Research Progress in Hemicellulose-Based Nanocomposite Film as Food Packaging
Abstract
:1. Introduction
2. Hemicellulose
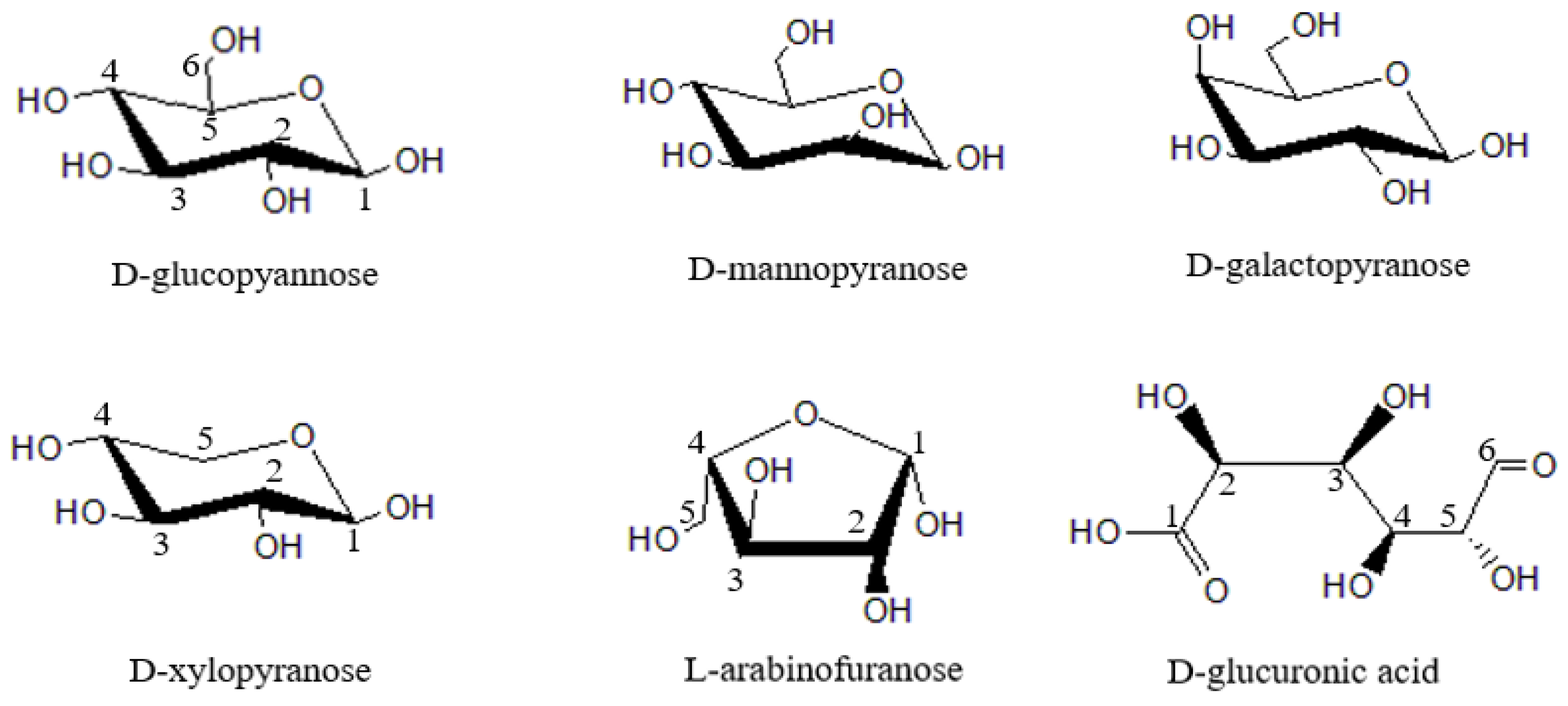
2.1. Composition and Structure of Hemicellulose
2.2. Hemicellulose Film
| Hemicellulose | Plasticizer | Oxygen Permeability (cm3·µm·m−2·24 h−1·0.1 MPa−1) | Reference |
|---|---|---|---|
| Feruloylated arabinoxylan | - | 78.6 | [44] |
| Feruloylated arabinoxylan | 30% sorbitol | 1.0 | [44] |
| Arabinoxylan | 9% glycerol | 3.0 | [45] |
| Glucuronoxylan | 35% sorbitol | 0.21 | [46] |
| Arabino-glucuronoxylan | 25% sorbitol | 0.17 | [47] |
| O-acetyl-galactoglucomannan | 40% sorbitol | 4.0 | [48] |
| Galactomannan | 29% sorbitol | 18 | [49] |
| Glucomannan | 29% sorbitol | 8.1 | [49] |
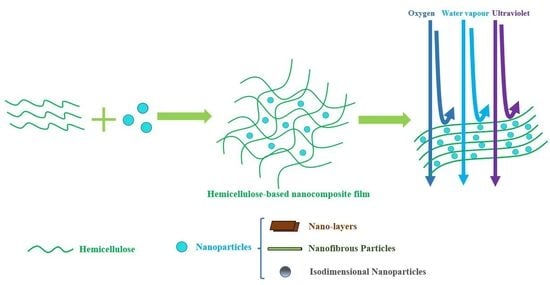
3. Nanocomposites and Nanoparticles
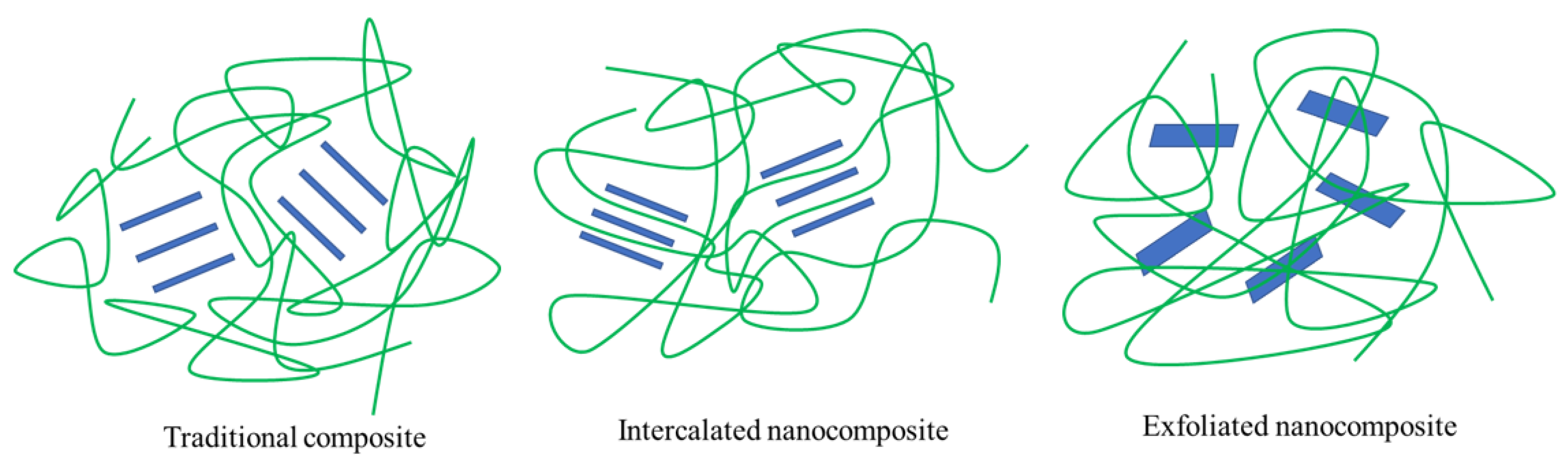
3.1. Nanocomposite
3.2. Nanoparticles
3.2.1. Nanolayers
3.2.2. Nanofibrous Particles
3.2.3. Isodimensional Nanoparticles
4. Hemicellulose-Based Nanocomposite Film
4.1. Layered Silicate and Hemicellulose Nanocomposite Film
4.2. Inorganic Nanoparticle and Hemicellulose Nanocomposite Film
4.3. Organic Nanomaterials and Hemicellulose Nanocomposite Film
4.3.1. Nanocellulose and Hemicellulose Film
4.3.2. Other Organic Nanoparticles and Hemicellulose Blend Film
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Tondi, G.; Schnabel, T. Bio-Based Polymers for Engineered Green Materials. Polymers 2020, 12, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebringerova, A.; Heinze, T. Xylan and xylan derivatives—Biopolymers with valuable properties, 1—Naturally occurring xylans structures, procedures and properties. Macromol. Rapid. Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Ebringerova, A. Structural diversity and application potential of hemicelluloses. Macromol. Symp. 2006, 232, 1–12. [Google Scholar] [CrossRef]
- Li, Z.Q.; Pan, X.J. Strategies to modify physicochemical properties of hemicelluloses from biorefinery and paper industry for packaging material. Rev. Environ. Sci. Bio/Technol. 2018, 17, 47–69. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Siddiqui, M.A.; Chandio, A.D.; Shar, M.A.; Alhazaa, A. Multi-Shaded Edible Films Based on Gelatin and Starch for the Packaging Applications. Polymers 2022, 14, 5020. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Xu, J.; Xia, R.; Zheng, L.; Yuan, T.; Sun, R. Plasticized hemicelluloses/chitosan-based edible films reinforced by cellulose nanofiber with enhanced mechanical properties. Carbohyd. Polym. 2019, 224, 115164. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Luo, W.H.; Xiao, N.Y.; Chen, M.J.; Liu, C.F. Construction of functional composite films originating from hemicellulose reinforced with poly(vinyl alcohol) and nano-ZnO. Cellulose 2020, 27, 1341–1355. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008, 54, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Peng, P.; Xu, F.; Sun, R.-C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant cell walls throughout evolution: Towards a molecular understanding of their design principles. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebringerova, A.; Hromadkova, Z.; Heinze, T. Hemicellulose. Adv. Polym. Sci. 2005, 186, 1–67. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, J.; Zhao, J.; Xu, H.; Wang, K.; Zhang, N.; Yang, J. Method for Co-Producing Biogas and Organic Fertilizer by Solid Anaerobic Fermentation of Agricultural and Forestry Residue. CN 115433742 A, 6 December 2022. [Google Scholar]
