Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration
Abstract
:1. Introduction
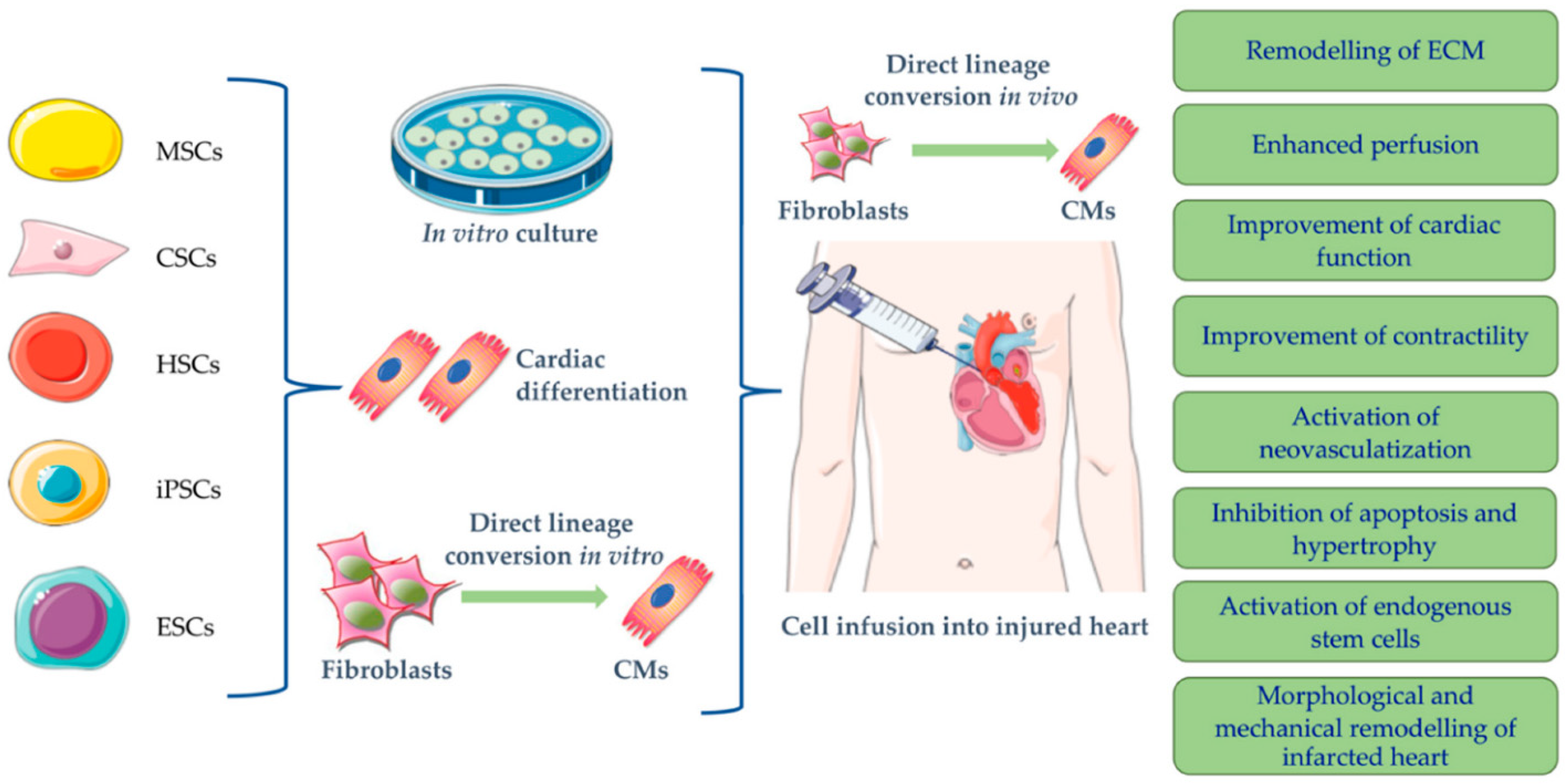
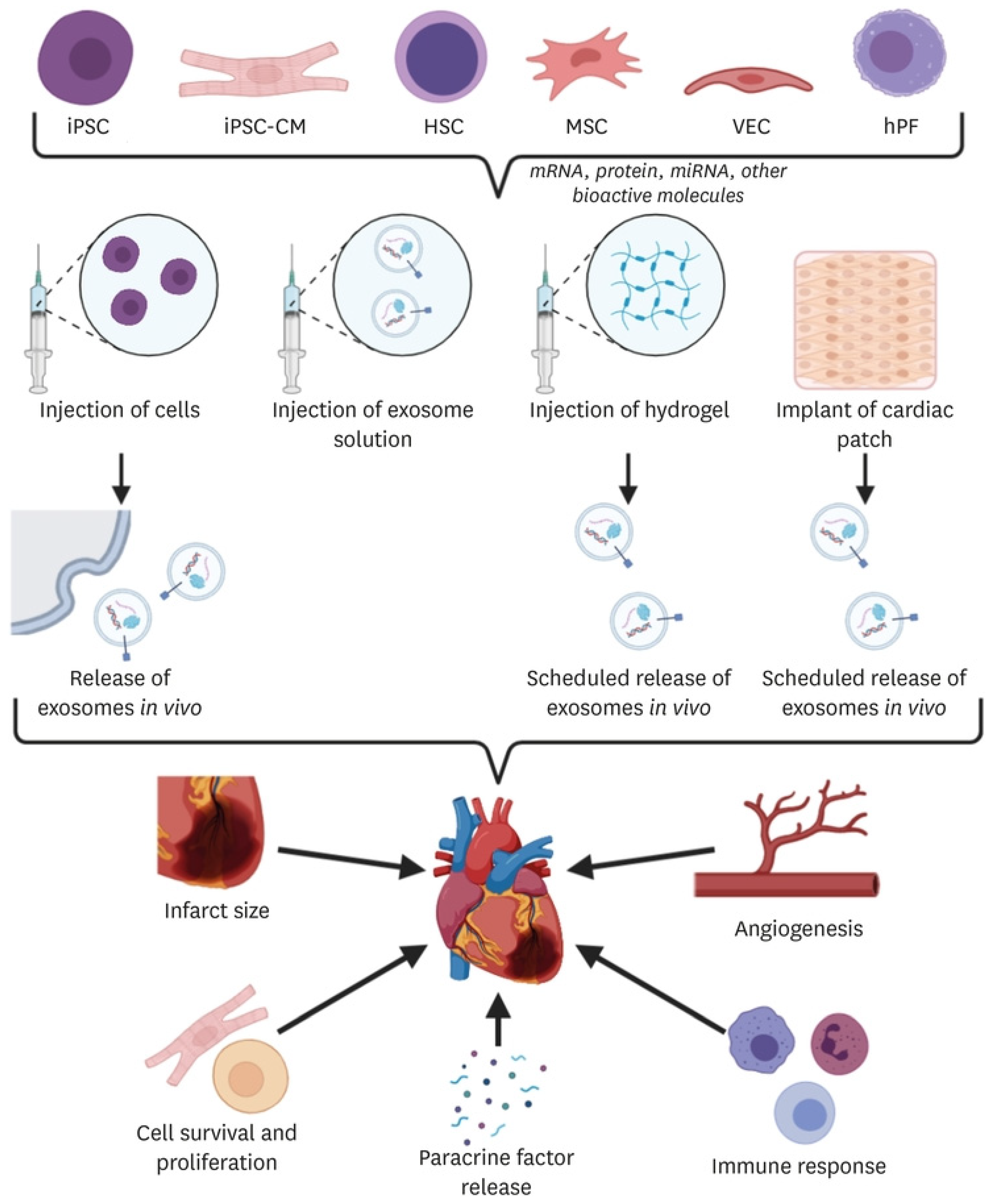
2. Cell-Based Therapies
3. Biomaterial-Based Approaches
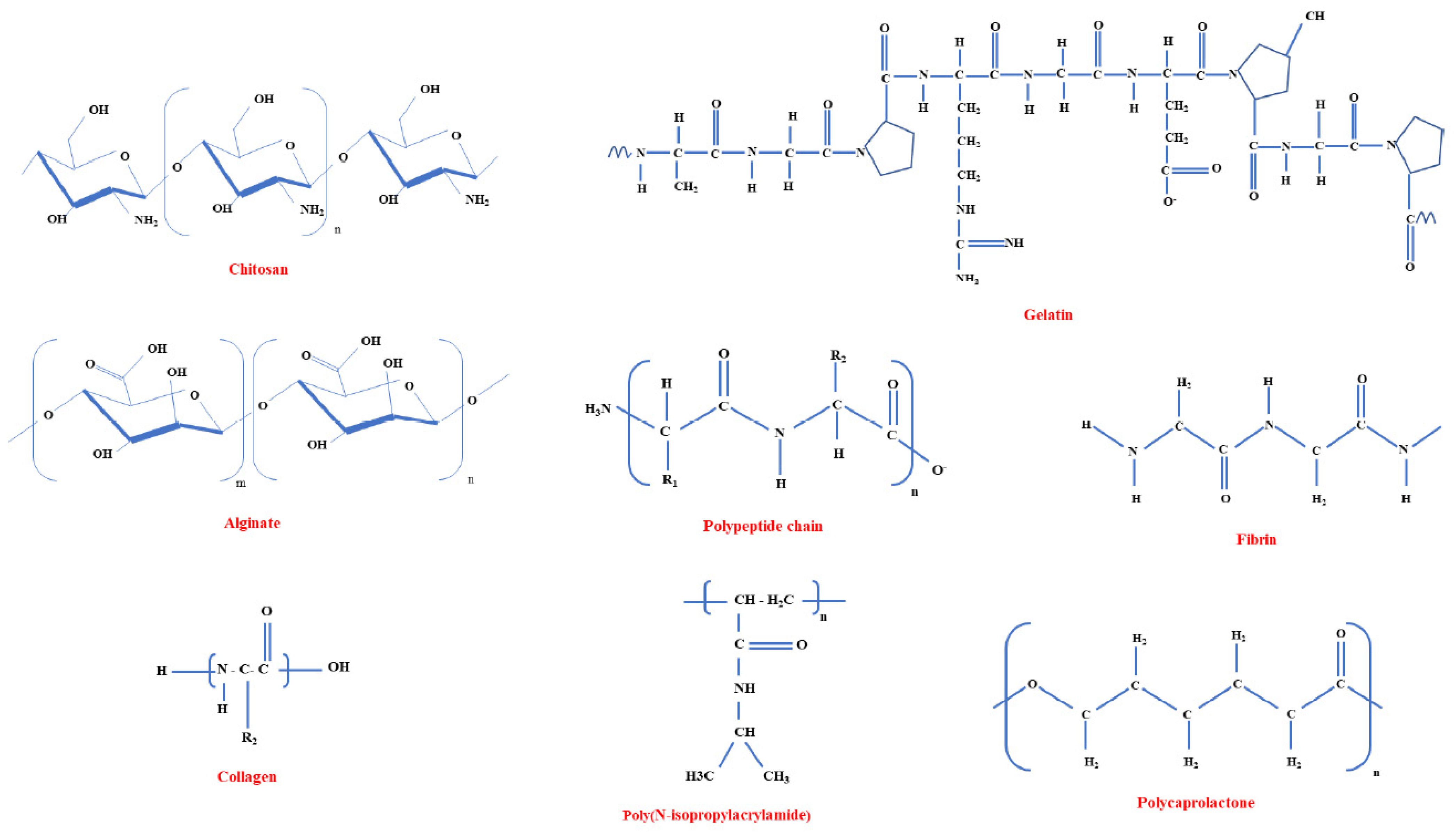
3.1. Biomaterials—Brief Overview
3.2. Approaches for Cardiac Tissue Engineering and Regeneration
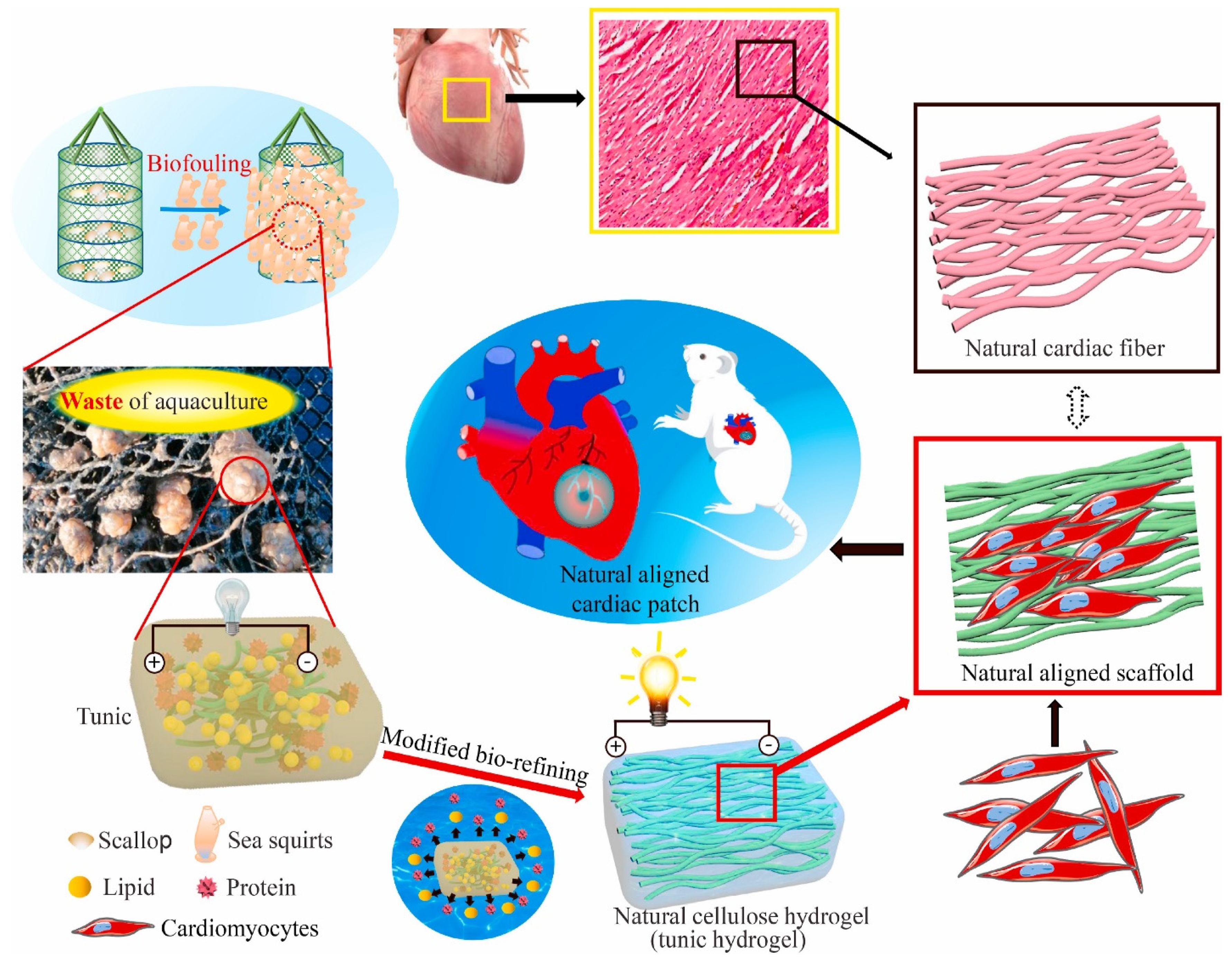
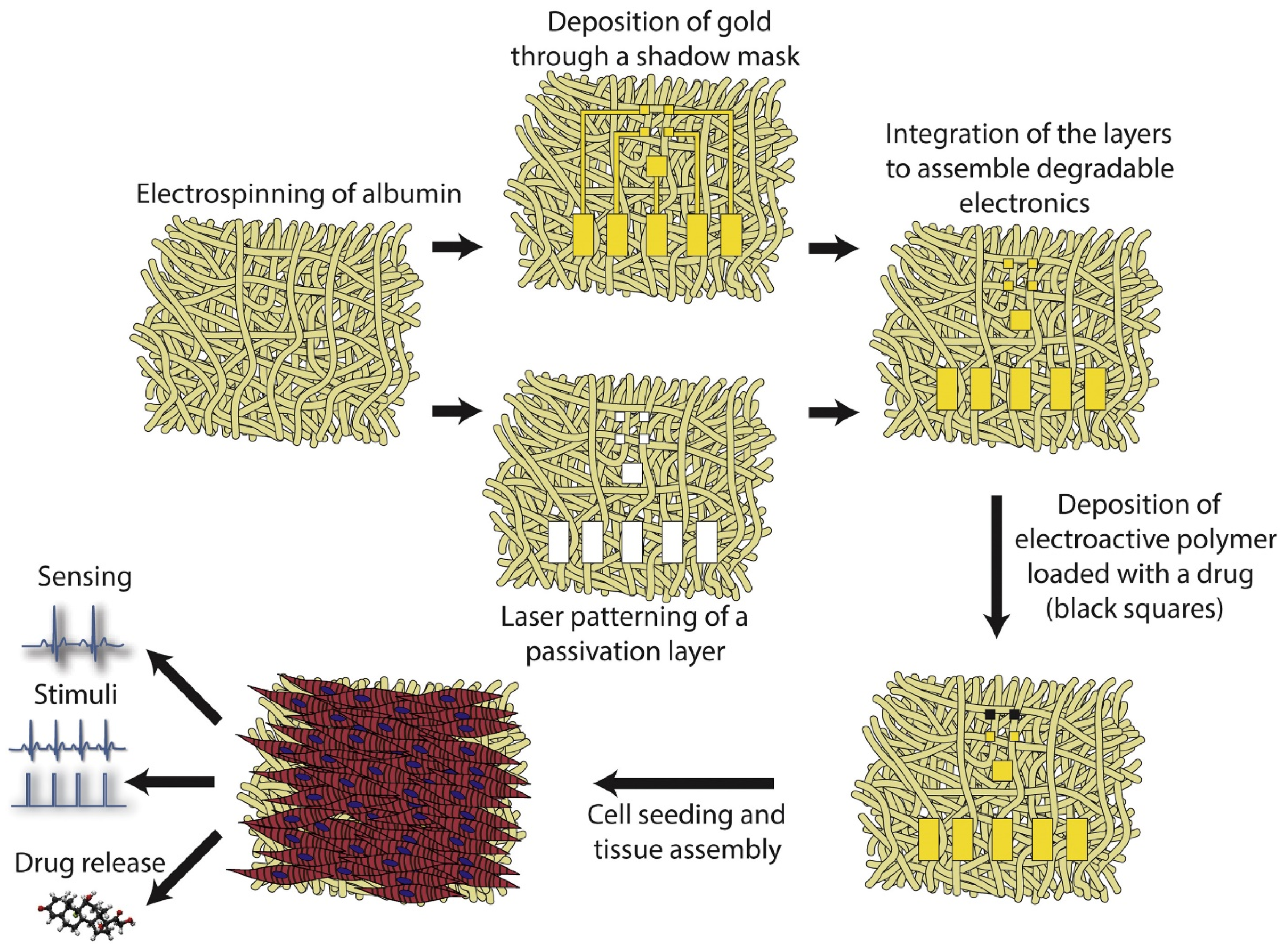
3.2.1. Cardiac Patches
3.2.2. Injectable Hydrogels
3.2.3. Extracellular Vesicles
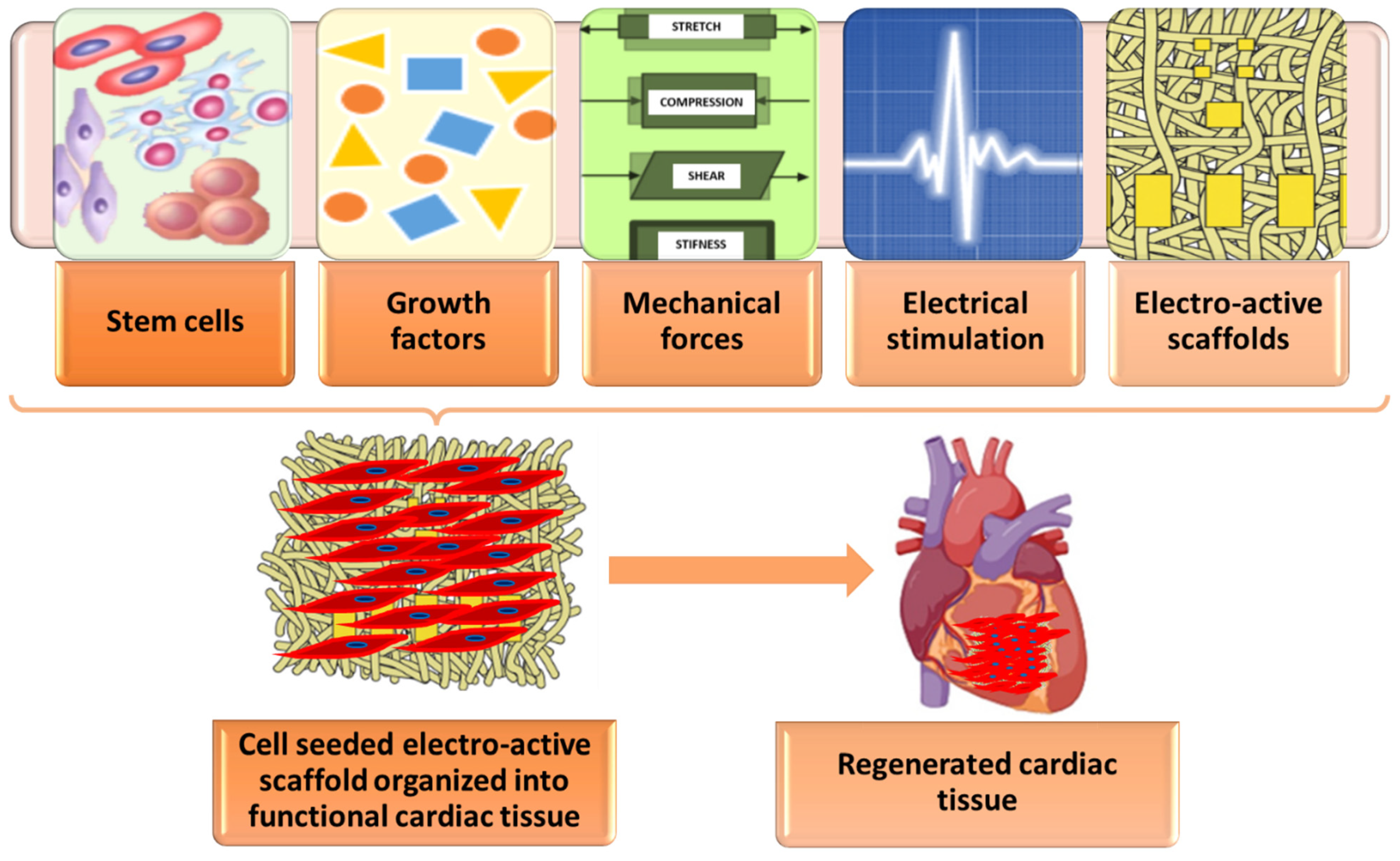
3.2.4. Scaffolds
4. Summative Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pournemati, B.; Tabesh, H.; Jenabi, A.; Mehdinavaz Aghdam, R.; Hossein Rezayan, A.; Poorkhalil, A.; Ahmadi