Formation of a Conducting Polymer by Different Electrochemical Techniques and Their Effect on Obtaining an Immunosensor for Immunoglobulin G
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electrochemical and Spectroscopic Measurements
- Cyclic voltammetry (CV) between −0.4 and +0.8 V at performed at different scan rates.
- Square wave voltammetry (SWV) The optimized parameters were: potential step 5 mV, amplitude 25 mV, and frequency 10 Hz, and the potential range was between −0.2 V and +0.65 V, for the initial and final potential, respectively.
- Electrochemical impedance spectroscopy (EIS) measurements were carried out to formal potential, E0’, which was determined from the average of the anodic and cathodic peak potentials, over a frequency range of 100 kHz to 0.01 Hz, at 8 step/decade, using a perturbation of 10 mV.
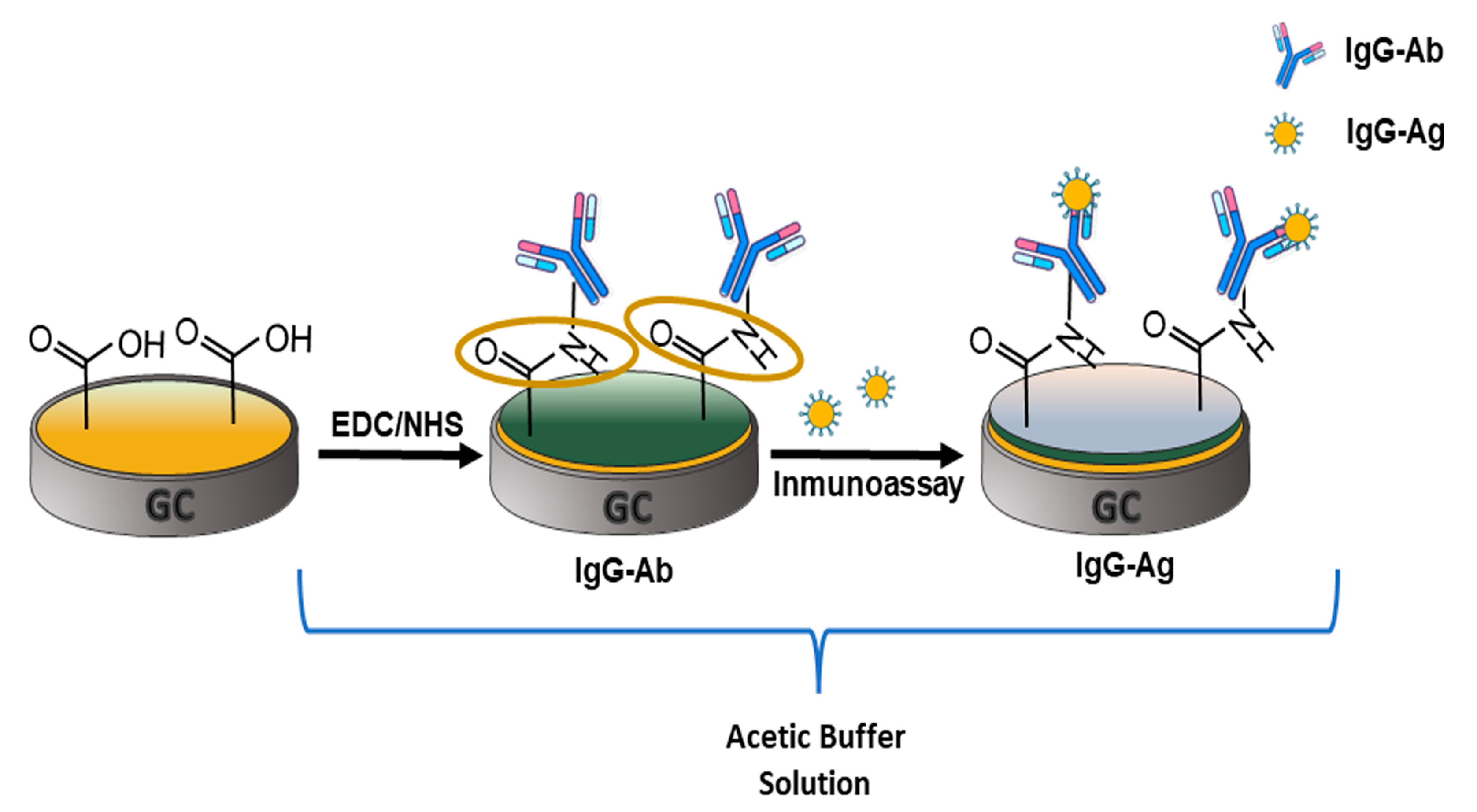
2.3. Obtention of Immunosensor Based on 6-PICA
2.4. Analytical Procedure
2.5. Optimization of Anti-IgG Antibody and IgG Antigen Incubation
2.6. Human Serum Analysis
3. Results
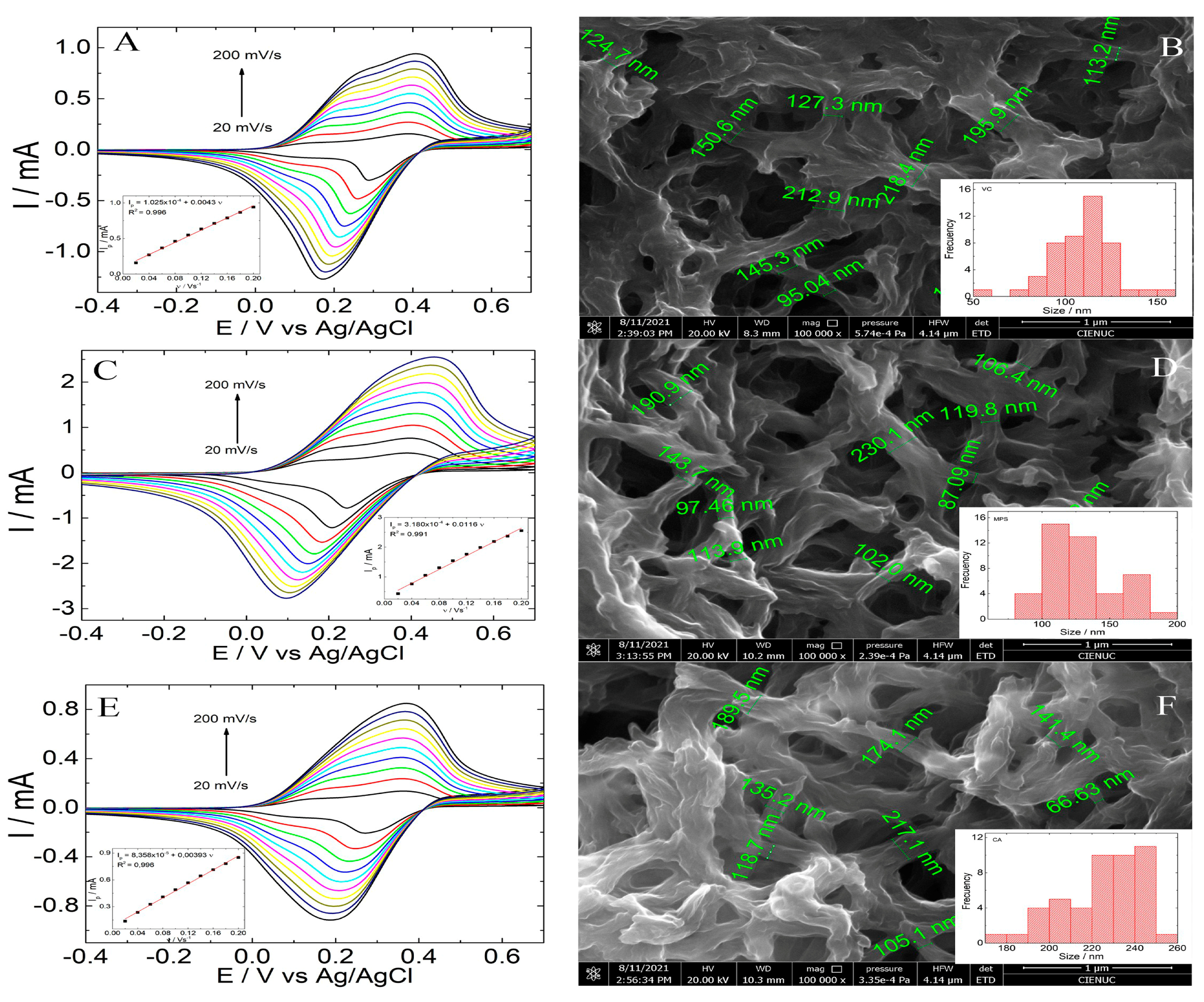
3.1. Morphological and Electrochemical Characterization of 6-PICA Platform
3.2. Label-Free Electrochemical Immunosensor Fabrication
3.3. Optimization of Method Label-Free Electrochemical Immunosensor
3.4. Quantitative Detection of IgG-Ag
3.5. Selectivity, Stability, and Affinity Constant of the Immunosensor and Serum Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, R.; Pan, Y.; Li, Z.; Qin, Z.; Rini, J.M.; Liu, X. SPEEDS: A portable serological testing platform for rapid electrochemical detection of SARS-CoV-2 antibodies. Biosens. Bioelectron. 2022, 197, 113762. [Google Scholar] [CrossRef] [PubMed]
- Tollånes, M.C.; Kran, A.-M.B.; Abildsnes, E.; Jenum, P.A.; Breivik, A.C.; Sandberg, S. Evaluation of eleven rapid tests for detection of antibodies against SARS-CoV-2. Clin. Chem. Lab. Med. 2020, 58, 1595–1600. [Google Scholar] [CrossRef]
- Chuchalin, A.G. Disease associated with immunoglobulin G. Terapevticheskii Arkhiv. 2018, 90, 4–9. [Google Scholar] [CrossRef]
- Janwan, P.; Sadaow, L.; Rodpai, R.; Yamasaki, H.; Luvira, V.; Sukeepaisarnjaroen, W.; Kitkhuandee, A.; Paonariang, K.; Sanpool, O.; Boonroumkaew, P.; et al. Evaluation of total immunoglobulin G and subclass antibodies in an enzyme-linked immunosorbent assay for serodiagnosis of human amebic liver abscess. PeerJ 2022, 10, e14085. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.-Q.; Deng, H.-H.; He, S.-B.; Peng, H.-P.; Lin, Z.; Xia, X.-H.; Chen, W. Immunoglobulin G-Encapsulated Gold Nanoclusters as Fluorescent Tags for Dot-Blot Immunoassays. ACS Appl. Mater. Interfaces 2019, 11, 31729–31734. [Google Scholar] [CrossRef]