- Peng, P.; She, D. Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: A review. Carbohyd. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef]
- Cyran, M.R.; Snochowska, K.K. Evidence of intermolecular associations of β-glucan and high-molar mass xylan in a hot water extract of raw oat groat. Carbohydr. Polym. 2021, 272, 118463. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, F.; Ma, C.; Wang, H.; Wen, J.; Yu, Y. The alkaline extraction efficiency of bamboo cell walls is related to their structural differences on both anatomical and molecular level. Ind. Crops Prod. 2022, 178, 114628. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkila, M.I.; Willfor, S.M.; Tenkanen, M. Films from Glyoxal-Crosslinked Spruce Galactoglucomannans Plasticized with Sorbitol. Int. J. Polym. Sci. 2012, 2012, 482810. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Takeuchi, M.; Isogai, A. Changes in neutral sugar composition, molar mass and molar mass distribution, and solid-state structures of birch and Douglas fir by repeated sodium chlorite delignification. Cellulose 2022, 29, 2119–2129. [Google Scholar] [CrossRef]
- Kochumalayil, J.J.; Berglund, L.A. Water-soluble hemicelluloses for high humidity applications—Enzymatic modification of xyloglucan for mechanical and oxygen barrier properties. Green Chem. 2014, 16, 1904–1910. [Google Scholar] [CrossRef]
- da Silva Nascimento, F.G.; de Souza Ferreira Bringel, P.H.; Maia, F.W.S.; Lima, C.P.C.; Alves, R.C.; Feitosa, J.P.A.; Mota, M.R.L.; Assreuy, A.M.S.; Castro, R.R. Galactomannan of Delonix regia seeds reduces nociception and morphological damage in the rat model of osteoarthritis induced by sodium monoiodoacetate. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 491–501. [Google Scholar] [CrossRef]
- Cyran, M.R.; Dynkowska, W.M.; Ceglinska, A.; Bonikowski, R. Improving rye bread antioxidant capacity by bread-making methodology: Contribution of phosphate-buffered saline- and methanol-soluble phenolic phytochemicals with different molecular profiles. J. Cereal Sci. 2021, 100, 103262. [Google Scholar] [CrossRef]
- Nesic, A.; Cabrera-Barjas, G.; Dimitrijevic-Brankovic, S.; Davidovic, S.; Radovanovic, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Guo, M.; Wang, D.; Zhao, D.; Wang, M. Advances in extraction, purification, structural characteristics and biological activities of hemicelluloses: A review. Int. J. Biol. Macromol. 2023, 225, 467–483. [Google Scholar] [CrossRef]
- Curry, T.M.; Pena, M.J.; Urbanowicz, B.R. An update on xylan structure, biosynthesis, and potential commercial applications. Cell Surf. 2023, 9, 100101. [Google Scholar] [CrossRef]
