Magnetodielectric Properties of Ordered Microstructured Polydimethylsiloxane-Based Magnetorheological Elastomer with Fe3O4@rGO Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Fe3O4@rGO Electromagnetic Biphasic Composite Particles
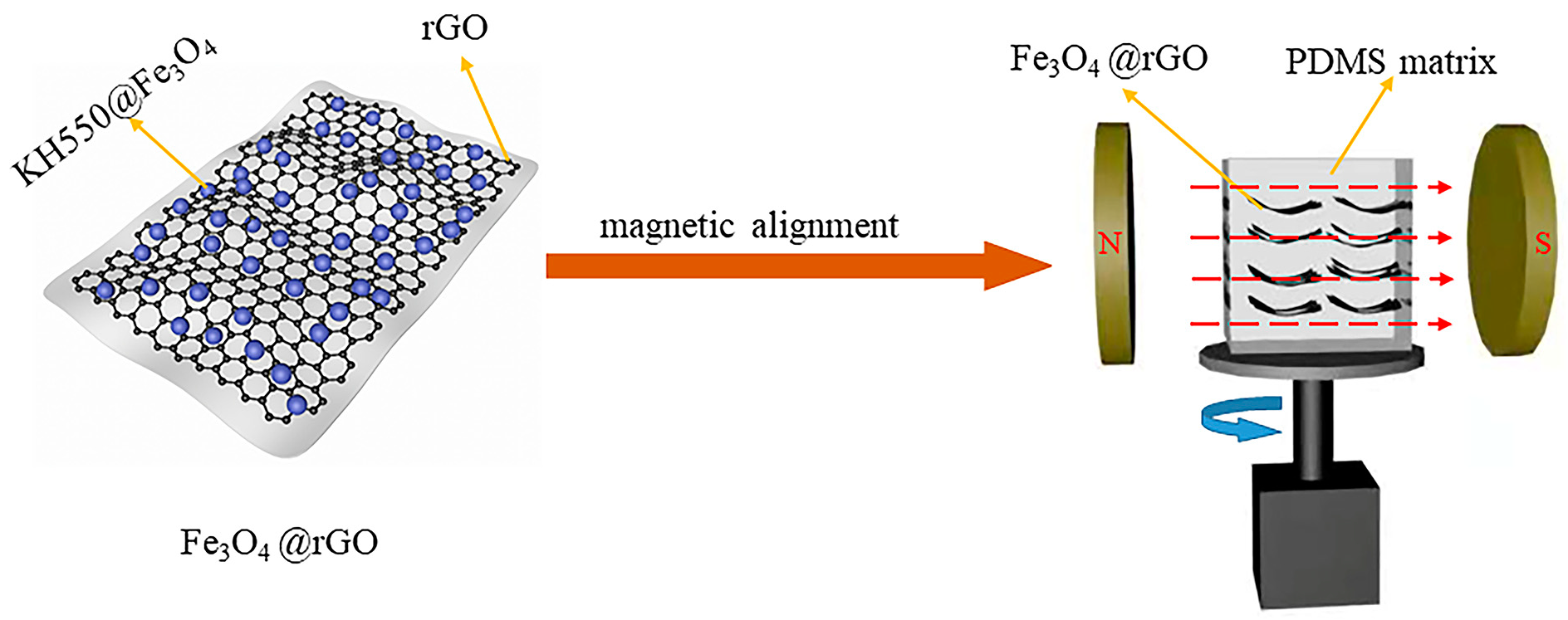
2.3. Fabrication of Highly Ordered Fe3O4@rGO/PDMS-MRE
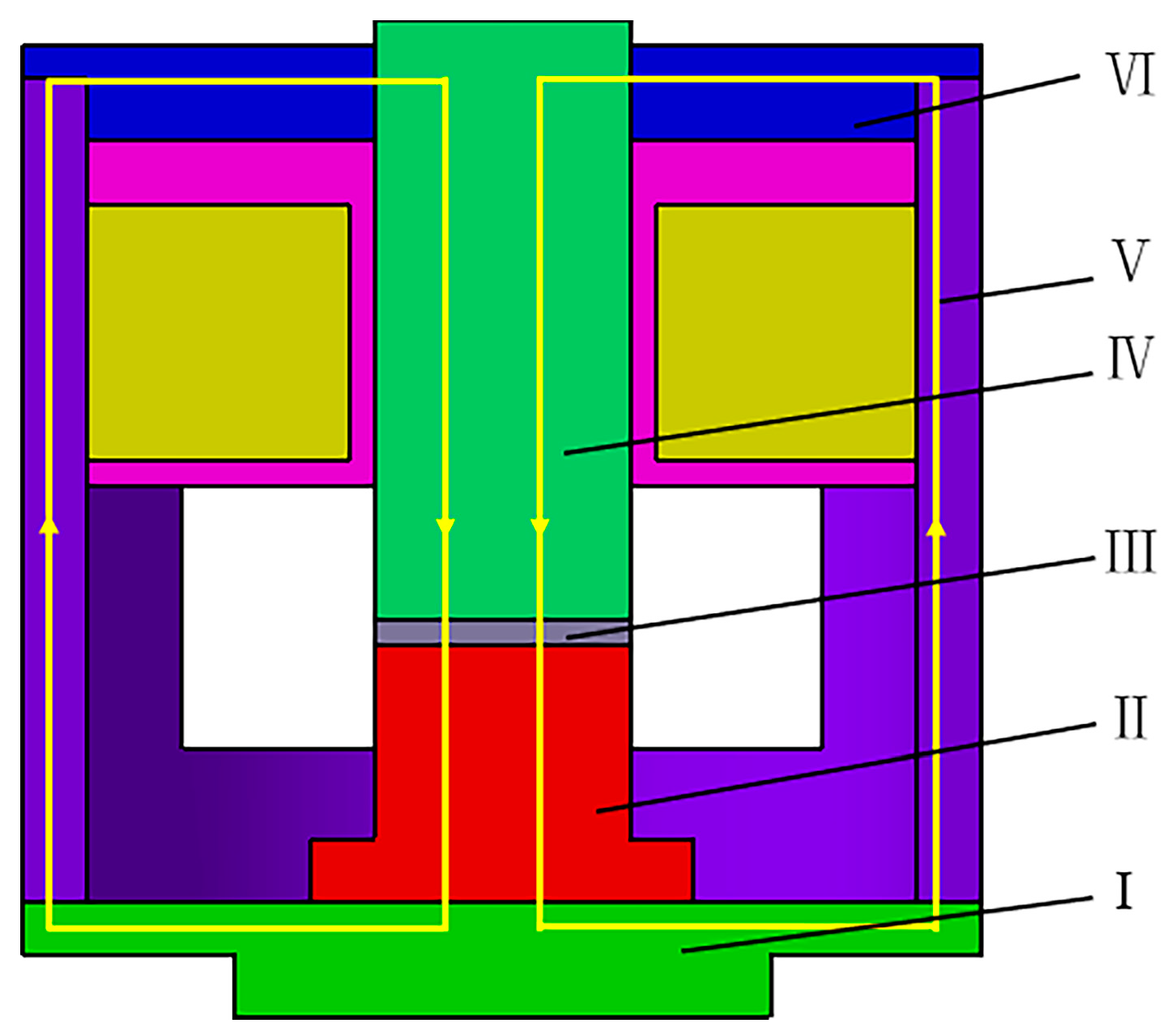
