Comparison of Solidification Characteristics between Polymer-Cured and Bio-Cured Fly Ash in the Laboratory
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
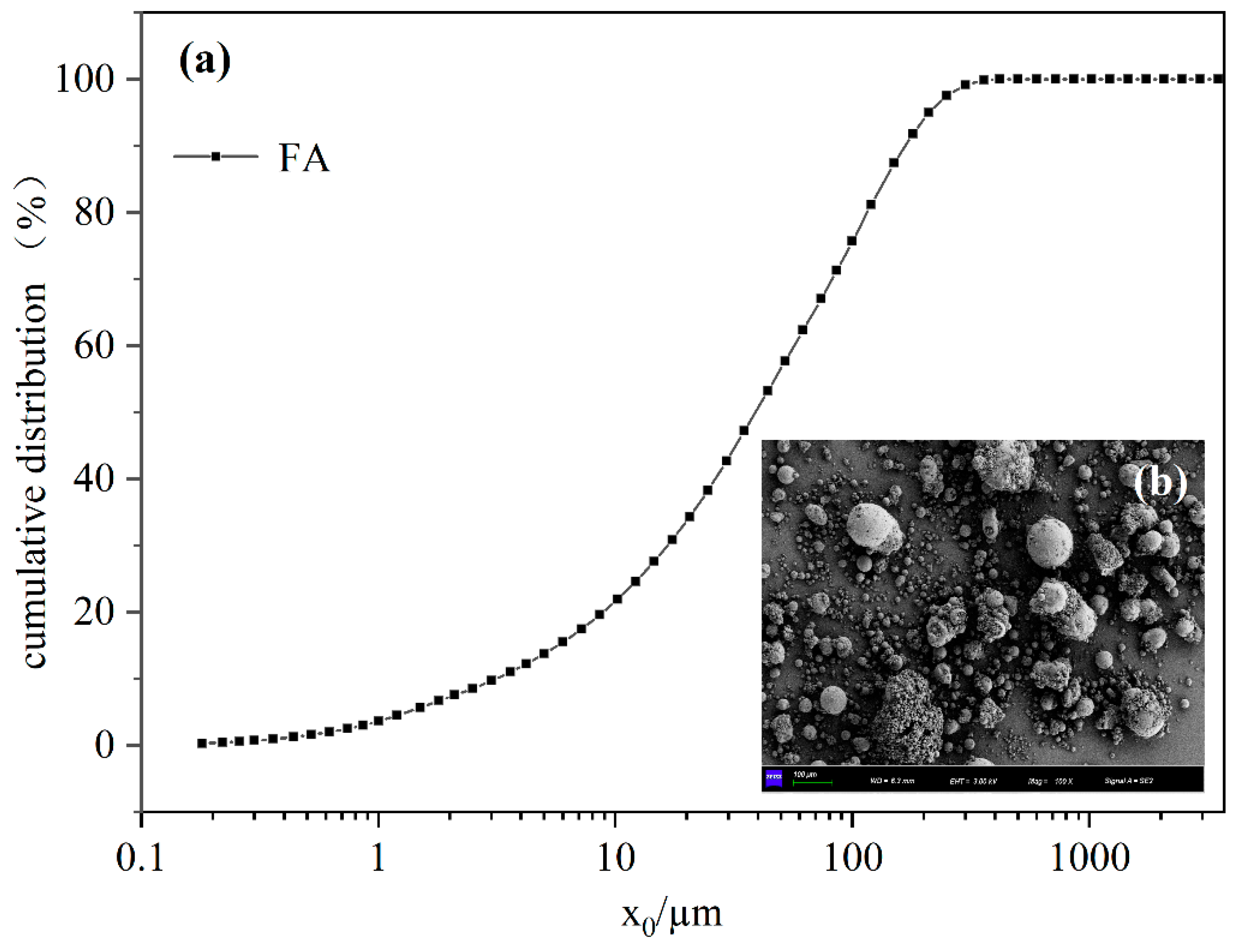
2.1.1. FA
2.1.2. Chemical Reagents
2.1.3. Urease
2.2. Sample Preparation
2.2.1. Preparation
2.2.2. Solution
2.2.3. Maintenance
2.3. Curing Performance Test
2.3.1. UCS
2.3.2. Screening Test
2.3.3. Wind Tunnel Test
2.3.4. Water-Stability Test
2.3.5. Freeze-Thaw Test
2.3.6. Particle Size Test
2.3.7. SEM
2.3.8. X-ray Diffraction (XRD) Analysis
2.3.9. Chemical Component Analysis
2.4. Statistical Analysis
3. Results and Discussion
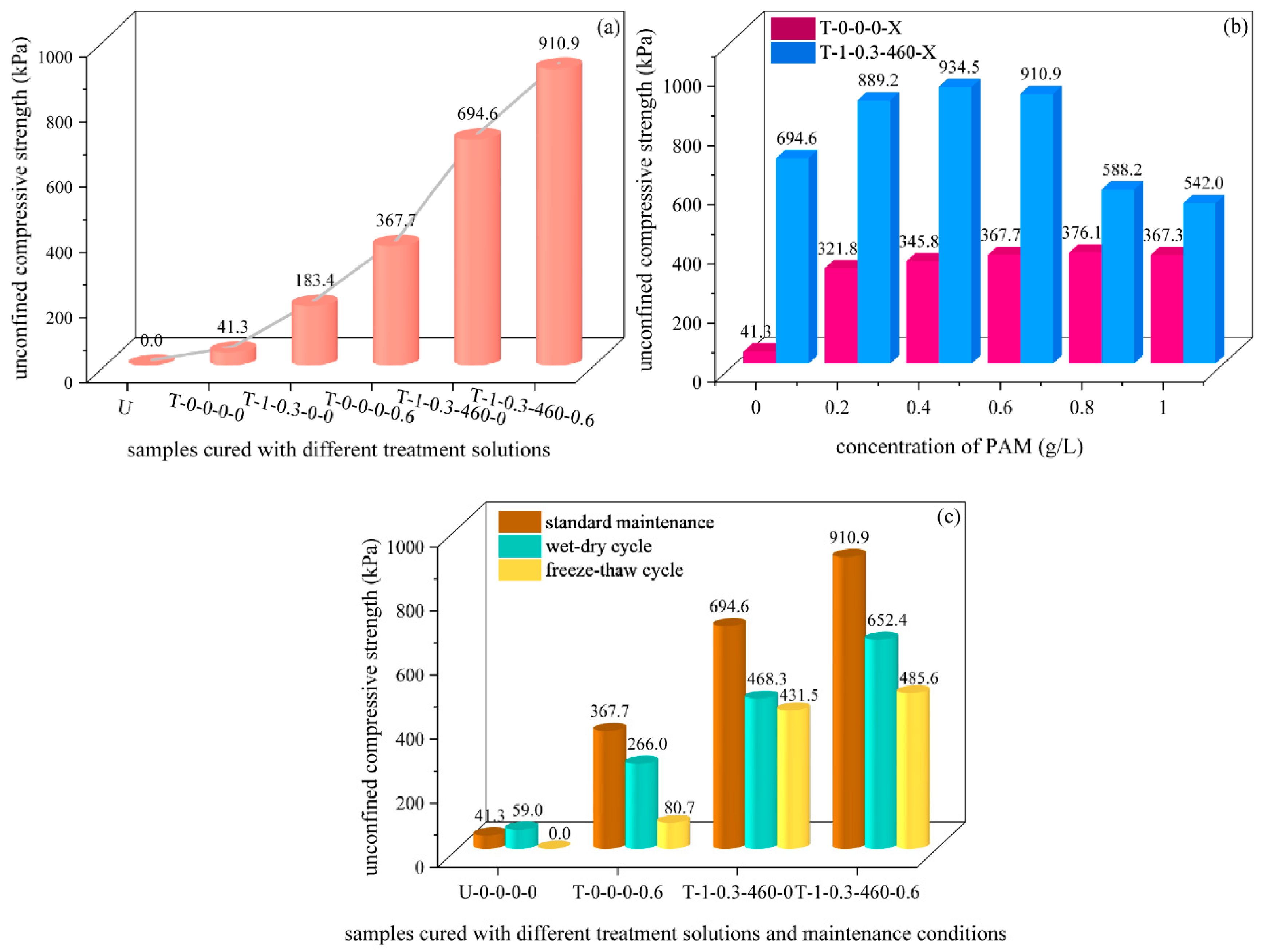
3.1. Results of UCS
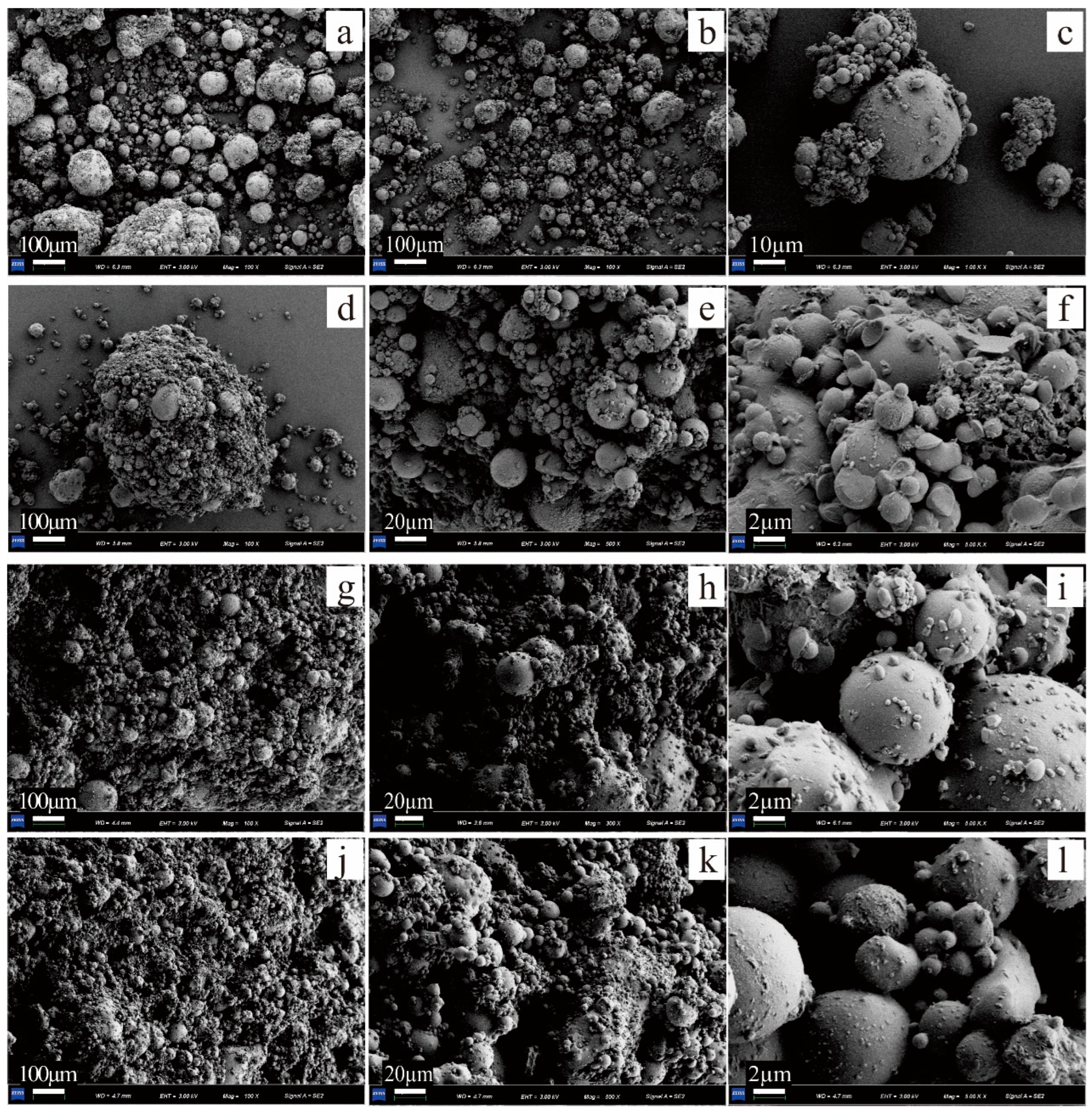
3.2. Analysis of Agglomerated Particles
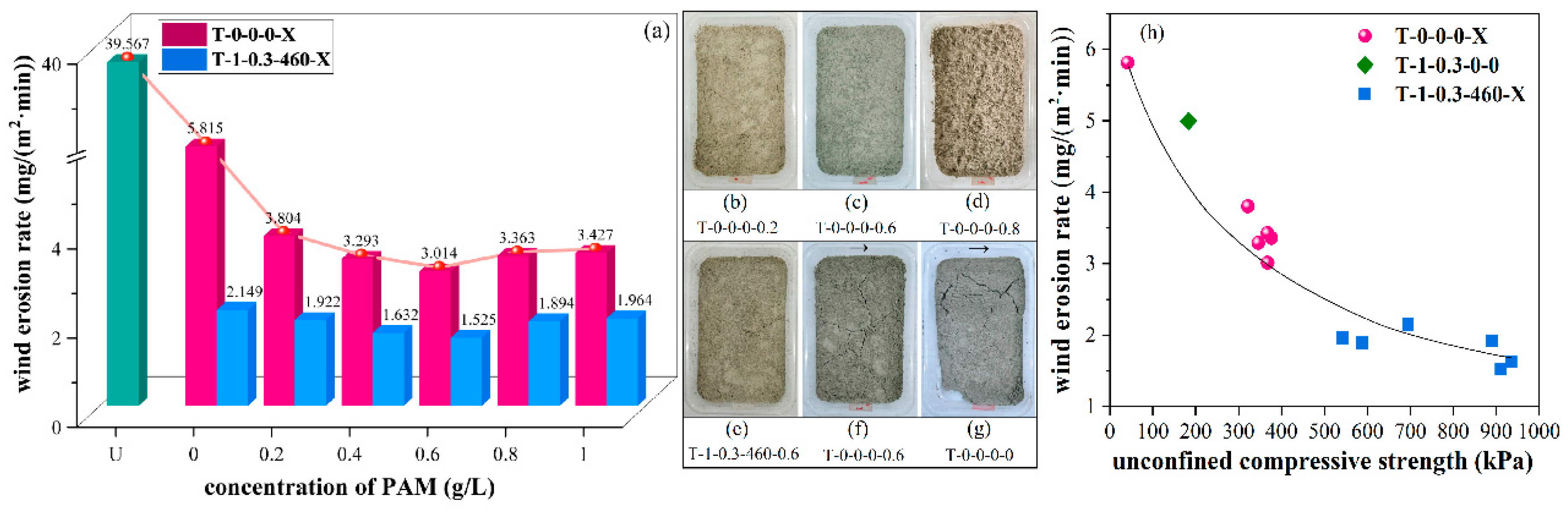
3.3. Analysis of Wind Erosion Resistance
3.4. Microscopic Analysis
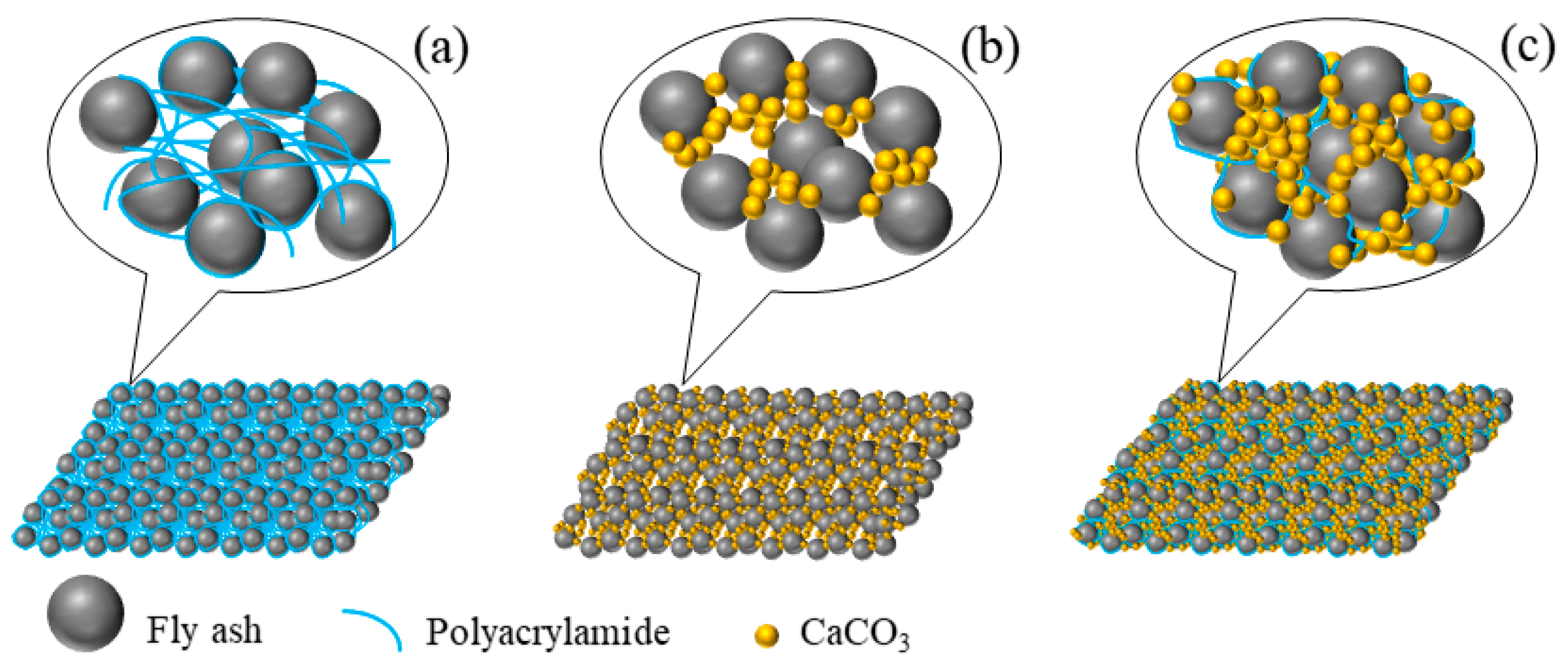
3.5. Analysis of Curing Mechanism
4. Conclusions
- (1)
- Compared with the deionized-treated sample, the curing effect of the sample treated by salt, PAM, EICP, and PAM-EICP composite solution was enhanced sequentially.
- (2)
- The spatial network structure formed by PAM improved the mechanics, wind erosion resistance, and water stability of the FA samples. When the concentration of PAM ranged from 0.6 g/L to 0.8 g/L, the UCS and wind erosion resistance of the sample appeared to be the optimal value. However, it reduced when the concentration of PAM was increased continuously.
