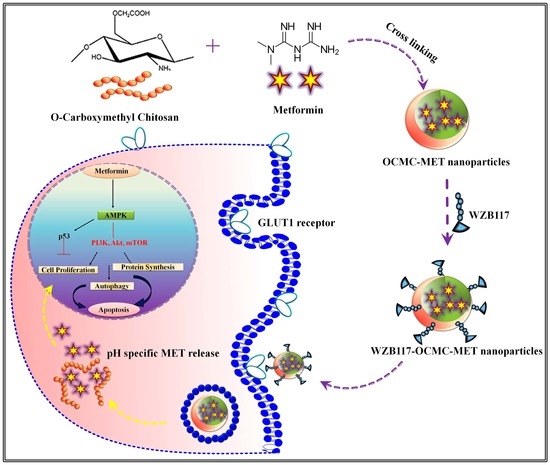
WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism
Abstract
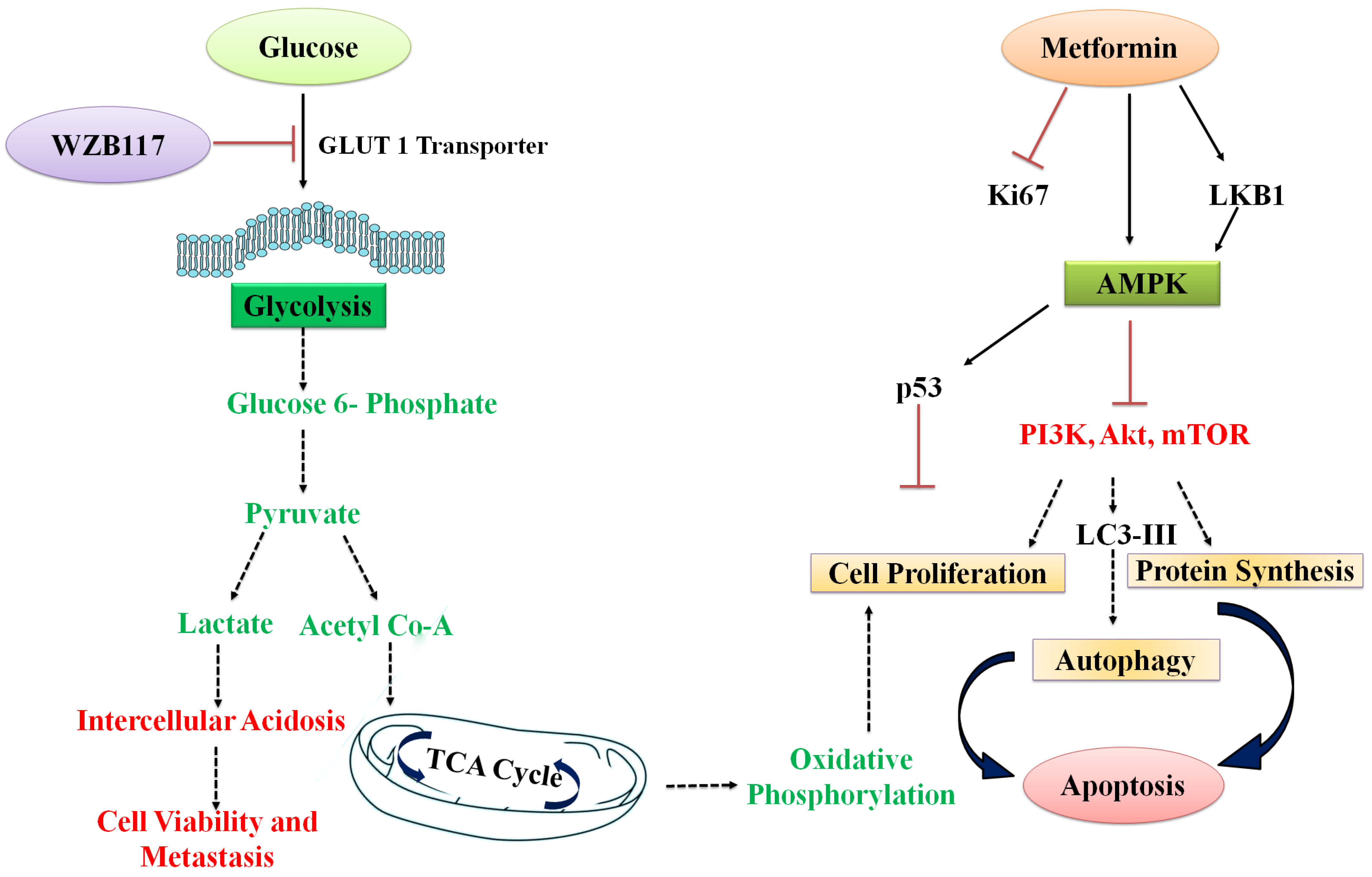
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
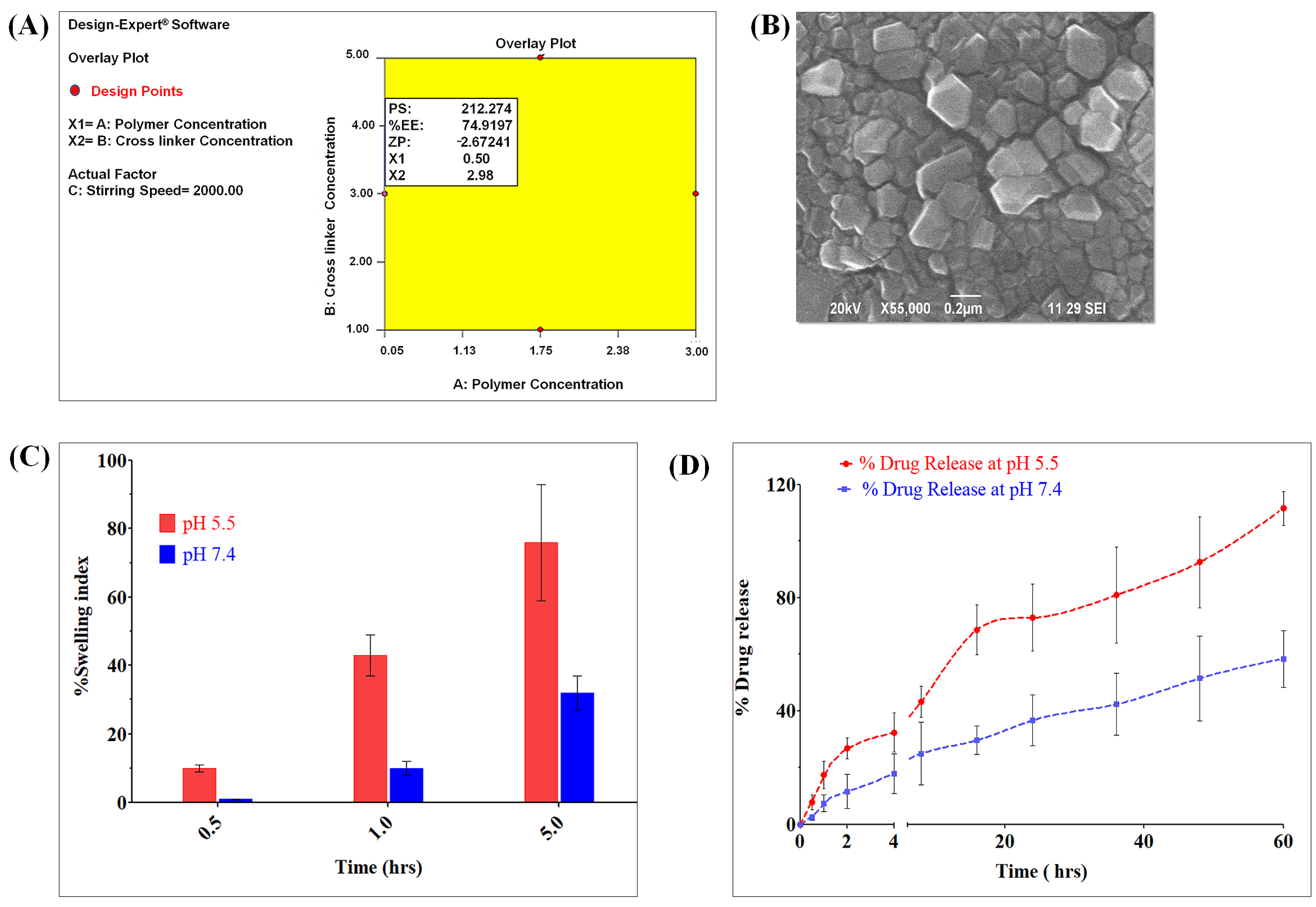
2.2.1. Formulation and Evaluation of OCMC-MET Nanoparticles
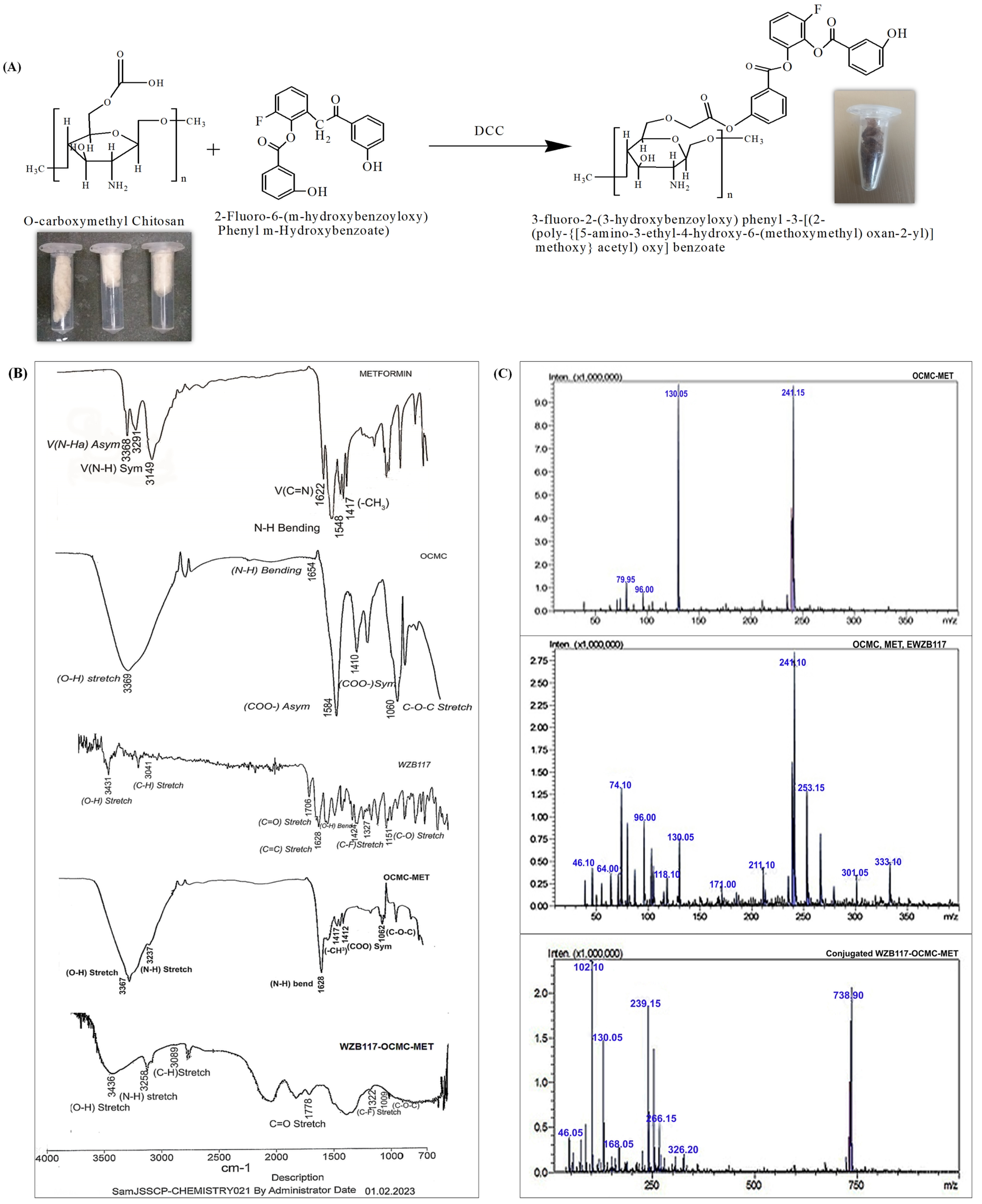
2.2.2. Synthesis and Characterization of WZB117-OCMC-MET
2.3. In Vitro Cytotoxicity Study to Evaluate the Efficacy of Conjugate Nanoparticles
2.4. Therapeutic Efficacy of WZB117-MET Combination by Compusyn® Software
2.5. Colony Formation Assay to Evaluate the Long Term Efficacy of Conjugate Nanoparticles
2.6. Cell Morphology Alteration Assay by AO/EB Staining to Evaluate the Efficacy of Conjugate Nanoparticles
2.7. DNA Fragmentation Analysis to Evaluate the Efficacy of Conjugate Nanoparticles
2.8. Apoptosis Assay to Evaluate the Efficacy of Conjugate Nanoparticles by Flow Cytometry
2.9. mTOR and BCL2 Downregulation Assay by Western Blotting to Evaluate the Efficacy of Conjugate Nanoparticles
2.10. Cellular Uptake Assay by Confocal Microscopy to Evaluate the Efficacy of Conjugate Nanoparticles
2.11. Cell Cycle Assay to Evaluate the Efficacy of Conjugate Nanoparticles
3. Results and Discussion
3.1. Formulation and Evaluation of OCMC-MET Nanoparticles
Synthesis and Characterization of WZB117-OCMC-MET
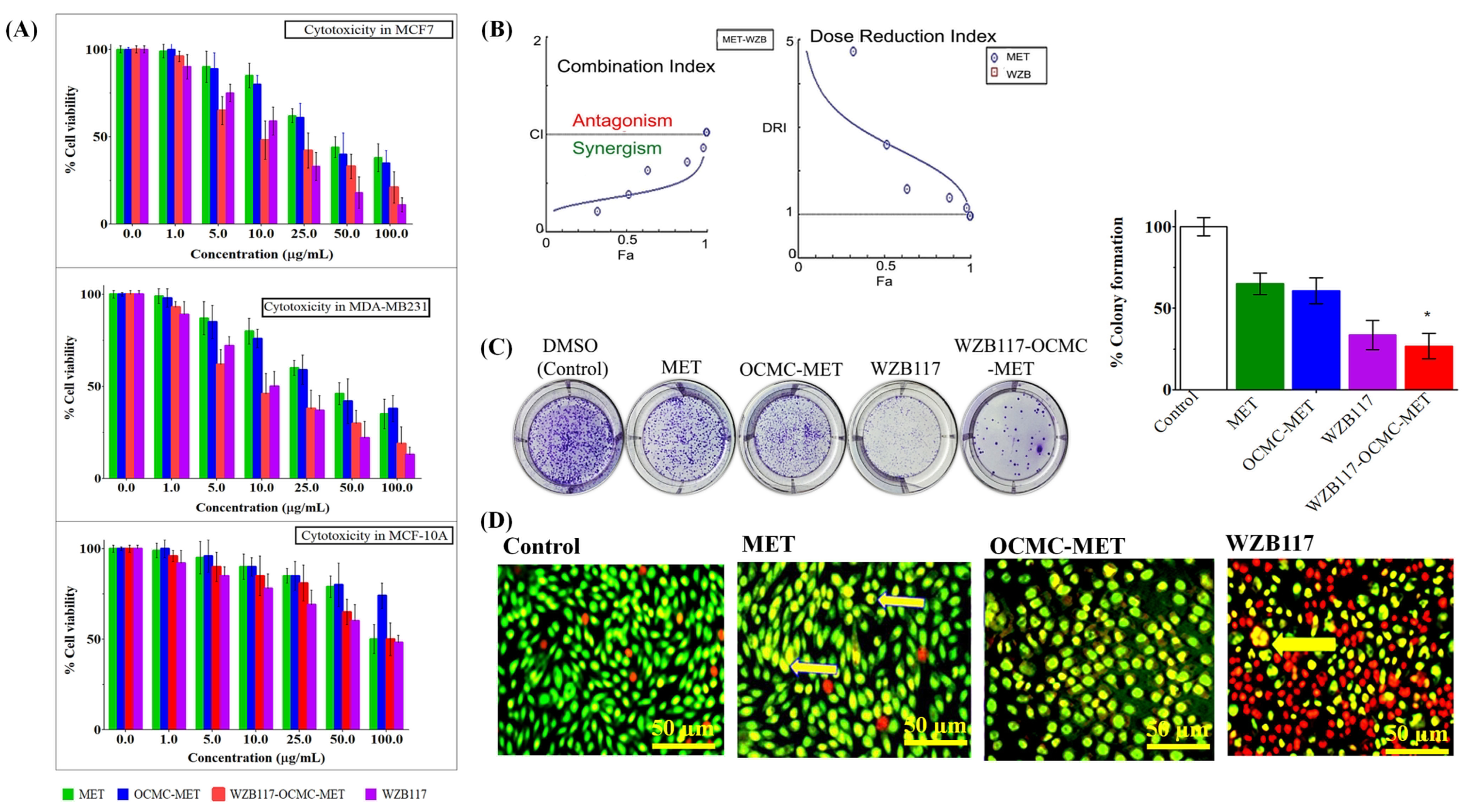
3.2. In Vitro Cytotoxicity Study to Evaluate the Efficacy of Conjugate Nanoparticles
3.3. Therapeutic Efficacy of WZB117-MET Combination by Compusyn® Software
3.4. Colony Formation Assay to Evaluate the Long Term Efficacy of Conjugate Nanoparticles
3.5. Cell Morphology Alteration Assay by AO/EB Staining to Evaluate the Efficacy of Conjugate Nanoparticles