- Keppler, B.D.; Showalter, A.M. IRX14 and IRX14-LIKE, Two Glycosyl Transferases Involved in Glucuronoxylan Biosynthesis and Drought Tolerance in Arabidopsis. Mol. Plant 2010, 3, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, X.; Li, P.; Li, K.; Yan, D.; Xu, H.; Huang, Y. A Kind of Rich in Mannan of Yeast Extract and Preparation Method and Application [Machine Translation]. CN 115678784 A, 3 February 2023. [Google Scholar]
- Buckeridge, M.S. Seed Cell Wall Storage Polysaccharides: Models to Understand Cell Wall Biosynthesis and Degradation. Plant Physiol. 2010, 154, 1017–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, A.J.; Tamma, P.D.; Zhang, S.X. Challenges with Utilizing the 1,3-Beta-D-Glucan and Galactomannan Assays To Diagnose Invasive Mold Infections in Immunocompromised Children. J. Clin. Microbiol. 2021, 59, e03276-20. [Google Scholar] [CrossRef] [PubMed]
- Yuting, X.; Fei, X.; Kao, W.; Xuewen, N. Research progress of konjac glucomannan-based antibacterial active packaging film. Sci. Technol. Food Ind. 2022, 43, 392–399. [Google Scholar] [CrossRef]
- Porter, D.; Peggs, D.; McGurk, C.; Martin, S.A.M. In-vivo analysis of Protec and β-glucan supplementation on innate immune performance and intestinal health of rainbow trout. Fish Shellfish Immunol. 2023, 134, 108573. [Google Scholar] [CrossRef] [PubMed]
- Colleoni-Sirghie, M.; Fulton, D.B.; White, P.J. Structural features of water soluble (1,3) (1,4)-beta-D-glucans from high-beta-glucan and traditional oat lines. Carbohyd. Polym. 2003, 54, 237–249. [Google Scholar] [CrossRef]
- Wood, P.J. Relationships between solution properties of cereal beta-glucans and physiological effects—A review. Trends Food Sci. Technol. 2004, 15, 313–320. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Rayon, C.; Urbanowicz, B.; Tine, M.A.S.; Carpita, N.C. Mixed linkage (1→3),(1→4)-beta-D-glucans of grasses. Cereal Chem. 2004, 81, 115–127. [Google Scholar] [CrossRef]
- Julian, J.D.; Zabotina, O.A. Xyloglucan Biosynthesis: From Genes to Proteins and Their Functions. Front. Plant Sci. 2022, 13, 920494. [Google Scholar] [CrossRef]
- Oliveira, J.P.d.; Bruni, G.P.; Fabra, M.J.; Zavareze, E.d.R.; Lopez-Rubio, A.; Martinez-Sanz, M. Development of food packaging bioactive aerogels through the valorization of Gelidium sesquipedale seaweed. Food Hydrocoll. 2019, 89, 337–350. [Google Scholar] [CrossRef]
- Huang, B.; Tang, Y.; Pei, Q.; Zhang, K.; Liu, D.; Zhang, X. Hemicellulose-Based Films Reinforced with Unmodified and Cationically Modified Nanocrystalline Cellulose. J. Polym. Environ. 2018, 26, 1625–1634. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef]
- Davis, G.; Song, J.H. Biodegradable packaging based on raw materials from crops and their impact on waste management. Ind Crop. Prod. 2006, 23, 147–161. [Google Scholar] [CrossRef]