- (3)
- The CaCO3 crystals enhanced the cohesion of the sample by cementing FA particles in the EICP process, which also showed good freeze-thaw stability.
- (4)
- The addition of PAM provided more nucleation sites for the formation of CaCO3 in the EICP process and increased the particle size of FA agglomerates. SEM revealed the stable and dense spatial structure formed by the cementing action of PAM and mineral crystals. At the same time, the uneven mineralization reaction and lots of small crystals were generated when the concentration of the PAM solution was too high.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cengizler, H.; Koç, M.; Şan, O. Production of ceramic glass foam of low thermal conductivity by a simple method entirely from fly ash. Ceram. Int. 2021, 47, 28460–28470. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mater. Res. Technol. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process Eng. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X.; Li, X.; Zhang, J. Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad. Dev. 2020, 31, 1317–1324. [Google Scholar] [CrossRef]
- Mahamaya, M.; Das, S.K.; Reddy, K.R.; Jain, S. Interaction of biopolymer with dispersive geomaterial and its characterization: An eco-friendly approach for erosion control. J. Clean. Prod. 2021, 312, 127778. [Google Scholar] [CrossRef]
- Feizi, Z.; Ayoubi, S.; Mosaddeghi, M.R.; Besalatpour, A.A.; Zeraatpisheh, M.; Rodrigo-Comino, J. A wind tunnel experiment to investigate the effect of polyvinyl acetate, biochar, and bentonite on wind erosion control. Arch. Agron. Soil Sci. 2019, 65, 1049–1062. [Google Scholar] [CrossRef]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Abd Majid, M.Z.; Tahir, M.M.; Mohamad, E.T. Improvement of Problematic Soils with Biopolymer An Environmentally Friendly Soil Stabilizer. J. Mater. Civ. Eng. 2017, 29, 04016204. [Google Scholar] [CrossRef]
- Mamedov, A.I.; Tsunekawa, A.; Haregeweyn, N.; Tsubo, M.; Fujimaki, H.; Kawai, T.; Kebede, B.; Mulualem, T.; Abebe, G.; Wubet, A.; et al. Soil Structure Stability under Different Land Uses in Association with Polyacrylamide Effects. Sustainability 2021, 13, 1407. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 92, p. 75. ISBN 978-0-12-373686-4. [Google Scholar]
- Kim, S.; Palomino, A.M. Polyacrylamide-treated kaolin: A fabric study. Appl. Clay Sci. 2009, 45, 270–279. [Google Scholar] [CrossRef]
- Bishop, M.D. Strength and Deformation of “Tunable” Clay-Polymer Composites. Appl. Clay Sci. 2013, 101, 265–271. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; McLaughlin, R.A.; Levy, G.J. Depositional seals in polyacrylamide-amended soils of varying clay mineralogy and texture. J. Soils Sediments 2010, 10, 494–504. [Google Scholar] [CrossRef]