3.6. DNA Fragmentation Analysis to Evaluate the Efficacy of Conjugate Nanoparticles
3.7. Apoptosis Assay to Evaluate the Efficacy of Conjugate Nanoparticles by Flow Cytometry
3.8. mTOR and BCL2 Downregulation Assay by Western Blotting to Evaluate the Efficacy of Conjugate Nanoparticles
3.9. Cellular Uptake Assay by Confocal Microscopy to Evaluate the Efficacy of Conjugate Nanoparticles
3.10. Cell Cycle Assay to Evaluate the Efficacy of Conjugate Nanoparticles
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A. Breast cancer statistics: Recent trends. Breast Cancer Metastasis Drug Resist; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–7. [Google Scholar]
- You, J.S.; Jones, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer cell. 2012, 22, 9–20. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Kuppusamy, G.; Karri, V.V.S.R. Affibody molecules for molecular imaging and targeted drug delivery in the management of breast cancer. Int. J. Biol. Macromol. 2018, 107, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Harris, A.L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012, 491, 364. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1. [Google Scholar]
- Jadhav, S.; Avila, J.; Schöll, M.; Kovacs, G.G.; Kövari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; de Silva, R. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Hao, Q.; Vadgama, J.V.; Wu, Y. Amp-activated protein kinase: A potential therapeutic target for triple-negative breast cancer. Breast Cancer Res. 2019, 21, 29. [Google Scholar] [CrossRef]