- Morittu, V.M.; Lopreiato, V.; Ceniti, C.; Spina, A.A.; Minuti, A.; Trevisi, E.; Britti, D.; Trimboli, F. Technical note: Capillary electrophoresis as a rapid test for the quantification of immunoglobulin G in serum of newborn lambs. J. Dairy Sci. 2020, 103, 6583–6587. [Google Scholar] [CrossRef]
- Niu, K.; Li, Y.; Bai, R.; Qu, Y.; Song, Y. Anion-exchange reactions: Facile and general access to sensitive photoelectrochemical platforms for biomarker immunosensing. J. Mater. Chem. B 2017, 5, 5145–5151. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Wang, J.; Zeng, C.; Wang, M.; Qiabc, W.; He, Z. AuNP array coated substrate for sensitive and homogeneous SERS-immunoassay detection of human immunoglobulin G. RSC Adv. 2021, 11, 22744–22750. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, A.; Song, J.; Wang, N.; Lam, P.; Li, Y.; Law, H.K.-w.; Yan, F. Ultrafast, sensitive, and portable detection of COVID-19 IgG using flexible organic electrochemical transistors. Sci. Adv. 2021, 7, eabg8387. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-P.; Wang, C.-C.; Hu, P.; Wu, Z.-S.; Shen, G.-L.; Yu, R.-Q. A label-free electrochemical immunoassay for IgG detection based on the electron transfer. Talanta 2010, 83, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Goode, J.; Dillon, G.; Millner, P.A. The development and optimisation of nanobody based electrochemical immunosensors for IgG. Sens. Actuators B Chem. 2016, 234, 478–484. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Kondzior, M.; Grabowska, I. Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors 2020, 20, 2014. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection. Chemosensors 2021, 9, 85. [Google Scholar] [CrossRef]
- Garyfallou, G.-Z.; Ketebu, O.; Sahin, S.; Mukaetova-Ladinska, E.B.; Catt, M.; Yu, E.H. Electrochemical Detection of Plasma Immunoglobulin as a Biomarker for Alzheimer’s Disease. Sensors 2017, 17, 2464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Peng, Y.-R.; Lin, H.-Y.; Hsueh, T.-Y.; Lai, C.-S.; Hua, M.-Y. Preparation and Characterization of Au/NiPc/Anti-p53/BSA Electrode for Application as a p53 Antigen Sensor. Chemosensors 2021, 9, 17. [Google Scholar] [CrossRef]