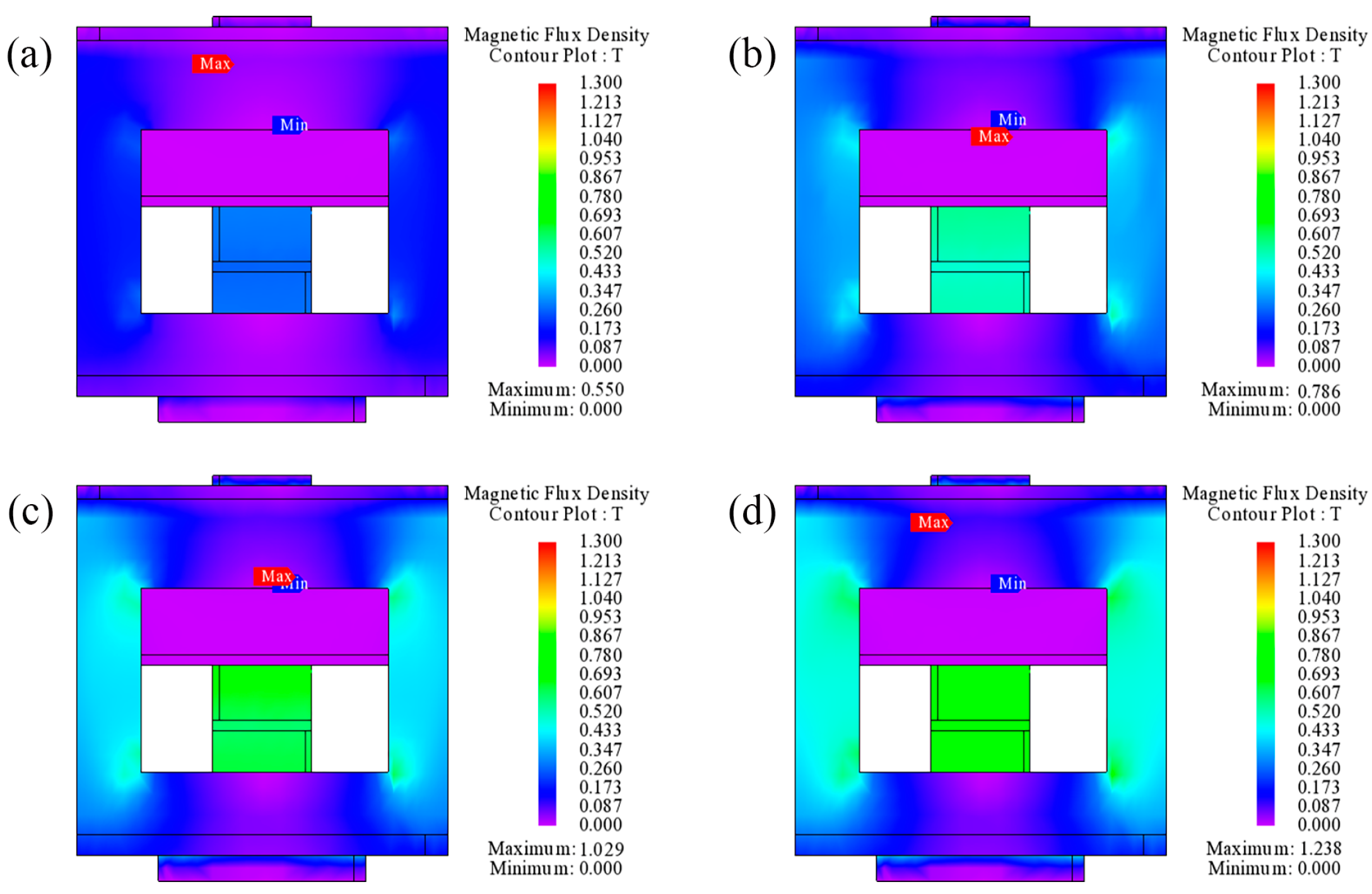
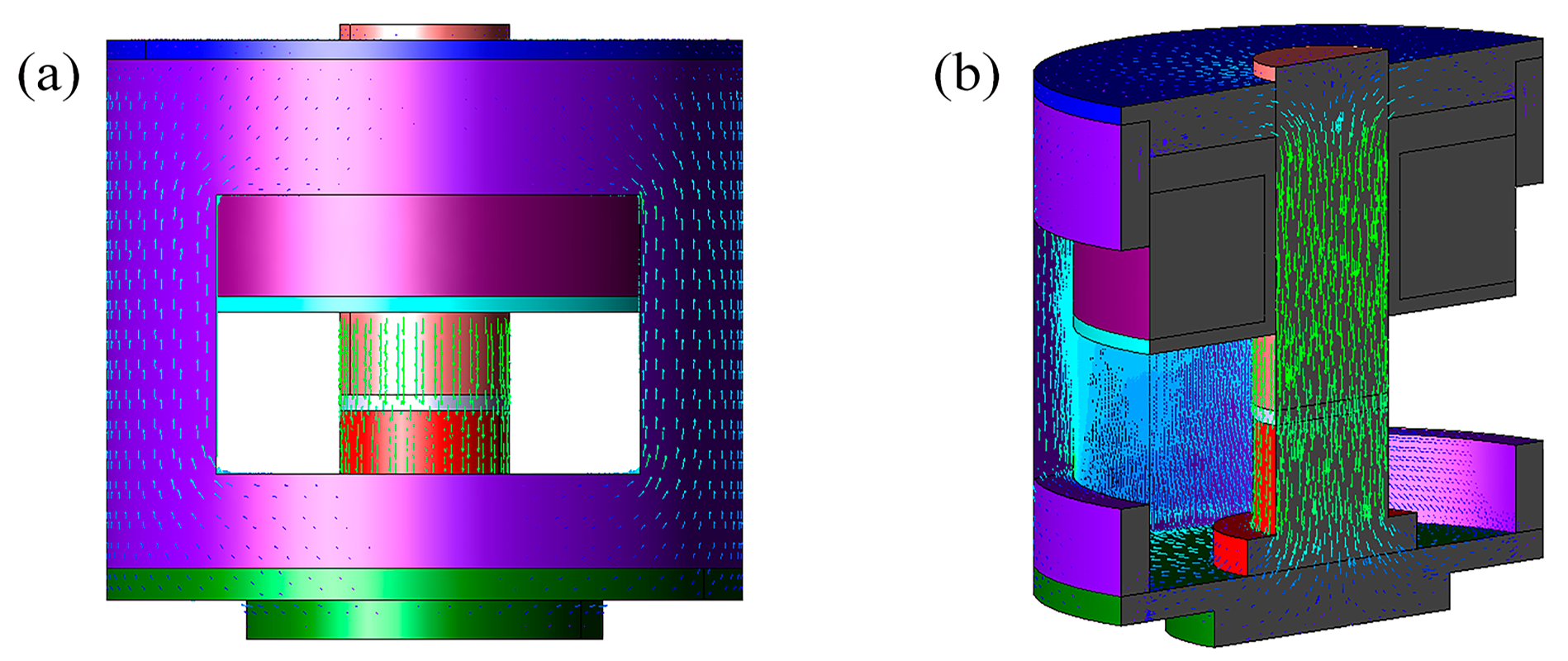
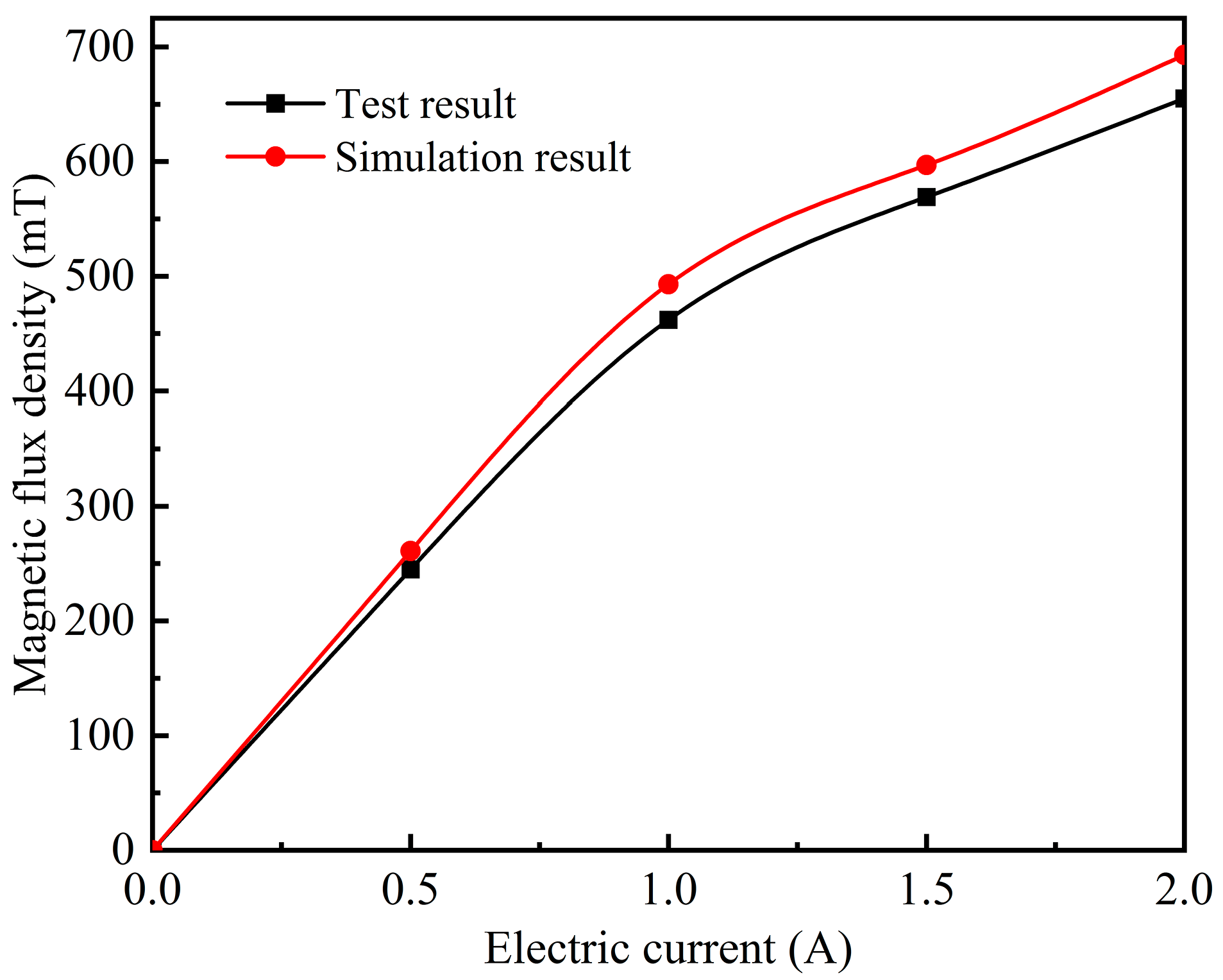
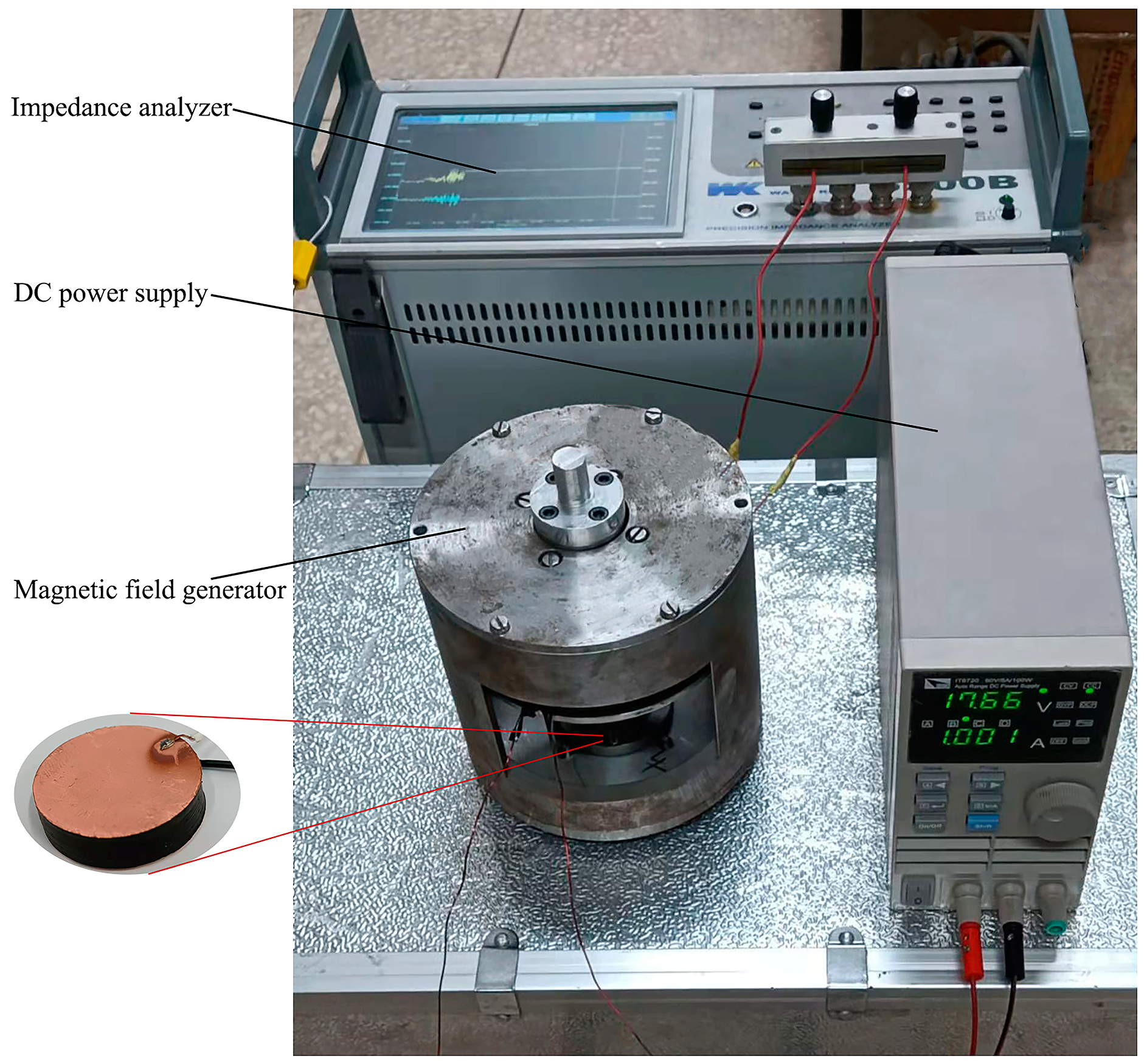
2.4. Design of Magnetic Field Generator
2.5. Characterization and Property Testing
3. Results and Discussion
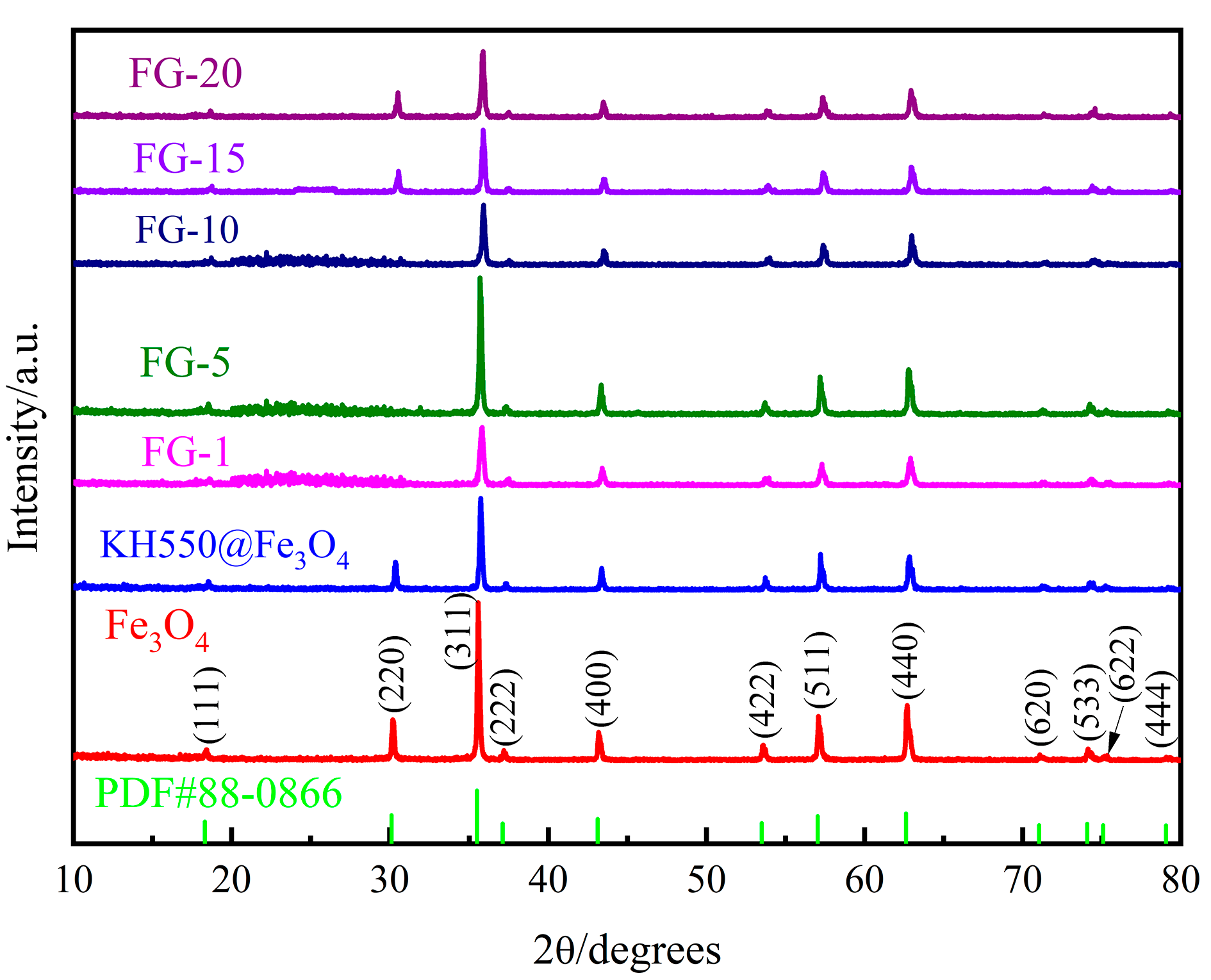
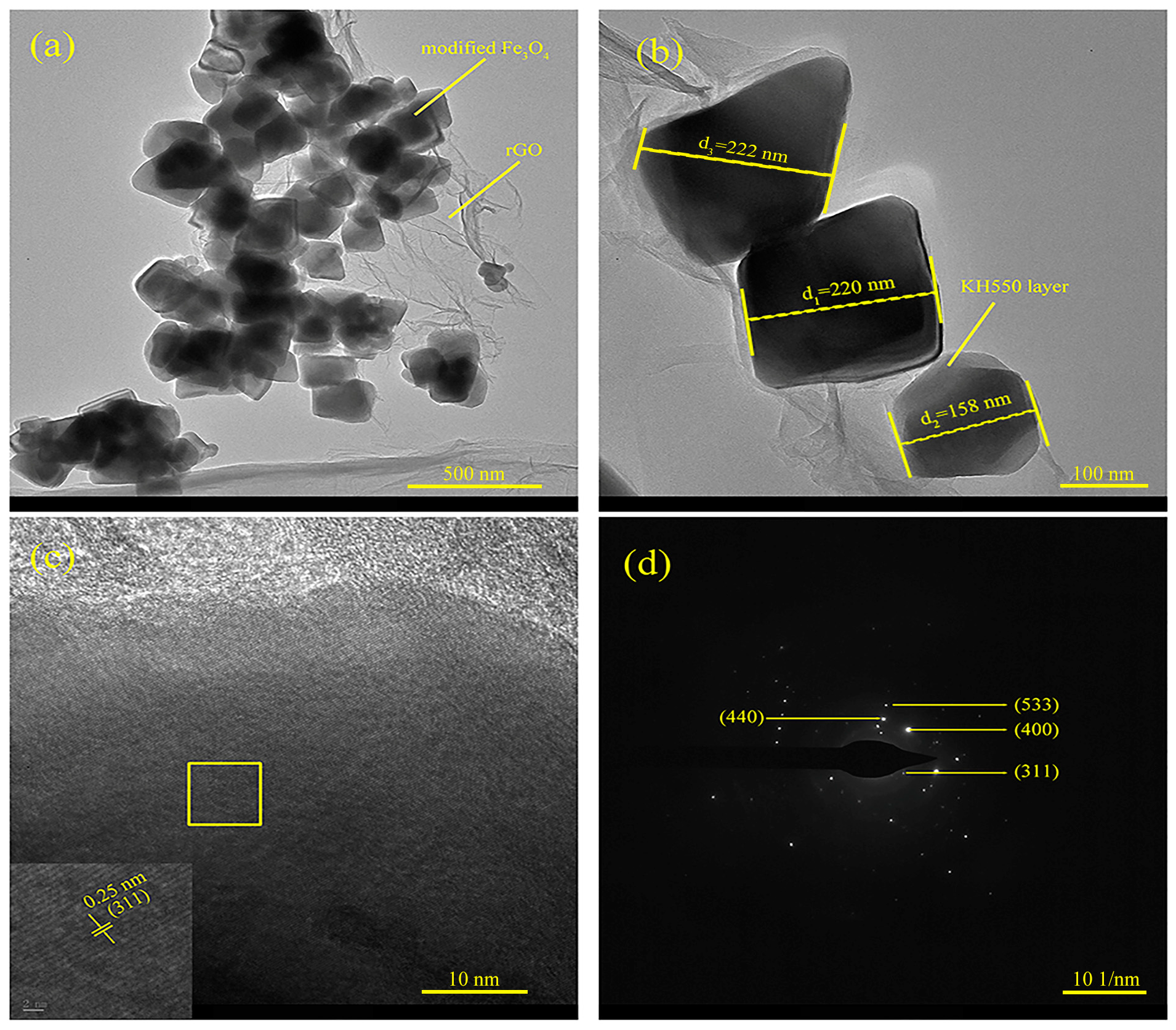