- De, A.; Kuppuswamy, G.; Jaiswal, A. Implementation of two different experimental designs for screening and optimization of process parameters for metformin-loaded carboxymethyl chitosan formulation. Drug Dev. Ind. Pharm. 2019, 45, 1821–1834. [Google Scholar] [CrossRef]
- De, A.; Kuppusamy, G. Metformin in breast cancer: Preclinical and clinical evidence. Curr. Probl. Cancer. 2020, 44, 100488. [Google Scholar] [CrossRef]
- Dowling, R.J.; Niraula, S.; Chang, M.C.; Done, S.J.; Ennis, M.; McCready, D.R.; Leong, W.L.; Escallon, J.M.; Reedijk, M.; Goodwin, P.J. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015, 17, 32. [Google Scholar] [CrossRef]
- Zakikhani, M.; Dowling, R.; Fantus, I.G.; Sonenberg, N.; Pollak, M. Metformin is an AMP kinase–Dependent growth inhibitor for breast cancer cells. Cancer Res. 2006, 66, 10269–10273. [Google Scholar] [CrossRef]
- Javidfar, S.; Pilehvar-Soltanahmadi, Y.; Farajzadeh, R.; Lotfi-Attari, J.; Shafiei-Irannejad, V.; Hashemi, M.; Zarghami, N. The inhibitory effects of nano-encapsulated metformin on growth and hTERT expression in breast cancer cells. J. Drug Deliv. Sci. Technol. 2018, 43, 19–26. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, Y.-S.; Wang, L.-C.; Huang, J.-B. Advances in metformin-based metabolic therapy for non-small cell lung cancer. Oncol. Rep. 2022, 47, 55. [Google Scholar] [CrossRef]
- Hung, H.-C.; Li, L.-C.; Guh, J.-H.; Kung, F.-L.; Hsu, L.-C. Discovery of New Glucose Uptake Inhibitors as Potential Anticancer Agents by Non-Radioactive Cell-Based Assays. Molecules 2022, 27, 8106. [Google Scholar] [CrossRef]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta-Rev. Cancer 2013, 1835, 164–169. [Google Scholar] [CrossRef]