- Gao, S.; Guisan, J.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms—A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Baniukevic, J.; Kirlyte, J.; Ramanavicius, A.; Ramanaviciene, A. Application of oriented and random antibody immobilization methods inimmunosensor design. Sens. Actuators B Chem 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Pâslaru, E.; Baican, M.C.; Hitruc, E.G.; Nistor, M.T.; Poncin-Epaillard, F.; Vasile, C. Immunoglobulin G immobilization on PVDF surface. Colloids Surf. B Biointerfaces 2014, 115, 139–149. [Google Scholar] [CrossRef]
- Puertas, S.; Villa, M.G.; Mendoza, E.; Jimenez-Jorquera, C.; Fernandez-Sanchez, C.; Grazu, V. Improving immunosensor performance through oriented immobilization of antibodies on carbon nanotube composite surfaces. Biosens. Bioelectron. 2013, 43, 274–280. [Google Scholar] [CrossRef]
- Martínez-Rojas, F.; Diculescu, V.C.; Armijo, F. Electrochemical Immunosensing Platform for the Determination of the 20S Proteasome Using an Aminophenylboronic/Poly-indole-6-carboxylic Acid-Modified Electrode. ACS Appl. Bio Mater. 2020, 3, 4941–4948. [Google Scholar] [CrossRef]
- Martínez-Rojas, F.; Castañeda, E.; Armijo, F. Conducting polymer applied in a label-free electrochemical immunosensor for the detection prostate-specific antigen using its redox response as an analytical signal. J. Electroanal. Chem. 2021, 880, 114877. [Google Scholar] [CrossRef]
- Song, Z.; Li, R.; Yang, X.; Zhang, Z.; Luo, X. Functional DNA-peptide conjugates with enhanced antifouling capabilities for electrochemical detection of proteins in complex human serum. Sens. Actuators B Chem. 2022, 367, 132110. [Google Scholar] [CrossRef]
- Li, Y.; Han, R.; Chen, M.; Zhang, L.; Wang, G.; Luo, X. Bovine Serum Albumin-Cross-Linked Polyaniline Nanowires for Ultralow Fouling and Highly Sensitive Electrochemical Protein Quantification in Human Serum Samples. Anal. Chem. 2021, 93, 4326–4333. [Google Scholar] [CrossRef]
- Liu, N.; Hui, N.; Davis, J.J.; Luo, X. Low fouling protein detection in complex biological media supported by a designed multifunctional peptide. ACS Sens. 2018, 3, 1210–1216. [Google Scholar] [CrossRef]
- Chen, M.; Song, Z.; Han, R.; Li, Y.; Luo, X. Low fouling electrochemical biosensors based on designed Y-shaped peptides with antifouling and recognizing branches for the detection of IgG in human serum. Biosens. Bioelectron. 2021, 178, 113016. [Google Scholar] [CrossRef]