- Sujatha, E.R.; Saisree, S. Geotechnical behaviour of guar gum-treated soil. Soils Found. 2019, 59, 2155–2166. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
- Su, F.; Yang, Y.; Qi, Y.; Zhang, H. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]
- Pan, L.; Li, Q.; Zhou, Y.; Song, N.; Yu, L.; Wang, X.; Xiong, K.; Yap, L.; Huo, J. Effects of different calcium sources on the mineralization and sand curing of CaCO3 by carbonic anhydrase-producing bacteria. RSC Adv. 2019, 9, 40827–40834. [Google Scholar] [CrossRef]
- Zhao, Z.; Hamdan, N.; Shen, L.; Nan, H.; Almajed, A.; Kavazanjian, E.; He, X. Biomimetic Hydrogel Composites for Soil Stabilization and Contaminant Mitigation. Environ. Sci. Technol. 2016, 50, 12401–12410. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y. Investigating the Factors Affecting the Properties of Coral Sand Treated with Microbially Induced Calcite Precipitation. Adv. Civ. Eng. 2018, 2018, 9590653. [Google Scholar] [CrossRef]
- Gao, Y.; He, J.; Tang, X.; Chu, J. Calcium carbonate precipitation catalyzed by soybean urease as an improvement method for fine-grained soil. Soils Found. 2019, 59, 1631–1637. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Ogbonnaya, U.; Wen, K.; Tian, A.; Amini, F. Influence of Fiber Addition on Mechanical Properties of MICP-Treated Sand. J. Mater. Civ. Eng. 2016, 28, 04015166. [Google Scholar] [CrossRef]