- Perez, J.; Munoz-Dorado, J.; de la Rubia, T.; Martinez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Gao, C.-d.; Ren, J.-l.; Wang, S.-y.; Sun, R.-c.; Zhao, L.-h. Preparation of Polyvinyl Alcohol/Xylan Blending Films with 1,2,3,4-Butane Tetracarboxylic Acid as a New Plasticizer. J. Nanomater. 2014, 2014, 764031. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz-Turan, S.; Jimenez-Quero, A.; Menzel, C.; de Carvalho, D.M.; Lindstrom, M.E.; Sevastyanova, O.; Moriana, R.; Vilaplana, F. Bio-based films from wheat bran feruloylated arabinoxylan: Effect of extraction technique, acetylation and feruloylation. Carbohyd. Polym. 2020, 250, 116916. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S.; Heikkinen, S.; Soovre, A.; Peura, M.; Serimaa, R.; Talja, R.A.; Helen, H.; Hyvonen, L.; Tenkanen, M. Films from Oat Spelt Arabinoxylan Plasticized with Glycerol and Sorbitol. J. Appl. Polym. Sci. 2009, 114, 457–466. [Google Scholar] [CrossRef]
- Grondahl, M.; Eriksson, L.; Gatenholm, P. Material properties of plasticized hardwood Xylans for potential application as oxygen barrier films. Biomacromolecules 2004, 5, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Goncalves, A.; Bodin, A.; Stepan, A.; Sandstrom, C.; Toriz, G.; Gatenholm, P. Flexible oxygen barrier films from spruce xylan. Carbohyd. Polym. 2012, 87, 2381–2387. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Schmidt, J.; Vesterinen, A.H.; Tenkanen, M. Crosslinking with ammonium zirconium carbonate improves the formation and properties of spruce galactoglucomannan films. J. Mater. Sci. 2013, 48, 4205–4213. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Mathew, A.P.; Pirkkalainen, K.; Serimaa, R.; Xu, C.; Willfor, S.; Oksman, K.; Tenkanen, M. Glucomannan composite films with cellulose nanowhiskers. Cellulose 2010, 17, 69–81. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.d. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Peponi, L.; Puglia, D.; Torre, L.; Valentini, L.; Kenny, J.M. Processing of nanostructured polymers and advanced polymeric based nanocomposites. Mater. Sci. Eng. R 2014, 85, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Dufresne, A.; Castano, J. Polysaccharide nanomaterial reinforced starch nanocomposites: A review. Starch-Starke 2017, 69, 1500307. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. Hemicellulose-Based Film: Potential Green Films for Food Packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef]
- Lincoln, D.M.; Vaia, R.A.; Wang, Z.G.; Hsiao, B.S. Secondary structure and elevated temperature crystallite morphology of nylon-6/layered silicate nanocomposites. Polymer 2001, 42, 1621–1631. [Google Scholar] [CrossRef]
- Osman, M.A.; Mittal, V.; Lusti, H.R. The aspect ratio and gas permeation in polymer-layered silicate nanocomposites. Macromol. Rapid Commun. 2004, 25, 1145–1149. [Google Scholar] [CrossRef]
- Jong-Whan, R.; Ng, P.K.W. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Pizzoli, A.P.; Marchiore, N.G.; De Souza, S.J.; de Freitas Santos, P.D.; Goncalves, O.H.; Yamashita, F.; Bracht, L.; Shirai, M.A.; Leimann, F.V. Antimicrobial PLA/TPS/gelatin sheets with enzymatically crosslinked surface containing silver nanoparticles. J. Appl. Polym. Sci. 2016, 133, 43039. [Google Scholar] [CrossRef]
