Bio-Based Polyurethane Foams from Kraft Lignin with Improved Fire Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rigid Polyurethane Foam Formulation
2.3. Rigid Polyurethane Foam Characterization
3. Results and Discussion
3.1. Effect of Blowing Agent, Catalyst and Surfactant Content on the Density and Thermal Conductivity of Bio-Based Rigid Polyurethane Foam
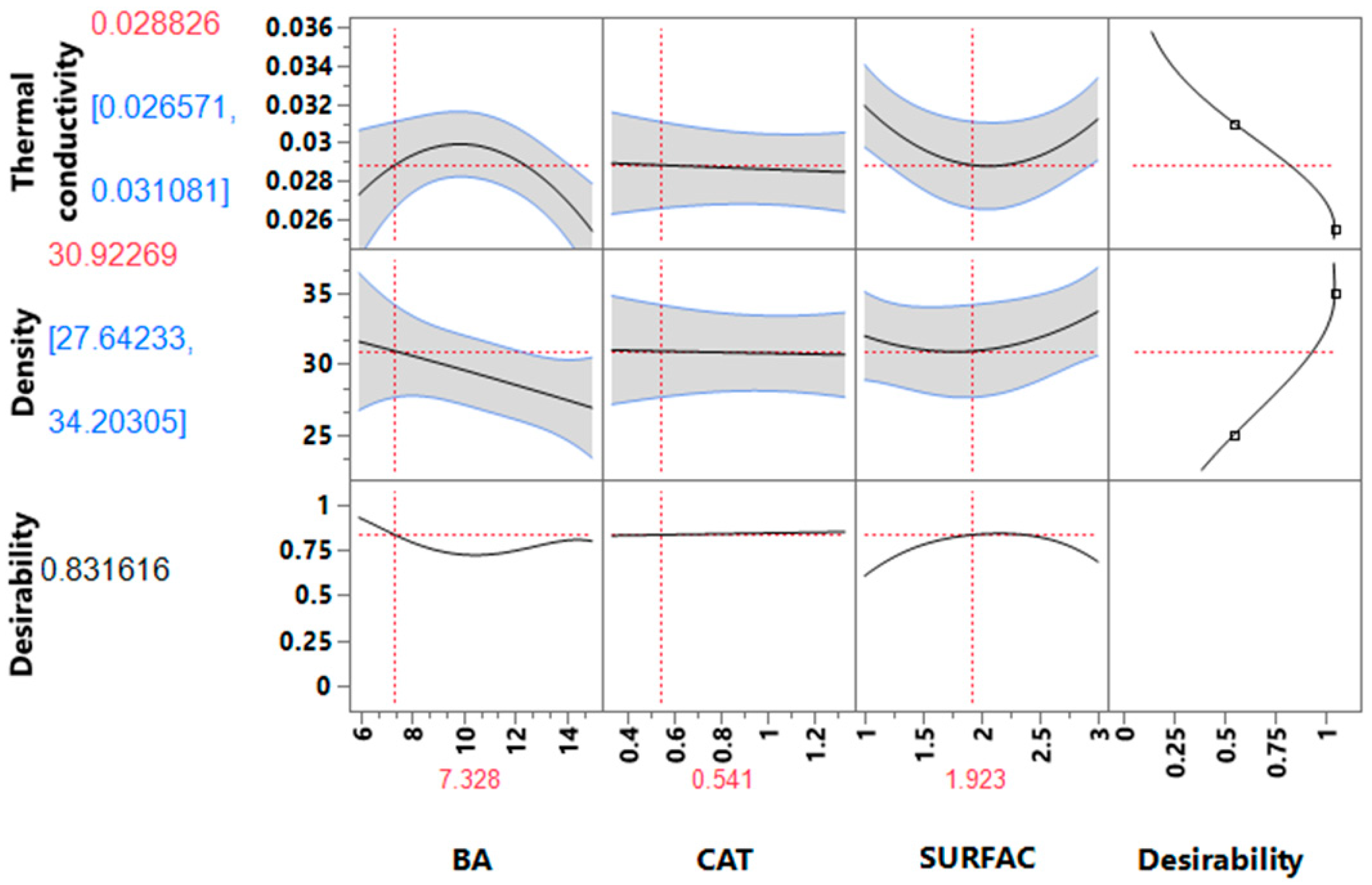
3.2. Optimization of Formulations
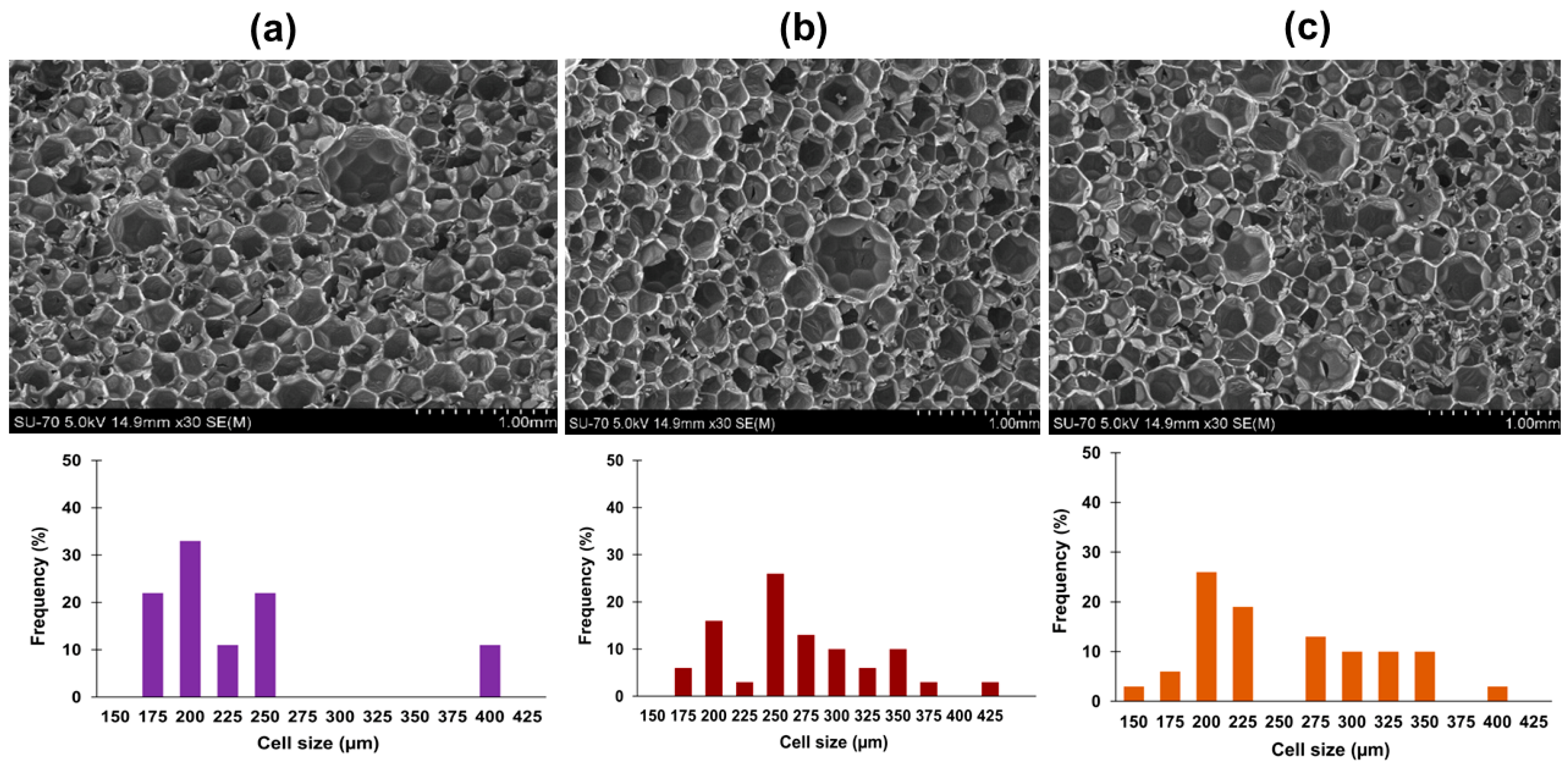
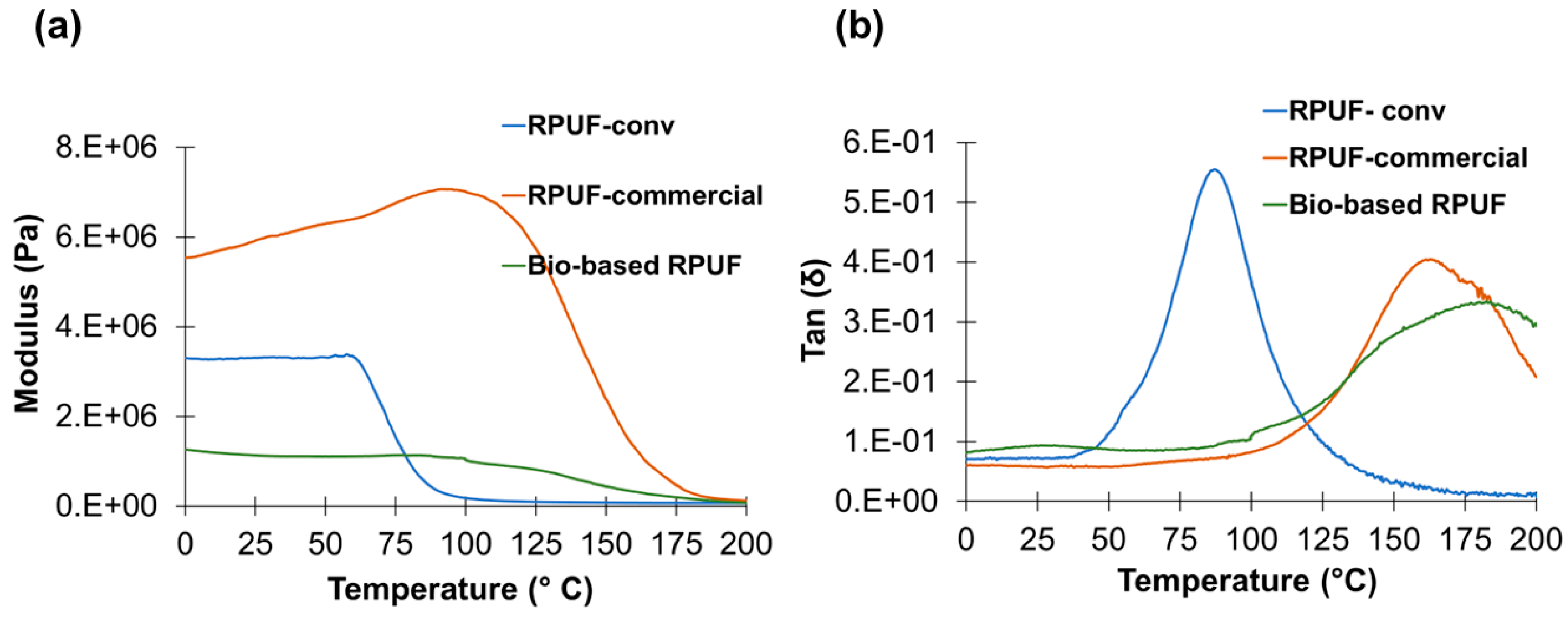
3.3. Characterization of Bio-Based Rigid Polyurethane Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.-T.; Ramesh, N.S. Polymeric Foams: Mechanisms and Materials; CRC Press: London, UK; New York, NY, USA, 2004. [Google Scholar]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane foams from vegetable oil-based polyols: A review. Polym. Bull. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environment impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the potential of hardwood kraft lignin to be a green platform material for emergence of the biorefinery. Polymers 2020, 12, 1795. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Hulteberg, C.P. Physicochemical Characterisation of Technical Lignins for Their Potential Valorisation. Waste Biomass Valorization 2017, 8, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Evtuguin, D.V.V.; Andreolety, J.P.P.; Gandini, A. Polyurethanes based on oxygen-organosolv lignin. Eur. Polym. J. 1998, 34, 1163–1169. [Google Scholar] [CrossRef]
- Luo, S.; Gao, L.; Guo, W. Effect of incorporation of lignin as bio-polyol on the performance of rigid lightweight wood–polyurethane composite foams. J. Wood Sci. 2020, 66, 23. [Google Scholar] [CrossRef] [Green Version]
- Kühnel, I.; Podschun, J.; Saake, B.; Lehnen, R. Synthesis of lignin polyols via oxyalkylation with propylene carbonate. Holzforschung 2015, 69, 531–538. [Google Scholar] [CrossRef]
- Duval, A.; Avérous, L. Cyclic Carbonates as Safe and Versatile Etherifying Reagents for the Functionalization of Lignins and Tannins. ACS Sustain. Chem. Eng. 2017, 5, 7334–7343. [Google Scholar] [CrossRef]
- Vieira, F.R.; Barros-timmons, A.; Evtuguin, D.V.; Pinto, P.C.R. Effect of different catalysts on the oxyalkylation of eucalyptus Lignoboost® kraft lignin. Holzforschung 2020, 74, 567–576. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, Y.; Elsayed, I.; Taylor, M.; Eberhardt, T.L.; Hassan, E.B.; Shmulsky, R. Rigid polyurethane foams containing lignin oxyalkylated with ethylene carbonate and polyethylene glycol. Ind. Crops Prod. 2019, 141, 111797. [Google Scholar] [CrossRef]