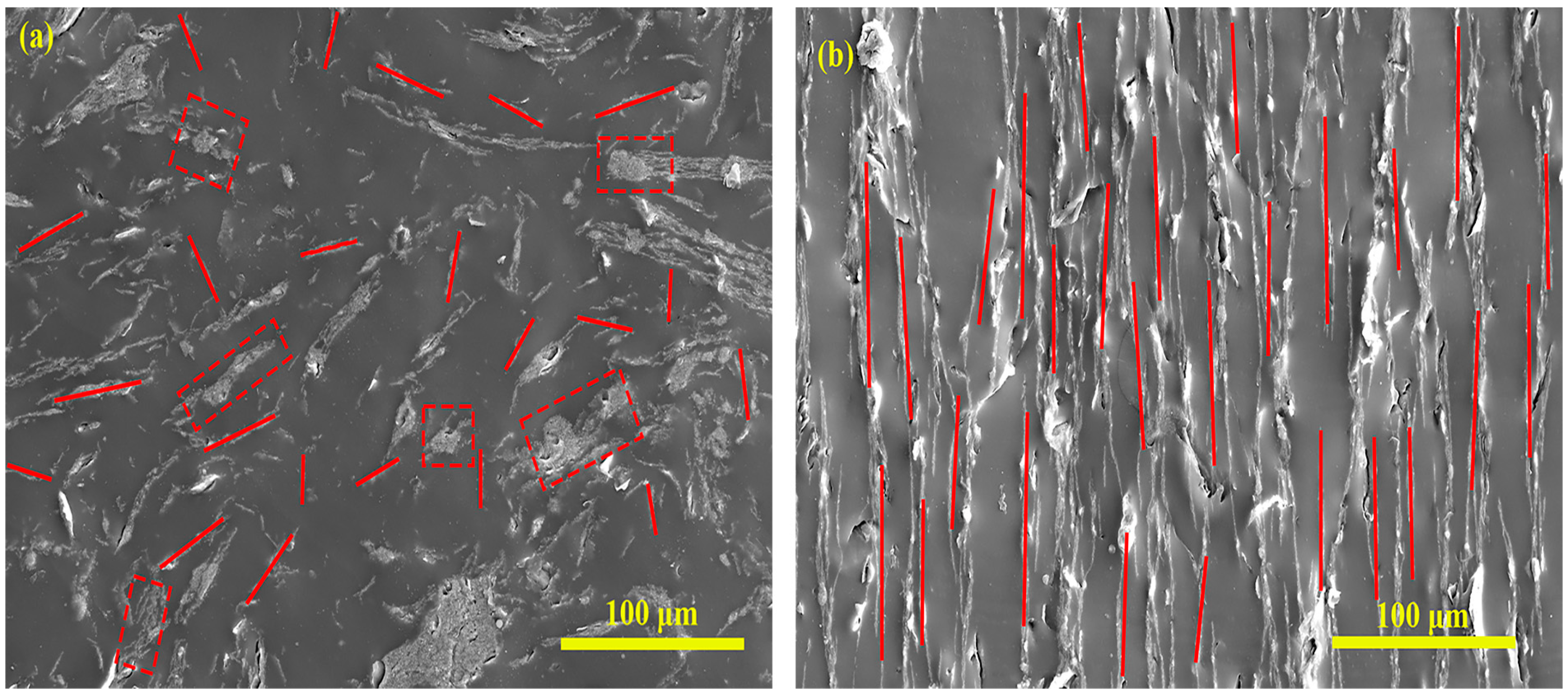
3.1. General Characterization
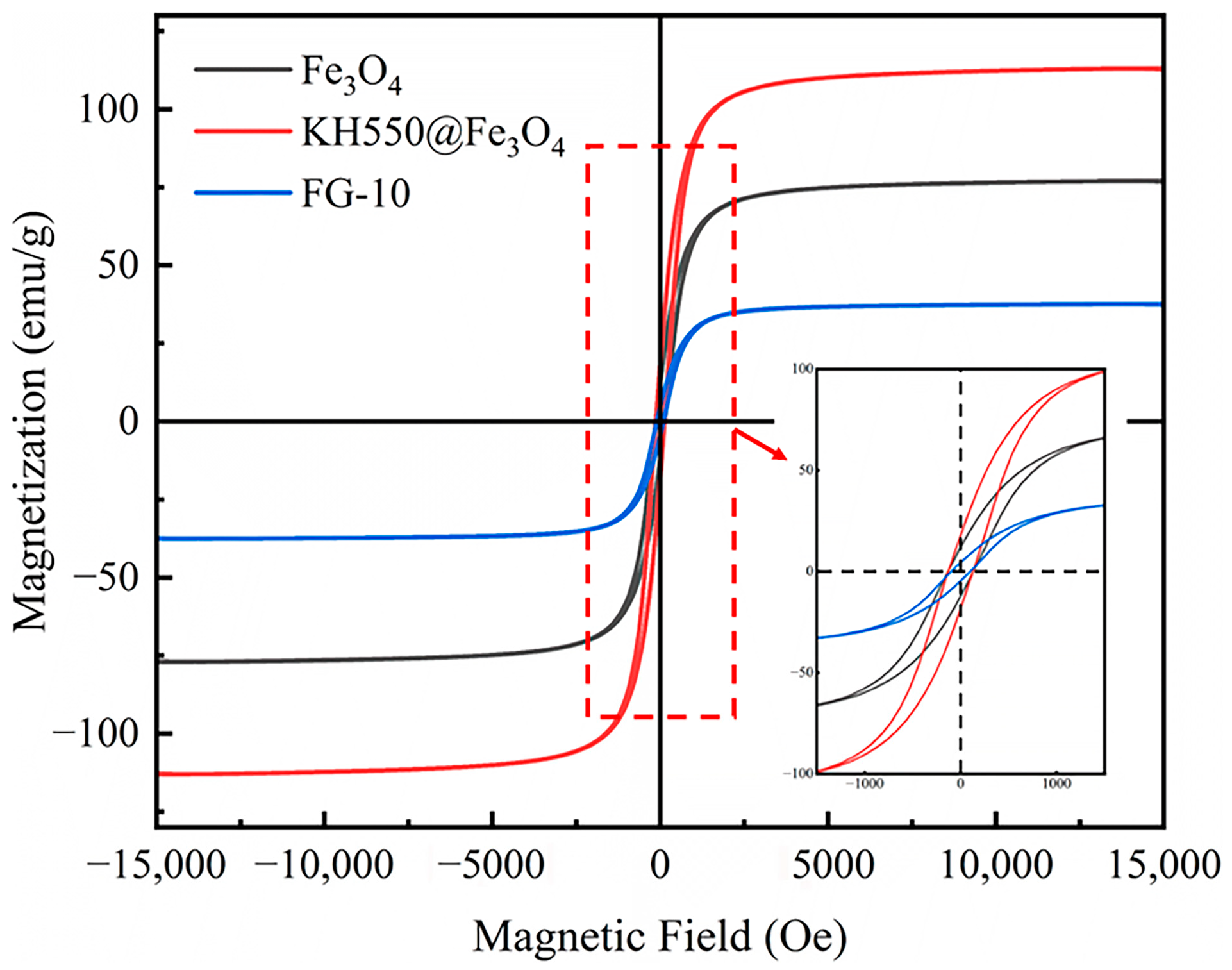
3.2. Magnetic Properties
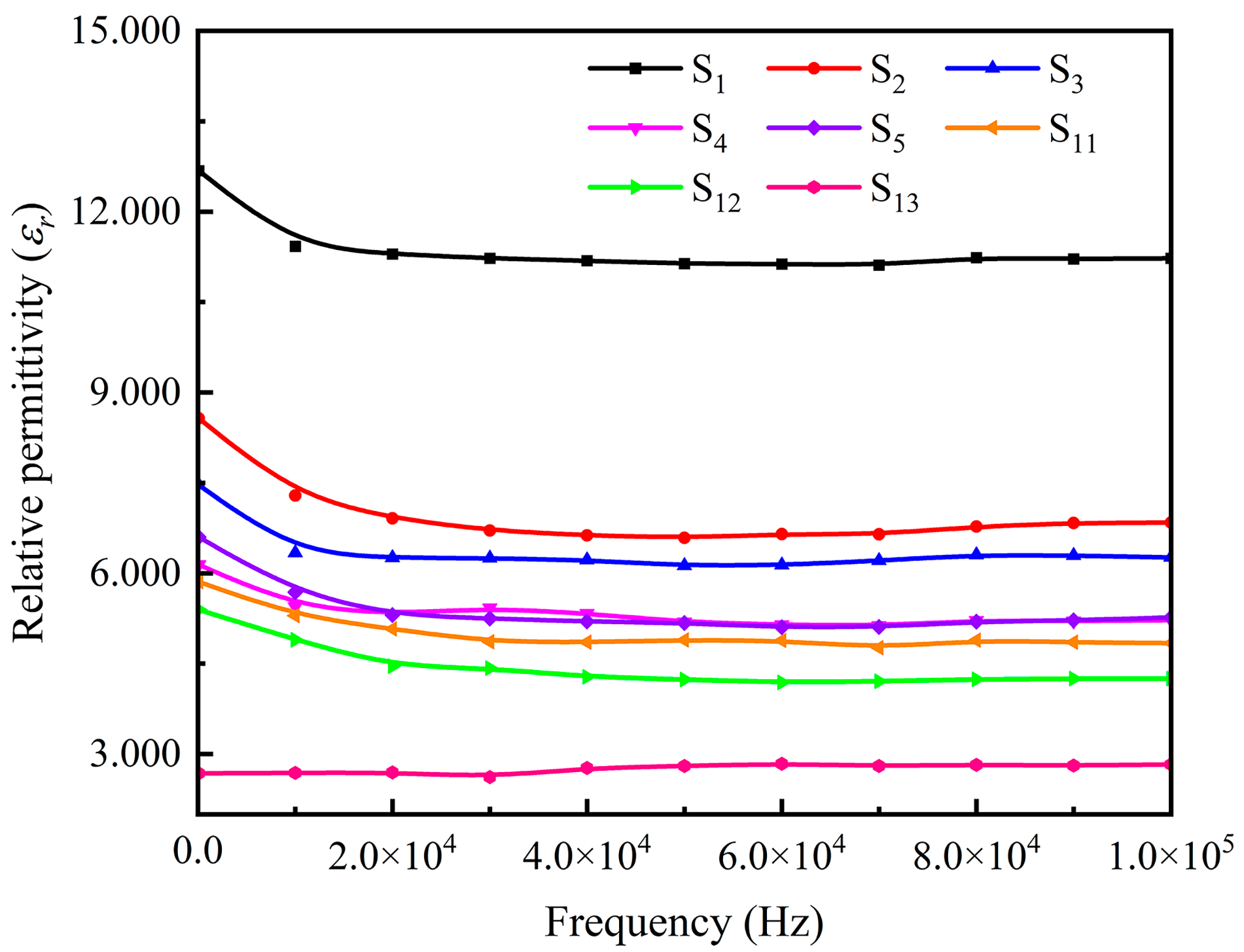
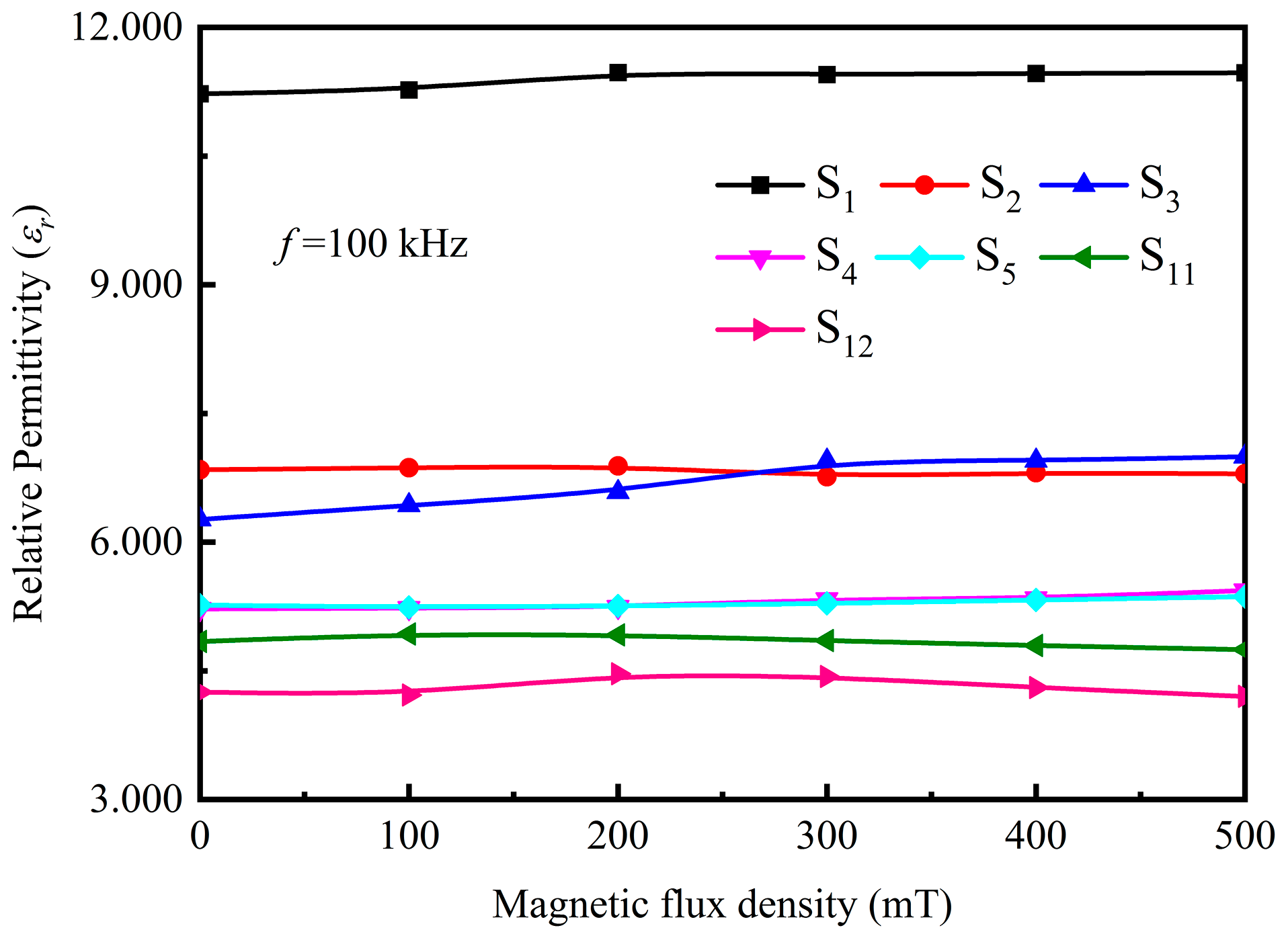
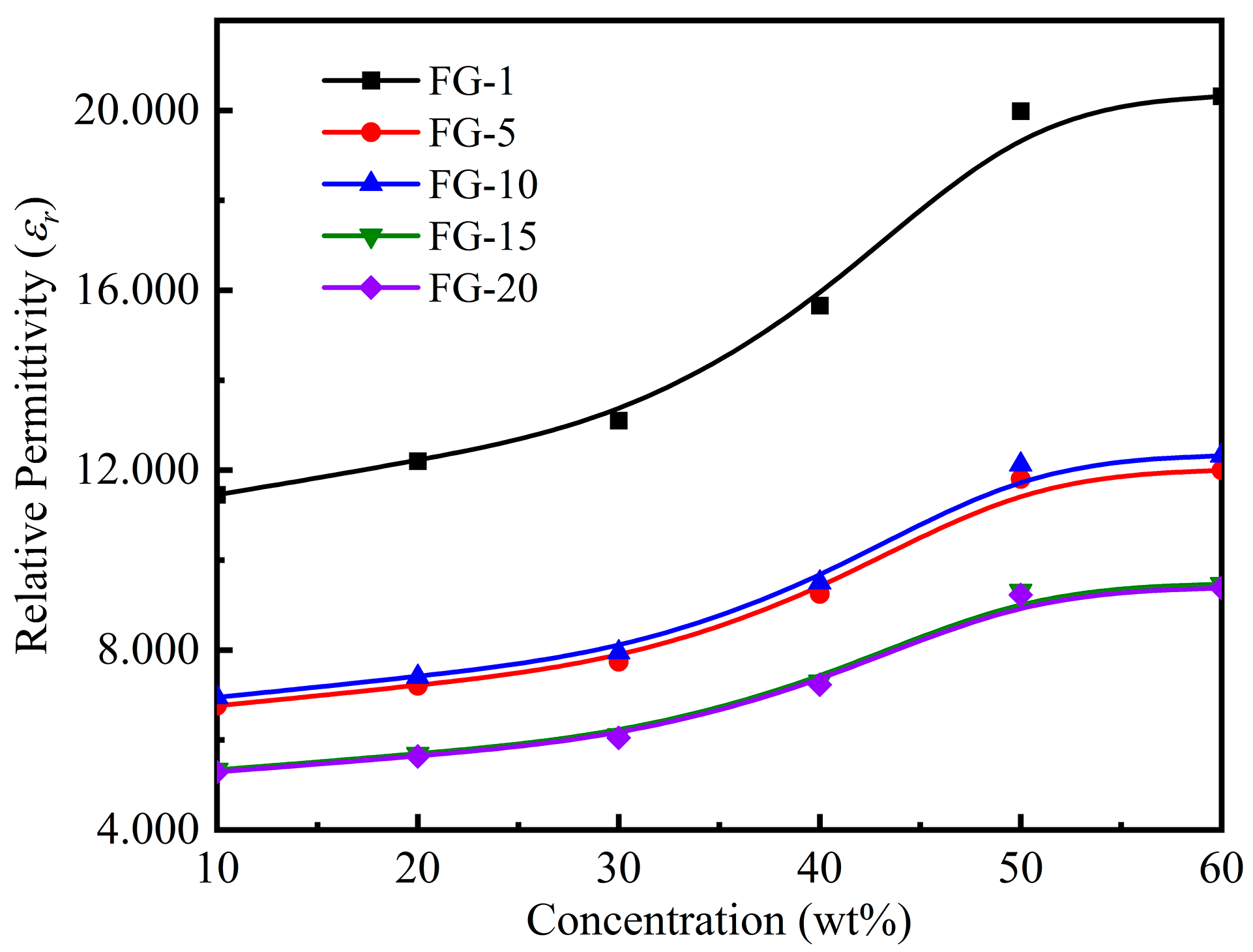
3.3. Magnetodielectric Properties of Ordered Microstructured Fe3O4@rGO/PDMS-MRE