- Cui, M.; Gong, Y.; Du, M.; Wang, K.; Li, T.; Zhu, X.; Wang, S.; Luo, X. An antifouling electrochemical biosensor based on a protein imprinted hydrogel for human immunoglobulin G recognition in complex biological media. Sens. Actuators B Chem. 2021, 337, 129820. [Google Scholar] [CrossRef]
- Liang, A.; Tang, S.; Liu, M.; Yi, Y.; Xie, B.; Huo, H.; Luo, A. A molecularly imprinted electrochemical sensor with tunable electro synthesized Cu-MOFs modification for ultrasensitive detection of human IgG. Bioelectrochemistry 2022, 146, 108154. [Google Scholar] [CrossRef]
- Liang, A.A.; Hou, B.H.; Tang, C.S.; Sun, D.L.; Luo, E.A. An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of Human IgG. Bioelectrochemistry 2021, 137, 107671. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.-Z.; Zheng, G.; Zhang, H.; Zhou, M.; Li, S.; Kong, Y. Dual-template molecularly imprinted electrochemical biosensor for IgG-IgM combined assay based on a dual-signal strategy. Bioelectrochemistry 2022, 148, 108267. [Google Scholar] [CrossRef]
- Aguirre, J.; Daille, L.; Fischer, D.A.; Galarce, C.; Pizarro, G.; Vargas, I.; Walczak, M.; de la Iglesia, R.; Armijo, F. Study of poly(3,4-ethylendioxythiphene) as a coating for mitigation of biocorrosion of AISI 304 stainless steel in natural seawater. Prog. Org. Coat. 2017, 113, 175–184. [Google Scholar] [CrossRef]
- Salgado, R.; del Valle, M.A.; Duran, B.G.; Pardo, M.A.; Armijo, F. Optimization of dopamine determination based on nanowires PEDOT/polydopamine hybrid film modified electrode. J. Appl. Electrochem. 2014, 44, 1289–1294. [Google Scholar] [CrossRef]
- Hernández, L.A.; del Valle, M.A.; Armijo, F. Electrosynthesis and characterization of nanostructured polyquinone for use in detection and quantification of naturally occurring dsDNA. Biosens. Bioelectron. 2016, 79, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M.A.; Gacitua, M.; Diaz, F.R.; Armijo, F.; Soto, J.P. Electro-synthesis and characterization of polythiophene nano-wires/platinum nano-particles composite electrodes. Study of formic acid electro-catalytic oxidation. Electrochim. Acta 2012, 71, 277–282. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Mo, D.; Hou, J.; Xu, J. Effect of substituent position on electrodeposition, morphology, and capacitance performance of polyindole bearing a carboxylic group. Electrochim. Acta 2015, 176, 1302–1312. [Google Scholar] [CrossRef]