- Liu, L.Y.; Cho, M.; Sathitsuksanoh, N.; Chowdhury, S.; Renneckar, S. Uniform Chemical Functionality of Technical Lignin Using Ethylene Carbonate for Hydroxyethylation and Subsequent Greener Esterification. ACS Sustain. Chem. Eng. 2018, 6, 12251–12260. [Google Scholar] [CrossRef]
- Duval, A.; Vidal, D.; Sarbu, A.; René, W.; Avérous, L. Scalable single-step synthesis of lignin-based liquid polyols with ethylene carbonate for polyurethane foams. Mater. Today Chem. 2022, 24, 100793. [Google Scholar] [CrossRef]
- Vieira, F.R.; Barros-timmons, A.; Evtuguin, D.V. Oxyalkylation of Lignoboost TM Kraft Lignin with Propylene Carbonate: Design of Experiments towards Synthesis Optimization. Materials 2022, 15, 1925. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Gama, N.V.; Barros-Timmons, A.; Evtuguin, D.V.; Pinto, P.C.O.R. Development of Rigid Polyurethane Foams Based on Kraft Lignin Polyol Obtained by Oxyalkylation Using Propylene Carbonate. ChemEngineering 2022, 6, 95. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Hu, S. Lignocellulosic Biomass-Based Polyols for Polyurethane Applications. In Bio-Based Polyols and Polyurethanes; Springer: New York, NY, USA, 2015; ISBN 9783319215389. [Google Scholar]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [Green Version]
- Peyrton, J.; Avérous, L. Structure-properties relationships of cellular materials from biobased polyurethane foams. Mater. Sci. Eng. R Rep. 2021, 145, 100608. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.Z.; Li, Y.M.; Tao, W.Q. Experimental study of the thermal conductivity of polyurethane foams. Appl. Therm. Eng. 2017, 115, 528–538. [Google Scholar] [CrossRef]
- Kirpluks, M.; Cabulis, U.; Zeltins, V.; Stiebra, L.; Avots, A. Rigid polyurethane foam thermal insulation protected with mineral intumescent mat. Autex Res. J. 2014, 14, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.N. Blowing Agents for Polyurethane Foams; iSmithers Rapra Publishing: Shrewsbury, UK, 2001; Volume 12, ISBN 1859573215. [Google Scholar]
- Wianowski, L.; Białkowska, A.; Dobrowolski, L.; Zarzyka, I. Physical blowing agents for polyurethanes. Polymers 2020, 65, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Grimminger, J.; Muha, K. Silicone Surfactants for Pentane Blown Rigid Foam. J. Cell. Plast. 1995, 31, 48–72. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, Y.H.; Kim, W.N.; Lee, H.S.; Sung, J.Y. Effects of Silicone Surfactant on the Cell Size and Thermal Conductivity of Rigid. Macromol. Res. 2009, 17, 44–50. [Google Scholar]
- Gama, N.V.; Silva, R.; Costa, M.; Barros-timmons, A.; Ferreira, A. Statistical evaluation of the effect of formulation on the properties of crude glycerol polyurethane foams. Polym. Test. 2016, 56, 200–206. [Google Scholar] [CrossRef]