- Balazs, A.C.; Singh, C.; Zhulina, E.; Lyatskaya, Y. Modeling the phase behavior of polymer/clay nanocomposites. Acc. Chem. Res. 1999, 32, 651–657. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nanostructured Materials Utilized in Biopolymer-based Plastics for Food Packaging Applications. Crit. Rev. Food Sci. 2015, 55, 1699–1723. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Samir, M.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Samir, M.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. Cellulose nanocrystals reinforced poly(oxyethylene). Polymer 2004, 45, 4149–4157. [Google Scholar] [CrossRef]
- Rayon, E.; Lopez, J.; Arrieta, M.P. Mechanical Characterization of Microlaminar Structures Extracted from Cellulosic Materials Using Nanoindentation Technique. Cell Chem. Technol. 2013, 47, 345–351. [Google Scholar]
- Mallakpour, S.; Naghdi, M. Fabrication and characterization of novel polyvinylpyrrolidone nanocomposites having SiO2 nanoparticles modified with citric acid and L(+)-ascorbic acid. Polymer 2016, 90, 295–301. [Google Scholar] [CrossRef]
- Yu, Y.W.; Zhang, S.Y.; Ren, Y.Z.; Li, H.; Zhang, X.N.; Di, J.H. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Chen, G.-G.; Qi, X.-M.; Li, M.-P.; Guan, Y.; Bian, J.; Peng, F.; Yao, C.-L.; Sun, R.-C. Hemicelluloses/montmorillonite hybrid films with improved mechanical and barrier properties. Sci. Rep. 2015, 5, 16405. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.G.; Qi, X.M.; Guan, Y.; Peng, F.; Yao, C.L.; Sun, R.C. High Strength Hemicellulose-Based Nanocomposite Film for Food Packaging Applications. Acs Sustain. Chem. Eng. 2016, 4, 1985–1993. [Google Scholar] [CrossRef]
- Naidu, D.S.; John, M.J. Effect of Clay Nanofillers on the Mechanical and Water Vapor Permeability Properties of Xylan-Alginate Films. Polymers 2020, 12, 2279. [Google Scholar] [CrossRef]
- Liu, X.X.; Chen, X.F.; Ren, J.L.; Chang, M.M.; He, B.; Zhang, C.H. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Ren, J.; Wang, S.; Gao, C.; Chen, X.; Li, W.; Peng, F. TiO2-containing PVA/xylan composite films with enhanced mechanical properties, high hydrophobicity and UV shielding performance. Cellulose 2015, 22, 593–602. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Y.; Chen, M.; Xiao, N.; Zhang, J.; Liu, C. Development of functional chitosan-based composite films incorporated with hemicelluloses: Effect on physicochemical properties. Carbohyd. Polym. 2020, 246, 116489. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Zhang, C. TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials 2018, 11, 1589. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.W.; Ren, J.L.; Zhong, L.X.; Sun, R.C. Nanocomposite Films Based on Xylan-Rich Hemicelluloses and Cellulose Nanofibers with Enhanced Mechanical Properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; de Brito, E.S.; Rodrigues, T.H.S.; Rosa, M.F.; Azeredo, H.M.C. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohyd. Polym. 2017, 164, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Zhang, H.; Luan, Q.; Zheng, M.; Tang, H.; Huang, F. Fabrication of cellulose nanowhiskers reinforced chitosan-xylan nanocomposite films with antibacterial and antioxidant activities. Carbohyd. Polym. 2018, 184, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mu, R.J.; Li, Y.Z.; Lin, L.Z.; Lin, Z.Y.