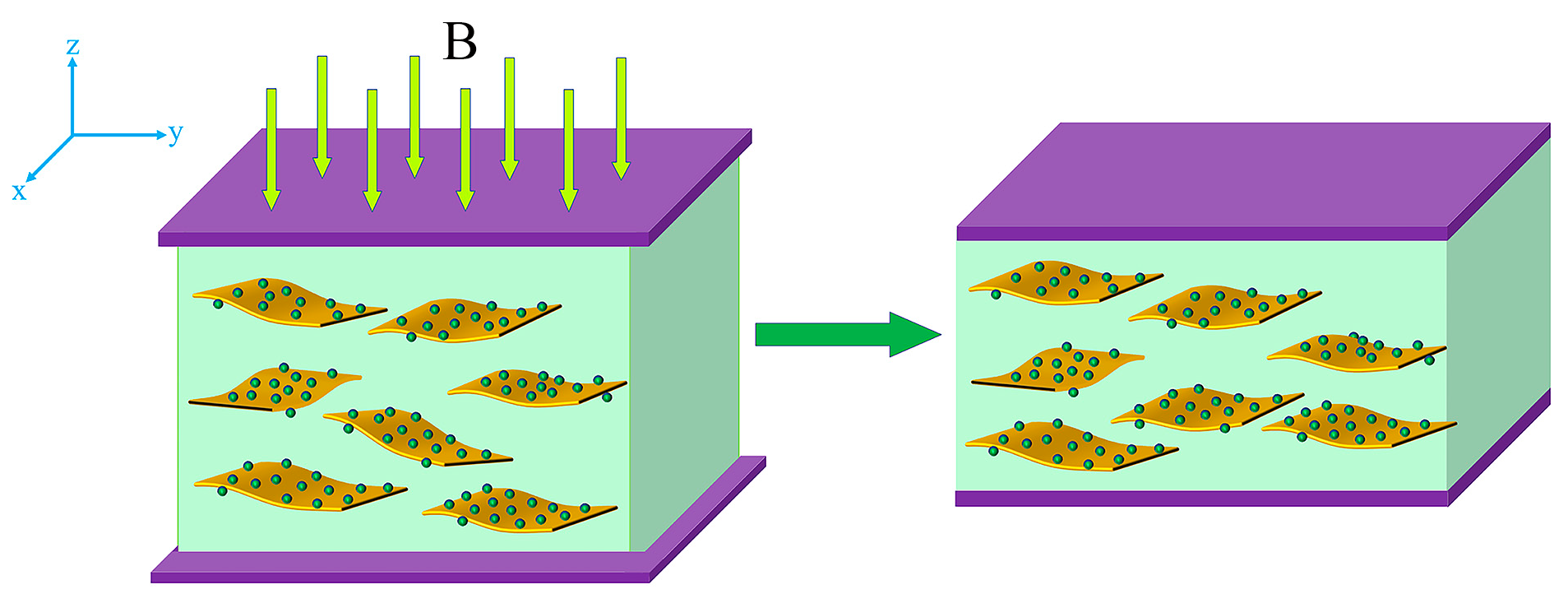
3.4. Mechanistic Analysis of Magnetodielectric Effect of Ordered Microstructured Fe3O4@rGO/PDMS-MRE
4. Conclusions
- (1)
- Chemical structure and microscopic morphological analysis indicate the successful synthesization of Fe3O4@rGO nanocomposite particles. It is shown that when the ratio of modified Fe3O4 to GO is 10:1, Fe3O4 particles are uniformly loaded on the surface of rGO, and the composite particles have a flaky structure. Moreover, Fe3O4@rGO composite particles are superparamagnetic at room temperature with a saturation magnetization of 42.36 emu/g, which is beneficial to the preparation of MRE with ordered microstructure.
- (2)
- It is shown that the relative permittivity of Fe3O4@rGO/PDMS-MRE is sensibly influenced by the magnetic flux density and the filler concentration. In particular, when the magnetic flux density is 500 mT and the filler concentration is 60 wt%, the relative permittivity reaches 12.934 and the magnetodielectric effect reaches 92.4%. It shows a strong magnetodielectric effect.