- Gonzalez-Menendez, P.; Hevia, D.; Alonso-Arias, R.; Alvarez-Artime, A.; Rodriguez-Garcia, A.; Kinet, S.; Gonzalez-Pola, I.; Taylor, N.; Mayo, J.C.; Sainz, R.M. GLUT1 protects prostate cancer cells from glucose deprivation-induced oxidative stress. Redox Biol. 2018, 17, 112–127. [Google Scholar] [CrossRef]
- Qian, Y. Internalization of Extracellular ATP in Cancer Cells and Development of New Generations of Anticancer Glucose Transport Inhibitors. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2014. [Google Scholar]
- Ta, H.T.; Dass, C.R.; Dunstan, D.E. Injectable chitosan hydrogels for localised cancer therapy. J. Control. Release 2008, 126, 205–216. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Cancer, chitosan nanoparticles and catalytic nucleic acids. J. Pharm. Pharmacol. 2009, 61, 3–12. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Wang, Y.; Tang, S.; Sun, X.; Feng, X.; Li, Y.; Bao, G.; Li, P.; Mao, X. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015, 6, 44579. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Maya, S.; Kumar, L.G.; Sarmento, B.; Rejinold, N.S.; Menon, D.; Nair, S.V.; Jayakumar, R. Cetuximab conjugated O-carboxymethyl chitosan nanoparticles for targeting EGFR overexpressing cancer cells. Carbohydr. Polym. 2013, 93, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Snima, K.; Jayakumar, R.; Lakshmanan, V.-K. In vitro and in vivo biological evaluation of O-carboxymethyl chitosan encapsulated metformin nanoparticles for pancreatic cancer therapy. Pharm. Res. 2014, 31, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Snima, K.; Jayakumar, R.; Unnikrishnan, A.; Nair, S.V.; Lakshmanan, V.-K. O-Carboxymethyl chitosan nanoparticles for metformin delivery to pancreatic cancer cells. Carbohydr. Polym. 2012, 89, 1003–1007. [Google Scholar] [CrossRef]