- Gao, Y.F.; Meng, H.; He, J.; Qi, Y.S.; Hang, L. Field trial on use of soybean crude extract for carbonate precipitation and wind erosion control of sandy soil. J. Cent. South Univ. 2020, 27, 3320–3333. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. The application of bio-cementation for improvement in collapsibility of loess. Int. J. Environ. Sci. Technol. 2021, 18, 2607–2618. [Google Scholar] [CrossRef]
- Li, G.; Wang, F.; Ma, W.; Fortier, R.; Mu, Y.; Mao, Y.; Hou, X. Variations in strength and deformation of compacted loess exposed to wetting-drying and freeze-thaw cycles. Cold Reg. Sci. Technol. 2018, 151, 159–167. [Google Scholar] [CrossRef]
- Azamian Jazi, M.; Ramezani SA, A.; Haddadi, S.A.; Ghaderi, S.; Azamian, F. In situ emulsion polymerization and characterization of PVAc nanocomposites including colloidal silica nanoparticles for wood specimens bonding. J. Appl. Polym. Sci. 2020, 137, 48570. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nusslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R.; Wang, H.; Xia, J. Surface rainfall erosion resistance and freeze-thaw durability of bio-cemented and polymer-modified loess slopes. J. Environ. Manag. 2022, 301, 113883. [Google Scholar] [CrossRef]
- Qureshi, M.U.; Chang, I.; Al-Sadarani, K. Strength and durability characteristics of biopolymer-treated desert sand. Geomech. Eng. 2017, 12, 785–801. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of Key Environmental Conditions on Microbially Induced Cementation for Soil Stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- Naeimi, M.; Chu, J. Comparison of conventional and bio-treated methods as dust suppressants. Environ. Sci. Pollut. Res. 2017, 24, 23341–23350. [Google Scholar] [CrossRef]