Tafti, S.H.; Mottaghy, K. Injectable conductive nanocomposite hydrogels for cardiac tissue engineering: Focusing on carbon and metal-based nanostructures. Eur. Polym. J. 2022, 174, 111336. [Google Scholar] [CrossRef]
- Wagner, K.T.; Nash, T.R.; Liu, B.; Vunjak-Novakovic, G.; Radisic, M. Extracellular Vesicles in Cardiac Regeneration: Potential Applications for Tissues-on-a-Chip. Trends Biotechnol. 2021, 39, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Turner, N.A. Channelling the Force to Reprogram the Matrix: Mechanosensitive Ion Channels in Cardiac Fibroblasts. Cells 2021, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Ruvinov, E.; Cohen, S. Biomaterials for Cardiac Tissue Engineering and Regeneration. In Adult and Pluripotent Stem Cells: Potential for Regenerative Medicine of the Cardiovascular System; Hescheler, J., Hofer, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 83–111. [Google Scholar]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, M.; Goran Orimi, H.; Ramzgouyan, M.R.; Nemati, A.; Deravi, N.; Beheshtizadeh, N.; Azami, M. Regenerative strategies for the consequences of myocardial infarction: Chronological indication and upcoming visions. Biomed. Pharmacother. 2022, 146, 112584. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, J.; Liu, Z.; Wang, C. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J. Cell. Mol. Med. 2010, 14, 