- Yakisich, J.S.; Azad, N.; Kaushik, V.; Iyer, A.K. The biguanides metformin and buformin in combination with 2-deoxy-glucose or WZB-117 inhibit the viability of highly resistant human lung cancer cells. Stem Cells Int. 2019, 2019, 6254269. [Google Scholar] [CrossRef]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 2015, 6, 25677. [Google Scholar] [CrossRef]
- Bordignon, C.; Canu, A.; Dyczko, A.; Leone, S.; Monti, P. T-cell metabolism as a target to control autoreactive T cells in β-cell autoimmunity. Curr Diab Rep. 2017, 17, 24. [Google Scholar] [CrossRef]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. In Seminars in Cell & Developmental Biology; Candiolo Cancer Institute: Turin, Italy, 2020; pp. 63–70. [Google Scholar]
- Sun, X.; Xue, Z.; Yasin, A.; He, Y.; Chai, Y.; Li, J.; Zhang, K. Colorectal cancer and adjacent normal mucosa differ in apoptotic and inflammatory protein expression. Eng. Regen. 2022, 2, 279–287. [Google Scholar] [CrossRef]
- De, A.; Ko, Y.T. Single pot organic solvent-free thermocycling technology for siRNA-ionizable LNPs: A proof-of-concept approach for alternative to microfluidics. Drug Deliv. 2022, 29, 2644–2657. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Chen, W.; Deng, X.; Zhao, J.; Huang, B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. AAPS PharmSciTech 2006, 7, E138–E143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Yang, J.; Wang, Y.; Chen, R.; Wu, J.; Liu, Y.; Zhang, N. Self-assembled nanoparticles of methotrexate conjugated O-carboxymethyl chitosan: Preparation, characterization and drug release behavior in vitro. Carbohydr. Polym. 2011, 86, 1665–1670. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Preparation, characterization, and insecticidal activity of avermectin-grafted-carboxymethyl chitosan. BioMed. Res. Int. 2016, 2016, 9805675. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Anitha, A.; Rani, V.D.; Krishna, R.; Sreeja, V.; Selvamurugan, N.; Nair, S.; Tamura, H.; Jayakumar, R. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O-carboxymethyl and N, O-carboxymethyl chitosan nanoparticles. Carbohydr. Polym. 2009, 78, 672–677. [Google Scholar] [CrossRef]
- De, A.; Roychowdhury, P.; Bhuyan, N.R.; Ko, Y.T.; Singh, S.K.; Dua, K.; Kuppusamy, G. Folic Acid Functionalized Diallyl Trisulfide–Solid Lipid Nanoparticles for Targeting Triple Negative Breast Cancer. Molecules 2023, 28, 1393. [Google Scholar] [CrossRef]
- Chou, T.-C. The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy 2018, 7, 49–50. [Google Scholar]
- Kaushik, S.; Shyam, H.; Sharma, R.; Balapure, A.K. Genistein synergizes centchroman action in human breast cancer cells. Indian J. Pharmacol. 2016, 48, 637. [Google Scholar]
- Afzali, F.; Nayeri, Z.; Minuchehr, Z.; Gardaneh, M. Investigating the Inhibitory Aspects of Metformin/Curcumin Co-Treatment through Convergence of In-Silico and In-Vitro Approaches. bioRxiv 2019, 568634. [Google Scholar] [CrossRef]
- Alimova, I.N.; Liu, B.; Fan, Z.; Edgerton, S.M.; Dillon, T.; Lind, S.E.; Thor, A.D. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009, 8, 909–915. [Google Scholar] [CrossRef]
- Lee, C.-M.; Jang, D.; Kim, J.; Cheong, S.-J.; Kim, E.-M.; Jeong, M.-H.; Kim, S.-H.; Kim, D.W.; Lim, S.T.; Sohn, M.-H. Oleyl-chitosan nanoparticles based on a dual probe for optical/MR imaging in vivo. Bioconjug. Chem. 2011, 22, 186–192. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Zhi, P.; You, J.; Gao, J.-Q. Metformin synergistically suppress tumor growth with doxorubicin and reverse drug resistance by inhibiting the expression and function of P-glycoprotein in MCF7/ADR cells and xenograft models. Oncotarget 2018, 9, 2158. [Google Scholar] [CrossRef]