- Lo, C.Y.; Tirkolaei, H.K.; Hua, M.; De Rosa, I.M.; Carlson, L.; Kavazanjian Jr, E.; He, X. Durable and ductile double-network material for dust control. Geoderma 2020, 361, 114090. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Nafisi, A.; Safavizadeh, S.; Montoya, B.M. Influence of Microbe and Enzyme-Induced Treatments on Cemented Sand Shear Response. J. Geotech. Geoenviron. Eng. 2019, 145, 06019008. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Valdes, J.R.; Cortes, D.D. Heat-Induced Bonding of Sands. In Geo-Congress 2014; American Society of Civil Engineers (ASCE): Reston, VA, USA, 2014; pp. 3721–3733. [Google Scholar]
- Zhang, W.; Ju, Y.; Zong, Y.; Qi, H.; Zhao, K. In Situ Real-Time Study on Dynamics of Microbially Induced Calcium Carbonate Precipitation at a Single-Cell Level. Environ. Sci. Technol. 2018, 52, 9266–9276. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | MnO | TiO2 | P2O5 | Loss on Ignition | FeO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 56.32 | 21.67 | 4.92 | 2.15 | 8.97 | 1.41 | 1.64 | 0.053 | 1.02 | 0.09 | 1.73 | 1.95 |
| No. | Composition | Urea (mol/L) | CaCl2 (mol/L) | Urease (Activity: u) | PAM (g/L) |
|---|---|---|---|---|---|
| 1 | U | 0 | 0 | 0 | 0 |