- Gustafsson, S.E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- ASTM 6226-05; Standard Test Method for Open Cell Content of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2005.
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- ASTM D1621-16; Standard Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 1991.
- ISO 11357-2:2020; Plastics—Differential Scanning Calorimetry (DSC)—Part 2: Determination of Glass Transition Temperature and Step Height. International Organization for Standardization: Geneva, Switzerland, 2020; Volume 2020, p. 9.
- Montgomery, D.C. Montgomery Design and Analysis of Experiments Eighth Edition. Arizona State University: Tempe, AZ, USA, 2013; Volume 2009, ISBN 9781118146927. [Google Scholar]
- Kirpluks, M.; Vanags, E.; Abolins, A.; Michalowski, S.; Fridrihsone, A.; Cabulis, U. High functionality bio-polyols from tall oil and rigid polyurethane foams formulated solely using bio-polyols. Materials 2020, 13, 1985. [Google Scholar] [CrossRef]
- Choe, K.H.; Soo, D.L.; Seo, W.J.; Kim, W.N. Properties of rigid polyurethane foams with blowing agents and catalysts. Polym. J. 2004, 36, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Boonachathong, R.; Kaewnok, B.; Widjaja, H.; Amornraksa, S. Development of Rigid Polyurethane Foam (RPUF) for Imitation Wood Blown by Distilled Water and Cyclopentane (CP). MATEC Web Conf. 2018, 187, 02001. [Google Scholar] [CrossRef] [Green Version]
- Eaves, D. Rigid polyurethane foam. In Handbook of Polymeric Foams and Foam Technology; Dave Eaves, D., Ed.; Rapra: Shawbury, UK, 2004; ISBN 1859573886. [Google Scholar]
- Jung, H.C.; Ryu, S.C.; Kim, W.N.; Lee, Y.B.; Choe, K.H.; Kim, S.B. Properties of rigid polyurethane foams blown by HCFC 141B and distilled water. J. Appl. Polym. Sci. 2001, 81, 486–493. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Kurańska, M.; Pinto, J.A.; Salach, K.; Barreiro, M.F.; Prociak, A. Synthesis of thermal insulating polyurethane foams from lignin and rapeseed based polyols: A comparative study. Ind. Crops Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Arbenz, A.; Frache, A.; Cuttica, F.; Avérous, L. Advanced biobased and rigid foams, based on urethane-modified isocyanurate from oxypropylated gambier tannin polyol. Polym. Degrad. Stab. 2016, 132, 62–68. [Google Scholar] [CrossRef]
- Pinto, J.A.; Fernandes, I.P.; Pinto, V.D.; Gomes, E.; Oliveira, C.F.; Pinto, P.C.R.; Mesquita, L.M.R.; Piloto, P.A.G.; Rodrigues, A.E.; Barreiro, M.F. Valorization of lignin side-streams into polyols and rigid polyurethane foams—A contribution to the pulp and paper industry biorefinery. Energies 2021, 14, 3825. [Google Scholar] [CrossRef]