- Nie, G.; Li, C.; Zhang, L.; Wang, L. Fabrication of a simple and sensitive QDs-based electrochemiluminescence immunosensor using a nanostructured composite material for the detection of tumor markers alpha-fetoprotein. Mater. Chem. B 2014, 2, 8321–8328. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Nie, G. Electrochemical immunosensor for the carcinoembryonic antigen based on a nanocomposite consisting of reduced graphene oxide, gold nanoparticles and poly(indole-6-carboxylic acid). Microchim. Acta 2016, 183, 2925–2932. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Peng, C.; Zhang, W. Construction of self-signal DNA electrochemical biosensor employing WS2 nanosheets combined with PIn6COOH. RSC Adv. 2019, 9, 9613–9619. [Google Scholar] [CrossRef]
- Yang, J.; Yin, X.; Zhang, W. Electrochemical determination of PIK3CA gene associated with breast cancer based on molybdenum disulfide nanosheet-supported poly(indole-6-carboxylic acid). Anal. Methods 2019, 11, 157–162. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Q.; Lu, Y.; Zhang, B.; Nie, G. Ultrasensitive “signal-on” electrochemiluminescence immunosensor for prostate-specific antigen detection based on novel nanoprobe and poly(indole-6-carboxylic acid)/flower-like au nanocomposite. Sens. Actuat. B Chem. 2020, 303, 127246. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, X.; Tian, Y.; Guo, Q.; Li, C.; Nie, G. An electrochemiluminescence aptasensor for the ultrasensitive detection of aflatoxin B1 based on gold nanorods/graphene quantum dots-modified poly(indole-6-carboxylic acid)/flower-gold nanocomposite. Microchem. J. 2020, 157, 104959. [Google Scholar] [CrossRef]
- Pan, D.; Zhou, Q.; Rong, S.; Zhang, G.; Zhang, Y.; Liu, F.; Li, M.; Chang, D.; Pan, H. Electrochemical immunoassay for the biomarker 8-hydroxy-2′-deoxyguanosine using a glassy carbon electrode modified with chitosan and poly(indole-5-carboxylic acid). Microchim. Acta 2016, 183, 361–368. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.-W.; Wan, G.-C.; Jiang, J.-H.; Wang, N.; Liu, Z.-Y.; Chang, D.; Pan, H.-Z. A Label-Free Electrochemical Biosensor Based on a Reduced Graphene Oxide and Indole-5-Carboxylic Acid Nanocomposite for the Detection of Klebsiella pneumoniae. J. AOAC Int. 2017, 100, 548–552. [Google Scholar] [CrossRef]