- Kalliola, S.; Repo, E.; Srivastava, V.; Zhao, F.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. Carboxymethyl chitosan and its hydrophobically modified derivative as pH-switchable emulsifiers. Langmuir 2018, 34, 2800–2806. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khan, G.; Bansal, M.; Vardhan, H.; Mishra, B. Screening of ionically crosslinked chitosan-tripolyphosphate microspheres using Plackett–Burman factorial design for the treatment of intrapocket infections. Drug Dev. Ind. Pharm. 2017, 43, 1801–1816. [Google Scholar] [CrossRef]
- Ahuja, M.; Yadav, M.; Kumar, S. Application of response surface methodology to formulation of ionotropically gelled gum cordia/gellan beads. Carbohydr. Polym. 2010, 80, 161–167. [Google Scholar] [CrossRef]
- Kalliola, S.; Repo, E.; Srivastava, V.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloids Surf. B Biointerfaces 2017, 153, 229–236. [Google Scholar] [CrossRef]
- Kalliola, S.; Repo, E.; Sillanpää, M.; Arora, J.S.; He, J.; John, V.T. The stability of green nanoparticles in increased pH and salinity for applications in oil spill-treatment. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 99–107. [Google Scholar] [CrossRef]
- Xu, J.; Patassini, S.; Rustogi, N.; Riba-Garcia, I.; Hale, B.D.; Phillips, A.M.; Waldvogel, H.; Haines, R.; Bradbury, P.; Stevens, A. Regional protein expression in human Alzheimer’s brain correlates with disease severity. Commun. Biol. 2019, 2, 43. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, J.-N.; Chou, T.-C. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: Experimental design and data analysis using the combination index method. Am. J. Cancer Res. 2016, 6, 97. [Google Scholar]