- Seo, W.J.; Jung, H.C.; Hyun, J.C.; Kim, W.N.; Lee, Y.B.; Choe, K.H.; Kim, S.B. Mechanical, morphological, and thermal properties of rigid polyurethane foams blown by distilled water. J. Appl. Polym. Sci. 2003, 90, 12–21. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Hatakeyema, H.; Tanamachi, N.; Matsumura, H.; Hirose, S.; Hatakeyama, T. Bio-based polyurethane composite foams with inorganic fillers studied by thermogravimetry. Thermochim. Acta 2005, 431, 155–160. [Google Scholar] [CrossRef]
- Goods, S.H.; Neuschwanger, C.L. & Whinnery, L.L. Mechanical Properties of a Structural Polyurethane Foam and the Effect of Particulate Loading. MRS Online Proc. Libr. 1998, 521, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Mazzuca, P.; Firmo, J.P.; Correia, J.R.; Castilho, E. Mechanical behaviour in shear and compression at elevated temperature of polyethylene terephthalate (PET) foam. J. Build. Eng. 2021, 42, 102526. [Google Scholar] [CrossRef]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.Y.; Chabert, E. Mechanical properties of high density polyurethane foams: I. Effect of the density. Compos. Sci. Technol. 2006, 66, 2700–2708. [Google Scholar] [CrossRef]
- Lin, H.R. The structure and property relationships of commercial foamed plastics. Polym. Test. 1997, 16, 429–443. [Google Scholar] [CrossRef]
- Federation of European Rigid Polyurethane Foam Associations. Thermal Insulation Materials Made of Rigid Polyurethane Foam (PUR/PIR). Available online: https://www.pu-europe.eu/library/pu-europe-reports/ (accessed on 10 June 2022).
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Ruiz-Herrero, J.L.; Villafañe, F.; Rodríguez-Pérez, M.Á. Long-term thermal conductivity of cyclopentane–water blown rigid polyurethane foams reinforced with different types of fillers. Polym. Int. 2019, 68, 1826–1835. [Google Scholar] [CrossRef]
- Mort, R.; Vorst, K.; Curtzwiler, G.; Jiang, S. Biobased foams for thermal insulation: Material selection, processing, modelling, and performance. RSC Adv. 2021, 11, 4375–4394. [Google Scholar] [CrossRef] [PubMed]
- Haridevan, H.; McLaggan, M.S.; Evans, D.A.C.; Martin, D.J.; Seaby, T.; Zhang, Z.; Annamalai, P.K. Dispersion Methodology for Technical Lignin into Polyester Polyol for High-Performance Polyurethane Insulation Foam. ACS Appl. Polym. Mater. 2021, 3, 3528–3537. [Google Scholar] [CrossRef]
- Peyrton, J.; Chambaretaud, C.; Sarbu, A.; Avérous, L. Biobased Polyurethane Foams Based on New Polyol Architectures from Microalgae Oil. ACS Sustain. Chem. Eng. 2020, 8, 12187–12196. [Google Scholar] [CrossRef]