- Jia, H.; Yang, T.; Xu, Q.; Xu, J.; Lu, L.; Yu, Y.; Li, P. Facile construction of poly (indole-5-carboxylic acid)@poly (3, 4-ethylenedioxythiophene) label-free immunosensing platform for sensitive detection of prostate specific antigen. J. Electroanal. Chem. 2019, 839, 202–209. [Google Scholar] [CrossRef]
- Yanga, T.; Rena, X.; Yanga, M.; Li, X.; He, K.; Raoa, A.; Wana, Y.; Yanga, H.; Wanga, S.; Luo, Z. A highly sensitive label-free electrochemical immunosensor based on poly(indole-5-carboxylicacid) with ultra-high redox stability. Biosens. Bioelectron. 2019, 141, 111406. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, B.; Tian, Y.; Guo, Q.; Nie, G. Ultrasensitive ratiometric photoelectrochemical immunoassay for prostate specific antigen based on nanoscale heterojunction. Sens. Actuators B Chem. 2021, 326, 128994. [Google Scholar] [CrossRef]
- Gan, X.; Han, D.; Wang, J.; Liu, P.; Li, X.; Zheng, Q.; Yan, Y. A highly sensitive electrochemiluminescence immunosensor for h-FABP determination based on self-enhanced luminophore coupled with ultrathin 2D nickel metal-organic framework nanosheets. Biosens. Bioelectron. 2021, 171, 112735. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, S.; Xu, J.; Farooq, U.; Yang, T.; Chen, W.; Zhou, L.; Gao, M.; Wang, S. Poly(indole-5-carboxylic acid)/reduced graphene oxide/gold nanoparticles/phage-based electrochemical biosensor for highly specific detection of Yersinia pseudotuberculosis. Microchim. Acta 2021, 188, 107. [Google Scholar] [CrossRef] [PubMed]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Suys, O.; Derenne, A.; Goormaghtigh, E. ATR-FTIR Biosensors for Antibody Detection and Analysis. Int. J. Mol. Sci. 2022, 23, 11895. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.W.; Rowland-Jones, R.C.; Farys, M.; Tran, R.; Kazarian, S.G.; Byrne, B. Insight into purification of monoclonal antibodies in industrial columns via studies of Protein A binding capacity by in situ ATR-FTIR spectroscopy. Analyst 2021, 146, 5177–5185. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, R.; Jarczewska, M.; Drozd, M.; Zasada, A.A.; Malinowska, E. Studies on the development of electrochemical Immunosensor for detection of diphtheria toxoid. J. Electrochem. Soc. 2019, 166, B472–B481. [Google Scholar] [CrossRef]




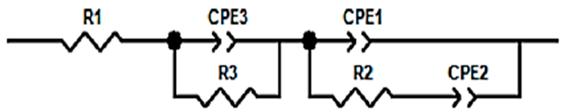 | |||
| Electrode | 6-PICA | 6-PICA/IgG-Ab | 6-PICA/IgG-Ab/IgG-Ag |
| R1 (Ω) | 49 | 48 | 46 |
| CPE1-T (mF) | 7.11 | 5.87 | 4.41 |
| CPE1-P | 0.97 | 0.69 | 0.55 |