- Cao, H.; Li, C.; Qi, W.; Meng, X.; Tian, R.; Qi, Y.; Yang, W.; Li, J. Synthesis, cytotoxicity and antitumour mechanism investigations of polyoxometalate doped silica nanospheres on breast cancer MCF-7 cells. PLoS ONE 2017, 12, e0181018. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | IC50 (µg/mL) Value at 48 h | |||
|---|---|---|---|---|
| MET | OCMC-MET | WZB117-OCMC-MET | WZB117 | |
| MCF 7 | >48.8 | >46.2 | 10.2 | 17.7 |
| MDA-MB-231 | >55.5 | >50.5 | 8.3 | 11.5 |
| MCF 10A | >96.7 | >98.6 | 93.3 | 78.1 |
| SI for MCF10A/MCF7 | 1.98 | 2.4 | 9.1 | 4.5 |
| SI for MCF10A/MDA-MB-231 | 1.75 | 2.1 | 11.2 | 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, A.; Wadhwani, A.; Sauraj; Roychowdhury, P.; Kang, J.H.; Ko, Y.T.; Kuppusamy, G. WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers 2023, 15, 976. https://doi.org/10.3390/polym15040976
De A, Wadhwani A, Sauraj, Roychowdhury P, Kang JH, Ko YT, Kuppusamy G. WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers. 2023; 15(4):976. https://doi.org/10.3390/polym15040976
Chicago/Turabian StyleDe, Anindita, Ashish Wadhwani, Sauraj, Parikshit Roychowdhury, Ji Hee Kang, Young Tag Ko, and Gowthamarajan Kuppusamy. 2023. "WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism" Polymers 15, no. 4: 976. https://doi.org/10.3390/polym15040976
APA StyleDe, A., Wadhwani, A., Sauraj, Roychowdhury, P., Kang, J. H., Ko, Y. T., & Kuppusamy, G. (2023). WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers, 15(4), 976. https://doi.org/10.3390/polym15040976