- Amran, U.A.; Zakaria, S.; Chia, C.H.; Roslan, R.; Jaafar, S.N.S.; Salleh, K.M. Polyols and rigid polyurethane foams derived from liquefied lignocellulosic and cellulosic biomass. Cellulose 2019, 26, 3231–3246. [Google Scholar] [CrossRef]
- Song, Z.L.; Ma, L.Q.; Wu, Z.J.; He, D.P. Effects of viscosity on cellular structure of foamed aluminum in foaming process. J. Mater. Sci. 2000, 35, 15–20. [Google Scholar] [CrossRef]
- Abdollahi Baghban, S.; Khorasani, M.; Mir Mohamad Sadeghi, G. Soundproofing flexible polyurethane foams: Effect of chemical structure of chain extenders on micro-phase separation and acoustic damping. J. Cell. Plast. 2020, 56, 167–185. [Google Scholar] [CrossRef]
- Cheng, B.X.; Gao, W.C.; Ren, X.M.; Ouyang, X.Y.; Zhao, Y.; Zhao, H.; Wu, W.; Huang, C.X.; Liu, Y.; Liu, X.Y.; et al. A review of microphase separation of polyurethane: Characterization and applications. Polym. Test. 2022, 107, 107489. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, W.; Liu, X.; Wang, Y.; Han, D.; Zhang, Q. Effect of bio-based polyols and chain extender on the microphase separation structure, mechanical properties and morphology of rigid polyurethane foams. Eur. Polym. J. 2022, 179, 111572. [Google Scholar] [CrossRef]
- Muller, L.C.; Marx, S.; Vosloo, H.C.; Fosso-Kankeu, E.; Chiyanzu, I. Rigid polyurethane foams from unrefined crude glycerol and technical lignins. Polym. Renew. Resour. 2018, 9, 111–132. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Polyurethane foams made from liquefied bark-based polyols. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Ottati, C.; Lopretti, M. Lignin-based rigid polyurethane foams with improved biodegradation. J. Cell. Plast. 2014, 50, 81–95. [Google Scholar] [CrossRef]
- Mazzuca, P.; Firmo, J.P.; Correia, J.R.; Garrido, M. Mechanical behaviour in shear and compression of polyurethane foam at elevated temperature. J. Sandw. Struct. Mater. 2022, 24, 1429–1448. [Google Scholar] [CrossRef]
- Garrido, M.; Correia, J.R.; Keller, T. Effects of elevated temperature on the shear response of PET and PUR foams used in composite sandwich panels. Constr. Build. Mater. 2015, 76, 150–157. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; MacOsko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Puszka, A. Thermal and mechanical behavior of new transparent thermoplastic polyurethane elastomers derived from cycloaliphatic diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef] [Green Version]
- Puszka, A.; Kultys, A. The influence of soft segments on some properties of new transparent segmented polyurethanes. Polym. Adv. Technol. 2017, 28, 1937–1944. [Google Scholar] [CrossRef]