- (3)
- A mechanism that can describe the magnetodielectric effect of Fe3O4@rGO/PDMS-MRE is proposed based on the rearrangement degree and the microcapacitance principle of the composite particles. The obtained effect can be used to design new magnetic sensors for detecting environmental parameters.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.; Dong, X.F.; Qi, M.; Wu, Z.J. Influence of Chain-like Cobalt Particles on the Properties of Magnetorheological Elastomers. Smart Mater. Struct. 2022, 31, 035007. [Google Scholar] [CrossRef]
- Zhong, H.M.; Pei, Y.; Hu, Z.J.; Zhang, P.; Guo, J.; Gong, X.L.; Zhao, Y. A Study of the Heat Transfer Properties of CIP Doped Magnetorheological Elastomers. Smart Mater. Struct. 2019, 28, 025027. [Google Scholar] [CrossRef]
- Saharuddin, K.D.; Afiff, M.H.M.; Bahiuddin, I.; Ubaidillah, U.; Mazlan, S.A.; Aziz, S.A.A.; Nazmi, N.; Fatah, A.Y.A.; Shapiai, M.I. Non-parametric Multiple inputs Prediction Model for Magnetic Field Dependent Complex Modulus of Magnetorheological Elastomer. Sci. Rep. 2022, 12, 2657. [Google Scholar] [CrossRef]
- Huang, X.G.; Yan, Z.Y.; Liu, C.; Li, G.H.; Wang, J. Study on the Resistance Properties of Magnetorheological Elastomer. Mater. Res. Innovations 2015, 19, 924–928. [Google Scholar] [CrossRef]
- Vatandoost, H.; Sedaghati, R.; Rakheja, S. A Novel Methodology for Accurate Estimation of Magnetic Permeability of Magnetorheological Elastomers. J. Magn. Magn. Mater. 2022, 560, 169669. [Google Scholar] [CrossRef]
- Vatandoost, H.; Sedaghati, R.; Rakheja, S. Development of New Phenomenological Models for Predicting Magnetic Permeability of Isotropic and Anisotropic Magneto-rheological Elastomers. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Chen, G.B.; Yang, H.; Zhang, X.M.; Liu, J.; Tang, J. Clarification of the Magnetocapacitance Mechanism for Fe3O4-PDMS Nanocomposites. J. Nanomater. 2015, 2015, 1–6. [Google Scholar]
- Mosallaei, H.; Sarabandi, K. Magneto-Dielectrics in Electromagnetics: Concept and Applications. IEEE Trans. Antennas Propag. 2004, 52, 1558–1567. [Google Scholar] [CrossRef]
- Martins, P.; Silva, D.; Silva, M.P.; Lanceros-Mendez, S. Improved Magnetodielectric Coefficient on Polymer Based Composites Through Enhanced Indirect Magnetoelectric Coupling. Appl. Phys. Lett. 2016, 109, 112905. [Google Scholar] [CrossRef]
- Stepanov, G.V.; Bakhtiiarov, A.V.; Lobanov, D.A.; Borin, D.Y.; Semerenko, D.A.; Storozhenko, P.A. Magnetoresistive and Magnetocapacitive Effects in Magnetic Elastomers. SN Appl. Sci. 2022, 4, 178. [Google Scholar] [CrossRef]
- Moucka, R.; Sedlacik, M.; Cvek, M. Dielectric Properties of Magnetorheological Elastomers with Different Microstructure. Appl. Phys. Lett. 2018, 112, 122901. [Google Scholar] [CrossRef]
- Bica, I. Influence of Magnetic Field Upon the Electric Capacity of a Flat Capacitor having Magnetorheological Elastomer as a Dielectric. J. Ind. Eng. Chem. 2009, 15, 605–609. [Google Scholar] [CrossRef]
- Bica, I.; Liu, Y.D.; Choi, H.J. Magnetic Field Intensity Effect on Plane Electric Capacitor Characteristics and Viscoelasticity of Magnetorheological Elastomer. Colloid and Polym. Sci. 2012, 290, 1115–1122. [Google Scholar] [CrossRef]
- Bica, I.; Anitas, E.M.; Averis, L.M.E.; Bunoiu, M. Magnetodielectric Effects in Composite Materials Based on Paraffin, Carbonyl Iron and Graphene. J. Ind. Eng. Chem. 2015, 21, 1323–1327. [Google Scholar] [CrossRef]
- Balasoiu, M.; Bica, I. Composite Magnetorheological Elastomers as Dielectrics for Plane Capacitors: Effects of Magnetic Field Intensity. Results Phys. 2016, 6, 199–202. [Google Scholar] [CrossRef]
- Bica, I.; Anitas, E.M. Magnetodielectric Effects in Membranes Based on Magnetorheological Bio-Suspensions. Mater. Des. 2018, 155, 317–324. [Google Scholar] [CrossRef]
- Bica, I.; Bunoiu, O.M. Magnetorheological Hybrid Elastomers Based on Silicone Rubber and Magnetorheological Suspensions with Graphene Nanoparticles: Effects of the Magnetic Field on the Relative Dielectric Permittivity and Electric Conductivity. Int. J. Mol. Sci. 2019, 20, 4201. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovici, A.M.; Anitas, E.M.; Chirigiu, L.; Bica, I.; Negrutiu, M.L. Magnetodielectric Effects in Magnetorheological Elastomers Based on Polymer Fabric, Silicone Rubber, and Magnetorheological Suspension. Adv. Poly. Technol. 2019, 2019, 1983547. [Google Scholar] [CrossRef]
- Semisalova, A.S.; Perov, N.S.; Stepanov, G.V.; Kramarenko, E.Y.; Khokhlov, A.R. Strong Magnetodielectric Effects in Magnetorheological Elastomers. Soft Matter 2013, 9, 11318–11324. [Google Scholar] [CrossRef]
- Danil, I.; Anna, S.; Yulia, A.; Makarova, L.; Perov, N. Simulation of Magnetodielectric Effect in Magnetorheological Elastomers. Int. J. Mol. Sci. 2019, 20, 1457. [Google Scholar]
- Wang, Y.; Xuan, S.H.; Ge, L.; Wen, Q.Q.; Gong, X.L. Conductive Magnetorheological Elastomer: Fatigue Dependent Impedance-Mechanic Coupling Properties. Smart Mater. Struct. 2017, 26, 015004. [Google Scholar] [CrossRef]