| 2 | T-0-0-0-0 | treated with deionized water | |||
| 3 | T-1-0.3-0-0 | 1 | 0.3 | 0 | 0 |
| 4 | T-0-0-0-0.2 | 0 | 0 | 0 | 0.2 |
| 5 | T-0-0-0-0.4 | 0 | 0 | 0 | 0.4 |
| 6 | T-0-0-0-0.6 | 0 | 0 | 0 | 0.6 |
| 7 | T-0-0-0-0.8 | 0 | 0 | 0 | 0.8 |
| 8 | T-0-0-0-1 | 0 | 0 | 0 | 1.0 |
| 9 | T-1-0.3-460-0 | 1 | 0.3 | 460 | 0 |
| 10 | T-1-0.3-460-0.2 | 1 | 0.3 | 460 | 0.2 |
| 11 | T-1-0.3-460-0.4 | 1 | 0.3 | 460 | 0.4 |
| 12 | T-1-0.3-460-0.6 | 1 | 0.3 | 460 | 0.6 |
| 13 | T-1-0.3-460-0.8 | 1 | 0.3 | 460 | 0.8 |
| 14 | T-1-0.3-460-1 | 1 | 0.3 | 460 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Liu, Y.; Chen, J. Comparison of Solidification Characteristics between Polymer-Cured and Bio-Cured Fly Ash in the Laboratory. Polymers 2023, 15, 1107. https://doi.org/10.3390/polym15051107
Jia Y, Liu Y, Chen J. Comparison of Solidification Characteristics between Polymer-Cured and Bio-Cured Fly Ash in the Laboratory. Polymers. 2023; 15(5):1107. https://doi.org/10.3390/polym15051107
Chicago/Turabian StyleJia, Yinggang, Yuhan Liu, and Jian Chen. 2023. "Comparison of Solidification Characteristics between Polymer-Cured and Bio-Cured Fly Ash in the Laboratory" Polymers 15, no. 5: 1107. https://doi.org/10.3390/polym15051107
APA StyleJia, Y., Liu, Y., & Chen, J. (2023). Comparison of Solidification Characteristics between Polymer-Cured and Bio-Cured Fly Ash in the Laboratory. Polymers, 15(5), 1107. https://doi.org/10.3390/polym15051107