- Gama, N.; Silva, R.; Carvalho, A.P.O.; Ferreira, A.; Barros-Timmons, A. Sound absorption properties of polyurethane foams derived from crude glycerol and liquefied coffee grounds polyol. Polym. Test. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Vieira, F.R.; Gama, N.; Magina, S.; Barros-Timmons, A.; Evtuguin, D.V.; Pinto, P.C.O.R. Polyurethane Adhesives Based on Oxyalkylated Kraft Lignin. Polymers 2022, 14, 5305. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green. Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Cui, C.; Sadeghifar, H.; Sen, S.; Argyropoulos, D.S. Toward thermoplastic lignin polymers; Part II: Thermal & polymer characteristics of kraft lignin & derivatives. BioResources 2013, 8, 864–886. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Sadeghifar, H.; Argyropoulos, D.S. Kraft lignin chain extension chemistry via propargylation, oxidative coupling, and claisen rearrangement. Biomacromolecules 2013, 14, 3399–3408. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.T.; Hull, T.R. The fire toxicity of polyurethane foams. Fire Sci. Rev. 2016, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J.-P. An Overview on the Use of Lignin and Its Derivatives in Fire Retardant Polymer Systems. In Lignin-Trends and Applications; BoD–Books on Demand: Norderstedt, Germany, 2018; pp. 207–231. [Google Scholar] [CrossRef] [Green Version]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Dai, P.; Liang, M.; Ma, X.; Luo, Y.; He, M.; Gu, X.; Gu, Q.; Hussain, I.; Luo, Z. Highly Efficient, Environmentally Friendly Lignin-Based Flame Retardant Used in Epoxy Resin. ACS Omega 2020, 5, 32084–32093. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, S.; Song, P.; Luo, X.; Jin, Y.; Lu, F.; Wu, Q.; Ye, J. Functionalized lignin by grafting phosphorus-nitrogen improves the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stab. 2012, 97, 541–546. [Google Scholar] [CrossRef]
- De Chirico, A.; Armanini, M.; Chini, P.; Cioccolo, G.; Provasoli, F.; Audisio, G. Flame retardants for polypropylene based on lignin. Polym. Degrad. Stab. 2003, 79, 139–145. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, J.; Liu, W.; Qiu, X.; Qian, Y.; Zhang, H.; Yang, Y.; Liu, M.; Yang, D. Pristine lignin as a flame retardant in flexible PU foam. Green Chem. 2021, 23, 5972–5980. [Google Scholar] [CrossRef]
- Koštial, P.; Jančíková, Z.K.; Frischer, R. Case study on fire resistance of sandwiches for means of transport. Coatings 2021, 11, 207. [Google Scholar] [CrossRef]




| Foams Codes | Polyol | pMDI | Surfactant | Catalyst | Blowing Agent | |
|---|---|---|---|---|---|---|
| n-Pentane | Water | |||||
| RPUF-1 | 100 | 93.7 | 1 | 0.5 | 7.0 | 2.0 |