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. Lwt-Food Sci. Technol. 2019, 113, 108293. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocoll. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Goksu, E.I.; Karamanlioglu, M.; Bakir, U.; Yilmaz, L.; Yilmazer, U. Production and characterization of films from cotton stalk xylan. J. Agric. Food Chem. 2007, 55, 10685–10691. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Marin, M.L.; Bello-Perez, L.A.; Yee-Madeira, H.; Zhong, Q.X.; Gonzalez-Soto, R.A. Nanocomposites of rice and banana flours blend with montmorillonite: Partial characterization. Mater. Sci. Eng. C-Mater. 2013, 33, 3903–3908. [Google Scholar] [CrossRef]
- Rao, J.; Gao, H.; Guan, Y.; Li, W.-q.; Liu, Q. Fabrication of hemicelluloses films with enhanced mechanical properties by graphene oxide for humidity sensing. Carbohyd. Polym. 2019, 208, 513–520. [Google Scholar] [CrossRef]
- Zhong, Y.; Janes, D.; Zheng, Y.; Hetzer, M.; De Kee, D. Mechanical and oxygen barrier properties of organoclay-polyethylene nanocomposite films. Polym. Eng. Sci. 2007, 47, 1101–1107. [Google Scholar] [CrossRef]
- Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E.Y.X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3559. [Google Scholar] [CrossRef] [Green Version]
- Peter, Z.; Kenyo, C.; Renner, K.; Krohnke, C.; Pukanszky, B. Decreased oxygen permeability of EVOH through molecular interactions. Express Polym. Lett. 2014, 8, 756–766. [Google Scholar] [CrossRef] [Green Version]
- El-Saftawy, A.A.; Ragheb, M.S.; Zakhary, S.G. Electron beam irradiation impact on surface structure and wettability of ethylene-vinyl alcohol copolymer. Radiat. Phys. Chem. 2018, 147, 106–113. [Google Scholar] [CrossRef]
- Mokwena, K.K.; Tang, J. Ethylene Vinyl Alcohol: A Review of Barrier Properties for Packaging Shelf Stable Foods. Crit. Rev. Food Sci. 2012, 52, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Jesus Cejudo-Bastante, M.; Cejudo-Bastante, C.; Cran, M.J.; Heredia, F.J.; Bigger, S.W. Optical, structural, mechanical and thermal characterization of antioxidant ethylene vinyl alcohol copolymer films containing betalain-rich beetroot. Food Packag. Shelf Life 2020, 24, 100502. [Google Scholar] [CrossRef]
- Chin, I.J.; Thurn-Albrecht, T.; Kim, H.C.; Russell, T.P.; Wang, J. On exfoliation of montmorillonite in epoxy. Polymer 2001, 42, 5947–5952. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, B.; Tan, X.; Qi, X.M.; Bian, J.; Peng, F.; Sun, R.G. Organic-Inorganic Composite Films Based on Modified Hemicelluloses with Clay Nanoplatelets. Acs Sustain. Chem. Eng. 2014, 2, 1811–1818. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Cao, J. Characterization and properties of organo-montmorillonite modified lignocellulosic fibers and their interaction mechanisms. Rsc Adv. 2015, 5, 76708–76717. [Google Scholar] [CrossRef]