- Belyaeva, I.A.; Kramarenko, E.Y.; Shamonin, M. Magnetodielectric Effect in Magnetoactive Elastomers: Transient Response and Hysteresis. Polymer 2017, 127, 119–128. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, X.M.; Chen, G.B.; Liu, J.; Tang, J.; Wang, W.J. Study on Magnetocapacitance Effect of Magnetic Particle Polymer Matrix Composite System by Finite Element Method. Results Phys. 2017, 7, 2334–2340. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.; Yang, H.; Oh, J.H. Fabrication of Hierarchically Porous structured PDMS Composites and Their Application as a Flexible Capacitive Pressure Sensor. Compos. Part B 2021, 211, 108607. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, X.M.; Chen, G.B.; Liu, J.; Tang, J.; Wang, W.J. Effect of Polymer Matrix Elasticity on the Magnetocapacitance Characteristics for Fe3O4/PDMS Nanocomposites: FEM Modelling and Experiment. Mater. Res. Express 2018, 6, 025010. [Google Scholar] [CrossRef]
- Zhang, C.J.; Liu, S.G.; Zhao, D.; Yang, S.; Ou, Y.Q. Pressure Sensing Properties for the Dielectric Layer of Graphene/Silicone Rubber-based Magnetorheological Elastomer. Polym. Int. 2022, 71, 999–1008. [Google Scholar] [CrossRef]
- Bica, I.; Anitas, E.M. Magnetic Field Intensity and Graphene Concentration Effects on Electrical and Rheological Properties of MREs-based Membranes. Smart Mater. Struct. 2017, 26, 105038. [Google Scholar] [CrossRef]
- Bica, I.; Anitas, E.M.; Chirigiu, L. Magnetic Field Intensity Effect on Plane Capacitors Based on Hybrid Magnetorheological Elastomers with Graphene Nanoparticles. J. Ind. Eng. Chem. 2017, 56, 407–412. [Google Scholar] [CrossRef]
- Tsai, P.J.; Nayak, S.; Ghosh, S.; Puri, I.K. Influence of Particle Arrangement on the Permittivity of an Elastomeric Composite. AIP Adv. 2017, 7, 015003. [Google Scholar] [CrossRef]
- Cahyana, A.H.; Liandi, A.R.; Maghdalena, M.; Yunarti, R.T.; Wendari, T.P. Magnetically Separable Fe3O4/graphene Oxide Nanocomposite: An Efficient Heterogenous Catalyst for Spirooxindole Derivatives Synthesis. Ceram. Int. 2022, 48, 18316–18323. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, L.H.; Zhu, Z.; Ni, Y.N. Functionalization of Fe3O4/rGO Magnetic Nanoparticles with Resveratrol and in Vitro DNA Interaction. Spectrochim. Acta Part A. 2022, 273, 121032. [Google Scholar] [CrossRef] [PubMed]
- Karimipour, M.; Dargahzadeh, M.; Molaei, M. Near Room Temperature Synthesis of rGO-Fe3O4 Nanosheets: Structural, Magnetic Ordering, Charge Transport, Dye Degradation Properties and Hydrogen Eevolution Reaction. Phys. E. 2020, 118, 113909. [Google Scholar] [CrossRef]
- Wei, B.G.; Chang, Q.; Bao, C.X.; Dai, L.; Zhang, G.Z.; Wu, F.P. Surface Modification of Filter Medium Particles with Silane Coupling Agent KH550. Colloids Surf. A 2013, 434, 276–280. [Google Scholar] [CrossRef]
- Xuan, S.H.; Hao, L.Y.; Jiang, W.Q.; Song, L.; Hu, Y.; Chen, Z.Y.; Fei, L.F.; Li, T.W. A FeCO3 Precursor-based Route to Microsized Peanutlike Fe3O4. Cryst. Growth Des. 2007, 7, 430–434. [Google Scholar] [CrossRef]
- Guan, X.C.; Dong, X.F.; Ou, J.P. Magnetostrictive Effect of Magnetorheological Elastomer. J. Magn. Magn. Mater. 2008, 320, 158–163. [Google Scholar] [CrossRef]
- Wendari, T.P.; Arief, S.; Mufti, N.; Blake, G.R.; Baas, J.; Suendo, V.; Prasetyo, A.; Insani, A.; Zulhadjri, Z. Lead-Free Aurivillius Phase Bi2LaNb1.5Mn0.5O9: Structure, Ferroelectric, Magnetic, and Magnetodielectric Effects. Inorg. Chem. 2022, 61, 8644–8652. [Google Scholar] [CrossRef] [PubMed]




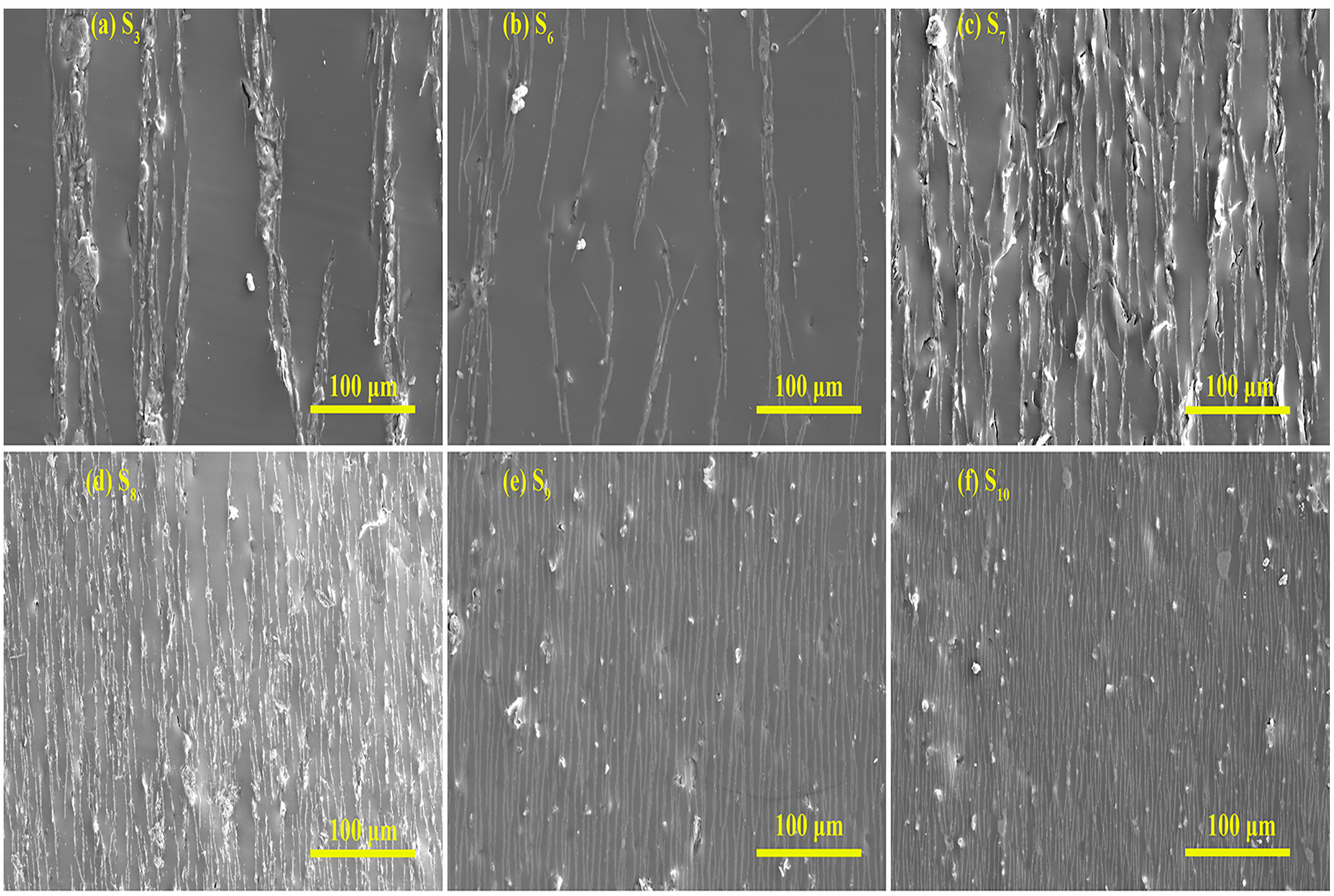


| Sample | Type of Particles | Weight Fraction (wt%) |
|---|---|---|
| S1 | FG-1 | 10% |
| S2 | FG-5 | 10% |
| S3 | FG-10 | 10% |
| S4 | FG-15 | 10% |
| S5 | FG-20 | 10% |
| S6 | FG-10 | 20% |
| S7 | FG-10 | 30% |
| S8 | FG-10 | 40% |
| S9 | FG-10 | 50% |
| S10 | FG-10 | 60% |
| S11 | FG-10 (random) | 10% |
| S12 | Fe3O4 | 10% |
| S13 | —— | —— |
| Parameter | Symbol | Value (mm) |
|---|---|---|
| Diameter of upper and lower end covers | D1 | 130 |
| Large diameter of coil frame | D2 | 110 |
| Small diameter of coil frame | D3 | 46 |
| Diameter of disk | D4 | 40 |
| Diameter of movable electromagnet core | D5 | 40 |
| Length of coil frame | L1 | 54 |
| Thickness of MRE | t1 | 2 |
| Length of shell | L2 | 130 |
| Thickness of shell | t2 | 10 |
| Length of disk | L3 | 60 |
| Length of movable electromagnet core | L4 | 94 |
| Parameter | Symbol | Value |
|---|---|---|
| Relative permeability of S10C steel | 1500 | |
| Total magnetomotive force | 2000 A | |
| Maximum input current | 2 A | |
| The coil number of turns | 1700 | |
| Nominal diameter of copper wire | 0.8 mm | |
| Resistance of copper wire | 27 Ω |
| Sample | |||
|---|---|---|---|
| Fe3O4 | 87.24 | 11.99 | 0.68 |
| KH550@Fe3O4 | 127.52 | 18.12 | 1.33 |
| FG-10 | 42.36 | 4.28 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, S.; Wei, F.; Dong, L.; Zhao, D.; Ou, Y. Magnetodielectric Properties of Ordered Microstructured Polydimethylsiloxane-Based Magnetorheological Elastomer with Fe3O4@rGO Nanoparticles. Polymers 2023, 15, 941. https://doi.org/10.3390/polym15040941
Zhang C, Liu S, Wei F, Dong L, Zhao D, Ou Y. Magnetodielectric Properties of Ordered Microstructured Polydimethylsiloxane-Based Magnetorheological Elastomer with Fe3O4@rGO Nanoparticles. Polymers. 2023; 15(4):941. https://doi.org/10.3390/polym15040941
Chicago/Turabian StyleZhang, Chunjie, Shaogang Liu, Fengmei Wei, Liqiang Dong, Dan Zhao, and Yuqing Ou. 2023. "Magnetodielectric Properties of Ordered Microstructured Polydimethylsiloxane-Based Magnetorheological Elastomer with Fe3O4@rGO Nanoparticles" Polymers 15, no. 4: 941. https://doi.org/10.3390/polym15040941