| RPUF-2 | 100 | 93.7 | 3 | 0.5 | 7.0 | 2.0 |
| RPUF-3 | 100 | 93.7 | 1 | 1.5 | 7.0 | 2.0 |
| RPUF-4 | 100 | 93.7 | 3 | 1.5 | 7.0 | 2.0 |
| RPUF-5 | 100 | 93.7 | 1 | 0.5 | 15 | 2.0 |
| RPUF-6 | 100 | 93.7 | 3 | 0.5 | 15 | 2.0 |
| RPUF-7 | 100 | 93.7 | 1 | 1.5 | 15 | 2.0 |
| RPUF-8 | 100 | 93.7 | 3 | 1.5 | 15 | 2.0 |
| RPUF-9 | 100 | 93.7 | 2 | 1.0 | 7 | 2.0 |
| RPUF- 10 | 100 | 93.7 | 2 | 1.0 | 15 | 2.0 |
| RPUF-11 | 100 | 93.7 | 2 | 0.5 | 11 | 2.0 |
| RPUF-12 | 100 | 93.7 | 2 | 1.5 | 11 | 2.0 |
| RPUF-13 | 100 | 93.7 | 1 | 1.0 | 11 | 2.0 |
| RPUF-14 | 100 | 93.7 | 3 | 1.0 | 11 | 2.0 |
| RPUF-15 | 100 | 93.7 | 2 | 1.0 | 11 | 2.0 |
| RPUF-16 | 100 | 93.7 | 2 | 1.0 | 11 | 2.0 |
| RPUF-conv | 100 | 93.7 | 1.5 | 1.0 | 8 | 2.0 |
| Foams Codes | Thermal Conductivity at 25 °C, W/m·K (Mean ± * SD) | Density, kg/m3 (Mean ± * SD) | Visual Inspection |
|---|---|---|---|
| RPUF-1 | 0.0313 ± 9.00 × 10−5 | 31.91 ± 1.85 | rigid, good dimensional stability |
| RPUF-2 | 0.0312 ± 1.10 × 10−4 | 36.20 ± 1.90 | slight shrinkage, rigid |
| RPUF-3 | 0.0314 ± 8.00 × 10−4 | 34.45 ± 2.40 | slight shrinkage, rigid |
| RPUF-4 | 0.0298 ± 2.00 × 10−4 | 32.87 ± 2.95 | rigid, good dimensional stability |
| RPUF-5 | 0.0288 ± 7.00 × 10−5 | 29.91 ± 2.90 | slight shrinkage |
| RPUF-6 | 0.0279 ± 1.40 × 10−4 | 27.61 ± 1.00 | rigid, slight shrinkage |
| RPUF-7 | 0.0308 ± 3.40 × 10−5 | 26.10 ± 1.00 | some holes |
| RPUF-8 | 0.0329 ± 3.40 × 10−4 | 23.62 ± 2.00 | less rigid, some holes |
| RPUF-9 | 0.0297 ± 9.00 × 10−5 | 27.55 ± 2.80 | less rigid, good dimensional stability |
| RPUF- 10 | 0.0264 ± 1.10 × 10−4 | 26.12 ± 1.00 | rigid, some holes |
| RPUF-11 | 0.0281 ± 8.00 × 10−5 | 28.21 ± 1.00 | rigid, good dimensional stability |
| RPUF-12 | 0.0313 ± 2.80 × 10−5 | 27.33 ± 2.00 | rigid, slight shrinkage |
| RPUF-13 | 0.0348 ± 1.30 × 10−4 | 28.60 ± 2.60 | rigid |
| RPUF-14 | 0.0314 ± 2.40 × 10−4 | 28.93 ± 2.90 | less rigid |
| RPUF-15 | 0.0316 ± 2.10 × 10−4 | 30.40 ± 2.10 | rigid, slight shrinkage |
| RPUF-16 | 0.0306 ± 2.10 × 10−4 | 29.40 ± 1.95 | rigid, some holes |
| Source | Responses | |||||
|---|---|---|---|---|---|---|
| Thermal Conductivity | Density | |||||
| DF | SS | MS | DF | SS | MS | |
| Model | 5 | 0.00004928 | 9.856 × 10−6 | 5 | 115.040 | 23.008 |
| Error | 10 | 1.8657 × 10−6 | 1.8657 × 10−6 | 10 | 44.129 | 4.412 |
| Total | 15 | 0.00006794 | - | 15 | 159.170 | - |
| F ratio | 5.283 | 5.213 | ||||
| p-value | <0.0124 | <0.0130 | ||||
| R2 | 0.730 | 0.722 | ||||
| R2 adjusted | 0.588 | 0.584 | ||||
| Mean of response | 0.0304 | 29.30 | ||||
| Properties | RPUF-1 | RPUF-6 | RPUF-11 |
|---|---|---|---|
| * Formulation | 1.0 Surf/0.5 cat/7:2 BA | 3.0 Surf/0.5 cat/15:2 BA | 2.0 Surf/1.5 cat/15:2 BA |