- Chandio, A.D.; Channa, I.A.; Rizwan, M.; Akram, S.; Javed, M.S.; Siyal, S.H.; Saleem, M.; Makhdoom, M.A.; Ashfaq, T.; Khan, S.; et al. Polyvinyl Alcohol and Nano-Clay Based Solution Processed Packaging Coatings. Coatings 2021, 11, 942. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; bin Jumah, M.N. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Li, X.W.; Song, R.G.; Jiang, Y.; Wang, C.; Jiang, D. Surface modification of TiO2 nanoparticles and its effect on the properties of fluoropolymer/TiO2 nanocomposite coatings. Appl. Surf. Sci. 2013, 276, 761–768. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid organic-inorganic materials—In search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Saxena, A.; Foston, M.; Kassaee, M.; Elder, T.J.; Ragauskas, A.J. Biopolymer Nanocomposite Films Reinforced with Nanocellulose Whiskers. J. Nanosci. Nanotechnol. 2012, 12, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Krajewska, B.; Wydro, P.; Janczyk, A. Probing the Modes of Antibacterial Activity of Chitosan. Effects of pH and Molecular Weight on Chitosan Interactions with Membrane Lipids in Langmuir Films. Biomacromolecules 2011, 12, 4144–4152. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.M.; Wang, X.H.; Sun, L.P. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci.-Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [Green Version]
- Lei, T.; Zhang, R.; Liu, Y.; Zhu, X.; Li, K.; Li, G.; Zheng, H. Effect of the high barrier and hydrophobic hemicellulose/montmorillonite film on postharvest quality of fresh green asparagus. Ind. Crop. Prod. 2022, 187, 115509. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Wang, J.; Cheng, M. Synthesis and Characterization of Konjac Glucomannan/Carrageenan/Nano-silica Films for the Preservation of Postharvest White Mushrooms. Polymers 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Louis, A.C.F.; Venkatachalam, S. Post-harvest quality and shelf life assessment of Agaricus bisporus influenced by nanocellulose/nanohemicellulose loaded starch based packaging. Polym. Compos. 2022, 43, 7538–7550. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G. Preparation Method of Vegetable Preserving Film Using Polyethylene and Ethylene-Vinyl Acetate. CN 110901111 A, 24 March 2020. [Google Scholar]





| Hemicellulose | Source | Solvent | Mw (g/mol) | Ref. |
|---|---|---|---|---|
| O-Acetyl-4-O-methyl-glucurono-xylan | Hardwood | Water or alkaline solution | 5000~130,000 | [15,17] |
| β-glucans | Oat | Water | 1,029,000~1,589,000 | [18] |
| Arabino-4-O-methyl-glucurono-xylan | Softwood, grain straw | Water | 30,000~370,000 | [15,19] |
| Glucomannan | Hardwood, konjac | Alkaline solution | 20,000~60,000 | [20,21] |
| Xyloglucan | Tamarind seed | Water | 1,500,000~2,000,000 | [22] |
| Galactomannan | Delonix regia seed | Water | 580,000 | [23] |
| Arabinoxylan | Rye | Water or alkaline solution | 443,000~556,000 | [24] |
| Films Composition | Reinforcing Agents | TS * (MPa) | EAB ** (%) | OP *** (cm3·µm·m−2·24 h−1·0.1 MPa−1) | WVP **** (10−11·g·s−1·m−1·Pa−1) | Ref. |
|---|---|---|---|---|---|---|
| MMT/QH | 1 wt% MMT | 19.8 | 0.5 | 12.26 | - | [75] |
| NCH blended MMT/QH | 1 wt% MMT | 24.2 | 1.7 | 44.41 | - | [75] |
| PVA blended MMT/QH | 1 wt% MMT | 31.4 | 1.1 | 5.54 | - | [75] |
| CS blended MMT/QH | 2 wt% MMT | 43.5 | 3.2 | 11.16 | 31.9 | [76] |
