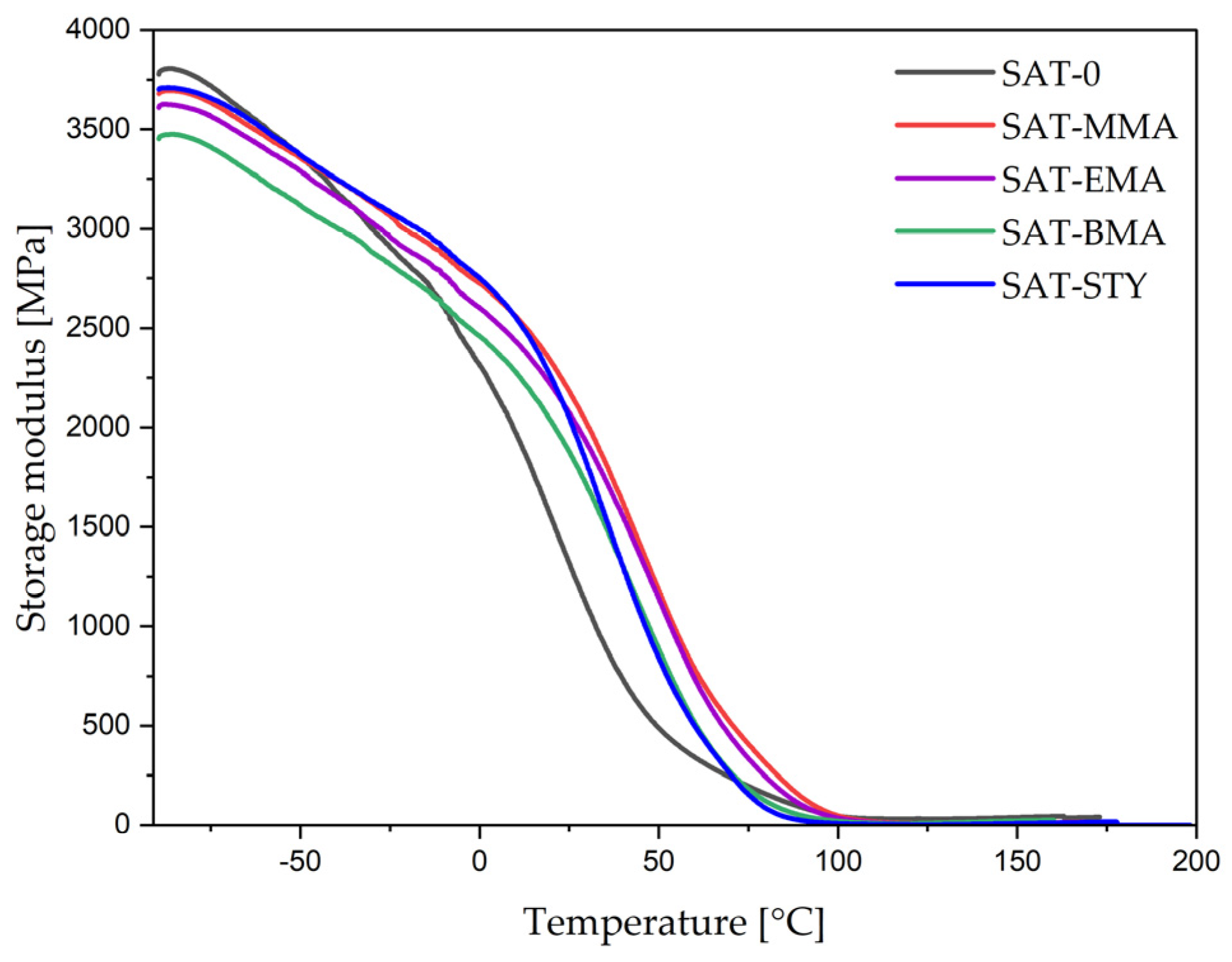
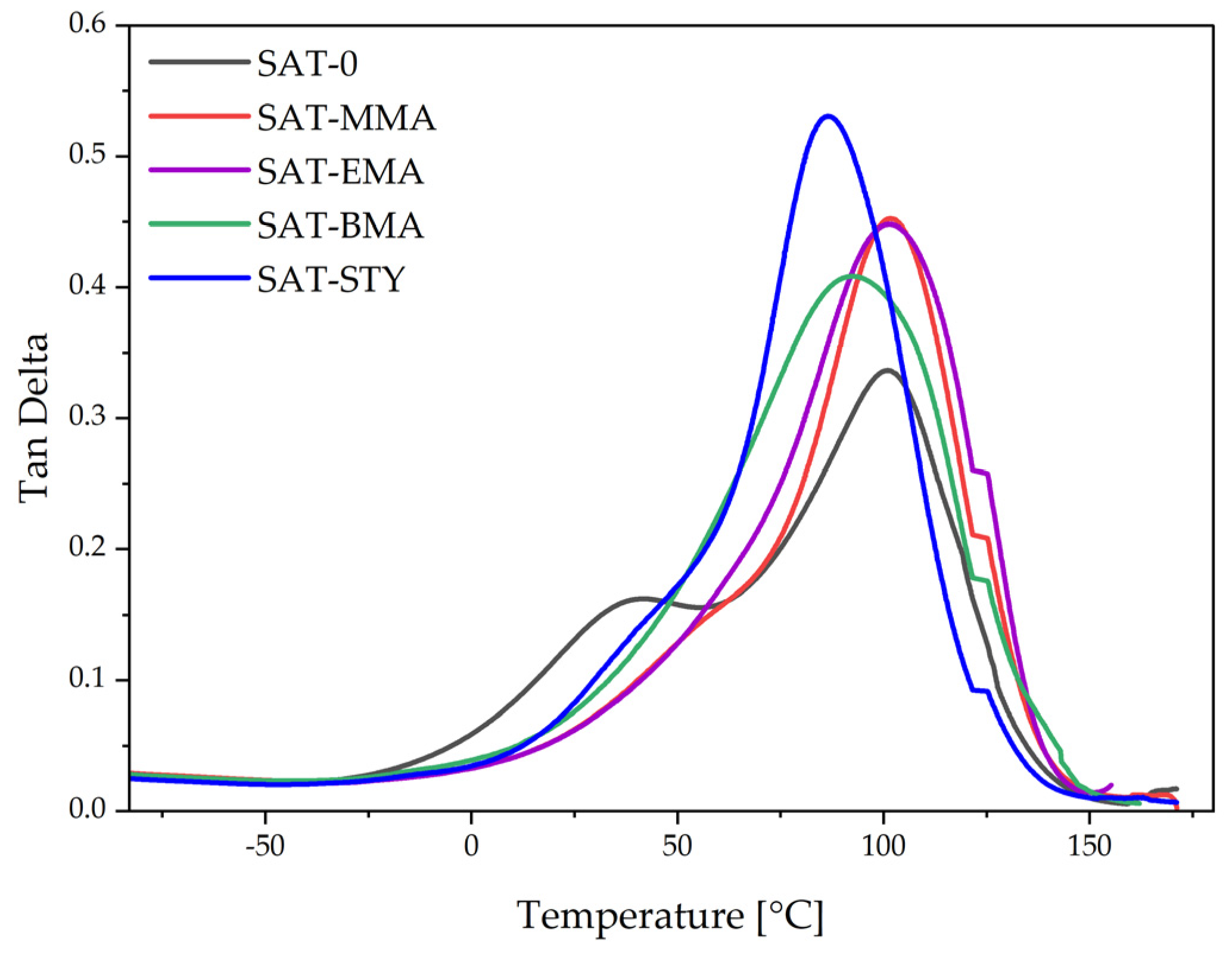
| SAT | E′ (−50 °C) (MPa) | E′ (25 °C) (MPa) | E′ (150 °C) (MPa) | Tg (°C) |
|---|---|---|---|---|
| SAT-0 | 3 358 | 1 310 | 38 | 30 and 102 |
| SAT-MMA | 3 350 | 2 165 | 10 | 101 |
| SAT-EMA | 3 285 | 2 070 | 15 | 100 |
| SAT-BMA | 3 112 | 1 875 | 21 | 92 |
| SAT-STY | 3 365 | 2 050 | 9 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gziut, K.; Kowalczyk, A.; Schmidt, B.; Idzik, T.J.; Sośnicki, J.G. Influence of Methacrylate and Vinyl Monomers on Radical Bulk Photopolymerization Process and Properties of Epoxy-Acrylate Structural Adhesives. Polymers 2023, 15, 926. https://doi.org/10.3390/polym15040926
Gziut K, Kowalczyk A, Schmidt B, Idzik TJ, Sośnicki JG. Influence of Methacrylate and Vinyl Monomers on Radical Bulk Photopolymerization Process and Properties of Epoxy-Acrylate Structural Adhesives. Polymers. 2023; 15(4):926. https://doi.org/10.3390/polym15040926
Chicago/Turabian StyleGziut, Konrad, Agnieszka Kowalczyk, Beata Schmidt, Tomasz J. Idzik, and Jacek G. Sośnicki. 2023. "Influence of Methacrylate and Vinyl Monomers on Radical Bulk Photopolymerization Process and Properties of Epoxy-Acrylate Structural Adhesives" Polymers 15, no. 4: 926. https://doi.org/10.3390/polym15040926
APA StyleGziut, K., Kowalczyk, A., Schmidt, B., Idzik, T. J., & Sośnicki, J. G. (2023). Influence of Methacrylate and Vinyl Monomers on Radical Bulk Photopolymerization Process and Properties of Epoxy-Acrylate Structural Adhesives. Polymers, 15(4), 926. https://doi.org/10.3390/polym15040926