| R2 (Ω) | 28 | 49 | 225 |
| CPE2-T (mF) | 7.22 | 0.0347 | 0.0288 |
| CPE2-P | 0.95 | 0.80 | 0.80 |
| R3 (Ω) | - | 38 | 64 |
| CPE3-T (mF) | - | 4.78 | 3.59 |
| CPE3-P | - | 0.92 | 0.95 |
| χ2 | 3.33 × 10−4 | 3.20 × 10−4 | 4.21 × 10−4 |
| Electrode | Analytical Probe | Electrochemical Method | Lineal Range ng·mL−1 | LOD ng·mL−1 | Ref. |
|---|---|---|---|---|---|
| DP/AuNPs/PEDOT/GCE | [Fe(CN)6]3−/4− | DPV | 0.1–10,000 | 0.037 | [23] |
| Pep/BSA/PANI-NW/GCE | Direct detection | DPV | 1.0–10,000 | 0.27 | [24] |
| Pep/PANI/GCE | Direct detection | DPV | 1.0–10,000 | 0.26 | [25] |
| Y-peptide/AuNPs/PEDOT/GCE | [Fe(CN)6]3−/4− | DPV | 0.1–10,000 | 0.032 | [26] |
| NIH/GCE | [Fe(CN)6]3−/4− | EIS | 0.5–200 | 0.03 | [27] |
| MIP/CS/Cu-MOF/GCE | [Fe(CN)6]3− | DPV | 0.01–10 | 0.003 | [28] |
| MoS2@N-GQDs-IL MIP Sensors | [Fe(CN)6]3− | DPV | 0.1–50 | 0.02 | [29] |
| DTMI electrochemical biosensor | Direct detection | DPV | 0.05–500 | 0.0288 | [30] |
| IgG-Ab/6-PICA/GCE | Direct detection | SWV | 2.0–16.0 | 0.8 | This work |
| Sample | Added Spiked (ng·mL−1) | Found (ng·mL−1) | Recovery % |
|---|---|---|---|
| 1 | 6 | 6.55 | 109 |
| 2 | 6 | 6.22 | 104 |
| 3 | 6 | 6.24 | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Sade, E.; Martinez-Rojas, F.; Ramos, D.; Aguirre, M.J.; Armijo, F. Formation of a Conducting Polymer by Different Electrochemical Techniques and Their Effect on Obtaining an Immunosensor for Immunoglobulin G. Polymers 2023, 15, 1168. https://doi.org/10.3390/polym15051168
Martinez-Sade E, Martinez-Rojas F, Ramos D, Aguirre MJ, Armijo F. Formation of a Conducting Polymer by Different Electrochemical Techniques and Their Effect on Obtaining an Immunosensor for Immunoglobulin G. Polymers. 2023; 15(5):1168. https://doi.org/10.3390/polym15051168
Chicago/Turabian StyleMartinez-Sade, Erika, Francisco Martinez-Rojas, Danilo Ramos, Maria Jesus Aguirre, and Francisco Armijo. 2023. "Formation of a Conducting Polymer by Different Electrochemical Techniques and Their Effect on Obtaining an Immunosensor for Immunoglobulin G" Polymers 15, no. 5: 1168. https://doi.org/10.3390/polym15051168
APA StyleMartinez-Sade, E., Martinez-Rojas, F., Ramos, D., Aguirre, M. J., & Armijo, F. (2023). Formation of a Conducting Polymer by Different Electrochemical Techniques and Their Effect on Obtaining an Immunosensor for Immunoglobulin G. Polymers, 15(5), 1168. https://doi.org/10.3390/polym15051168