| Density, kg/m3 | 31.9 ± 2.50 | 27.6 ± 1.30 | 28.2 ± 1.70 |
| Thermal conductivity, W/m·K | 0.0313 ± 2.00 × 10−4 | 0.0279 ± 1.2 × 10−4 | 0.0281 ± 3.8 × 10−4 |
| Cell size average, µm | 232 ± 82 | 266 ± 57 | 254 ± 69 |
| BET surface area, m2/g | 5.96 ± 1.20 | 4.30 ± 0.90 | 4.81 ± 0.75 |
| Pore volume, cm3/g | 9.70 × 10−3 | 9.62 × 10−3 | 4.90 × 10−3 |
| Run | Variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| BA, % | CAT, % | SURF, % | Predicted TC, W/m·K | Experimental TC, (Mean ± SD) | Predicted Density, kg/m3 | Experimental Density, kg/m3 (Mean ± SD) | |
| 1 | 8:2 | 0.80 | 1.5 | 0.0300 (0.0286–0.0312) * | 0.0290 ± 3.18 × 10−4 | 31.93 (28.9–34.7) * | 33.1 ± 1.00 |
| 2 | 5:2 | 1.0 | 1.5 | 0.0250 (0.0221–0.0 293) * | 0.0292 ± 4.38 × 10−4 | 32.90 (27.3–38.5) * | 28.5 ± 1.10 |
| Properties | Bio-Based RPUF | RPUF-Conv | RPUF-Commercial |
|---|---|---|---|
| Density, kg/m3 | 33.2 ± 1.00 | 44.5 ± 2.80 | 34.0 ± 1.00 |
| Thermal conductivity, W/m·K | 0.029 ± 3.18 × 10−4 | 0.034 ± 1.90 × 10−4 | 0.024 ± 1.65 × 10−4 |
| Cell size average, µm | 248 ± 88.0 | 344 ± 108 | 221 ± 69.0 |
| Closed cell content, % | 68.1 ± 1.50 | 67.9 ± 1.62 | 86.5 ± 0.90 |
| Compressive stress σ 10%, kPa | 127 ± 9.50 | 160 ± 13.0 | 209 ± 18.0 |
| Young’s modulus, kPa | 1954 ± 180 | 2449 ± 210 | 7645 ± 253 |
| Properties | Bio-Based RPUF | RPUF-Conv |
|---|---|---|
| Average HRR60seconds, kW/m2 | 20.9 ± 2.10 | 72.8 ± 6.20 |
| Average HRR300seconds, kW/m2 | 23.9 ± 4.55 | 29.3 ± 2.20 |
| MARHE, kW/m2 | 33.1 ± 3.60 | 110.8 ± 9.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, F.R.; Gama, N.V.; Evtuguin, D.V.; Amorim, C.O.; Amaral, V.S.; Pinto, P.C.O.R.; Barros-Timmons, A. Bio-Based Polyurethane Foams from Kraft Lignin with Improved Fire Resistance. Polymers 2023, 15, 1074. https://doi.org/10.3390/polym15051074
Vieira FR, Gama NV, Evtuguin DV, Amorim CO, Amaral VS, Pinto PCOR, Barros-Timmons A. Bio-Based Polyurethane Foams from Kraft Lignin with Improved Fire Resistance. Polymers. 2023; 15(5):1074. https://doi.org/10.3390/polym15051074
Chicago/Turabian StyleVieira, Fernanda R., Nuno V. Gama, Dmitry V. Evtuguin, Carlos O. Amorim, Vitor S. Amaral, Paula C. O. R. Pinto, and Ana Barros-Timmons. 2023. "Bio-Based Polyurethane Foams from Kraft Lignin with Improved Fire Resistance" Polymers 15, no. 5: 1074. https://doi.org/10.3390/polym15051074
APA StyleVieira, F. R., Gama, N. V., Evtuguin, D. V., Amorim, C. O., Amaral, V. S., Pinto, P. C. O. R., & Barros-Timmons, A. (2023). Bio-Based Polyurethane Foams from Kraft Lignin with Improved Fire Resistance. Polymers, 15(5), 1074. https://doi.org/10.3390/polym15051074