| Xylan–alginate | - | 8.87 | 51.29 | - | 39.4 | [77] |
| Nanoclays blended xylan | 5 wt% bentonite | 18.86 | 46.7 | - | 20.1 | [77] |
| PVA/xylan | - | - | - | 6.82 | 3.97 | [78] |
| Nano-ZnO blended PVA/xylan | 3 wt% nano-ZnO | 20.4 | - | 5.28 | 3.14 | [78] |
| Nano-SiO2 blended PVA/xylan | 3 wt% nano-SiO2 | 22.5 | - | 5.003 | 3.03 | [78] |
| Nano-ZnO blended HC/PVA | 1 wt% nano-ZnO | - | 87.18 | 2.24 | 38.9 | [11] |
| PVA/xylan | - | 23.54 | 327.23 | - | 4.3 | [79] |
| Nano-TiO2 blended PVA/xylan | 2 wt% nano-TiO2 | 30.73 | 192.91 | - | 3.46 | [79] |
| Chitosan/hemicellulose | - | 13.72 | 30.49 | - | 44.0 | [80] |
| Nano-TiO2/CS/hemicellulose | 20 wt% nano-TiO2 | 25.75 | 19.95 | - | 28.8 | [80] |
| PVA/xylan | - | 16.1 | - | 6.82 | 3.97 | [81] |
| TiO2-KH550/PVA/xylan | 1.5 wt% TiO2-KH550 | 27.3 | - | 4.013 | 2.75 | [81] |
| CNFs-reinforced hemicellulose | 15 wt% CNFs | 28.9 | 1.8 | - | - | [82] |
| Hemicellulose | - | 8.71 | 3.75 | - | 0.12 | [83] |
| CNCs-reinforced hemicellulose | 8 wt% CNCs | 14.98 | 2.36 | - | 0.071 | [83] |
| Chitosan/xylan | - | 4.9 | 6.47 | - | - | [84] |
| CS-xylan/NCW | 12 wt% NCW | 16.04 | 11.49 | - | - | [84] |
| Konjac glucomannan | - | 4.25 | - | - | - | [85] |
| Curcumin/KGM/ZNs | ZNs | 7.34 | - | - | - | [85] |
| KGM | - | 30-35 | 42.23 | - | 18.22 | [86] |
| KGM/CGNPs | 10 wt% CGNPs | 35~45 | 26.61 | - | 10~15 | [86] |
| Xylan/lignin | - | 1.39 | 56.76 | - | - | [87] |
| Banana flours film | - | 23.4 | 8.3 | - | 24.9 | [88] |
| Quaternized hemicellulose | - | 10.02 | 1.28 | - | - | [89] |
| EVA | - | 17.04 | 912 | 145.8 | - | [90] |
| LDPE | - | 15.18 | 289 | 78.2 | - | [90] |
| HDPE | - | 20.29 | 553 | 24 | - | [90] |
| PET | - | 45 | 335 | 58.34 | - | [91] |
| EVOH | - | 40 | 230 | 2.77 | - | [92,93,94,95] |
| Film | Food | Packaging Effects | Ref. |
|---|---|---|---|
| Hemicellulose/montmorillonite | Green asparagus | Delayed the loss of soluble protein, vitamin C and other nutrients; Extended the shelf life to 7 days | [109] |
| KGM/carrageenan/nano-silica | White mushrooms | Improved the quality of food preservation; Extended the shelf life to 12 days | [110] |
| Nanocellulose/nanohemicellulose/starch | Agaricus bisporus | Retained the quality of mushrooms; Extended the shelf life to 6 days | [111] |
| Hemicellulose/polyethylene/nano-silver | Vegetable | Extended preservation time | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Shi, K.; Sun, H. Research Progress in Hemicellulose-Based Nanocomposite Film as Food Packaging. Polymers 2023, 15, 979. https://doi.org/10.3390/polym15040979
Liu G, Shi K, Sun H. Research Progress in Hemicellulose-Based Nanocomposite Film as Food Packaging. Polymers. 2023; 15(4):979. https://doi.org/10.3390/polym15040979
Chicago/Turabian StyleLiu, Guoshuai, Kang Shi, and Hui Sun. 2023. "Research Progress in Hemicellulose-Based Nanocomposite Film as Food Packaging" Polymers 15, no. 4: 979. https://doi.org/10.3390/polym15040979
APA StyleLiu, G., Shi, K., & Sun, H. (2023). Research Progress in Hemicellulose-Based Nanocomposite Film as Food Packaging. Polymers, 15(4), 979. https://doi.org/10.3390/polym15040979