1044–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Li, A.; Wang, S.; Lu, Y.; Xie, J.; Zhang, H.; Zhang, D.; Ding, J.; Wang, Z.; Tu, C.; et al. Multifunctional elastomer cardiac patches for preventing left ventricle remodeling after myocardial infarction in vivo. Biomaterials 2022, 282, 121382. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Vasu, S.; Zhou, J.; Chen, J.; Johnston, P.V.; Kim, D.H. Biomaterials-based Approaches for Cardiac Regeneration. Korean Circ. J. 2021, 51, 943–960. [Google Scholar] [CrossRef]
- Gokce, C.; Gurcan, C.; Delogu, L.G.; Yilmazer, A. 2D Materials for Cardiac Tissue Repair and Regeneration. Front. Cardiovasc. Med. 2022, 9, 802551. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Gil, S.; Garbayo, E.; Díaz-Herráez, P.; Prosper, F.; Blanco-Prieto, M.J. Heart regeneration after myocardial infarction using synthetic biomaterials. J. Control Release 2015, 203, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feric, N.T.; Thavandiran, N.; Nunes, S.S.; Radisic, M. The Role of Tissue Engineering and Biomaterials in Cardiac Regenerative Medicine. Can. J. Cardiol. 2014, 30, 1307–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M. Natural biomaterials for cardiac tissue engineering: A highly biocompatible solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef]
- Esmaeili, H.; Patino-Guerrero, A.; Hasany, M.; Ansari, M.O.; Memic, A.; Dolatshahi-Pirouz, A.; Nikkhah, M. Electroconductive biomaterials for cardiac tissue engineering. Acta Biomater. 2022, 139, 118–140. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, B.; Li, R.-K. Application of Biomaterials in Cardiac Repair and Regeneration. Engineering 2016, 2, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Reis, L.; Chiu, L.L.Y.; Feric, N.; Fu, L.; Radisic, M. 3–Injectable biomaterials for cardiac regeneration and repair. In Cardiac Regeneration and Repair; Li, R.-K., Weisel, R.D., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 49–81. [Google Scholar]
- Vasanthan, V.; Fatehi Hassanabad, A.; Pattar, S.; Niklewski, P.; Wagner, K.; Fedak, P.W.M. Promoting cardiac regeneration and repair using acellular biomaterials. Front. Bioeng. Biotechnol. 2020, 8, 291. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37. [Google Scholar] [CrossRef]
- Huyer, L.D.; Montgomery, M.; Zhao, Y.; Xiao, Y.; Conant, G.; Korolj, A.; Radisic, M. Biomaterial based cardiac tissue engineering and its applications. Biomed. Mater. 2015, 10, 034004. [Google Scholar] [CrossRef] [Green Version]
- Parrotta, E.I.; Scalise, S.; Scaramuzzino, L.; Cuda, G. Stem Cells: The Game Changers of Human Cardiac Disease Modelling and Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 5760. [Google Scholar] [CrossRef] [Green Version]
- Narayan Alagarsamy, K.; Yan, W.; Srivastava, A.; Desiderio, V.; Dhingra, S. Application of injectable hydrogels for cardiac stem cell therapy and tissue engineering. RCM 2019, 20, 221–230. [Google Scholar]
- Lee, C.Y.; Kim, R.; Ham, O.; Lee, J.; Kim, P.; Lee, S.; Oh, S.; Lee, H.; Lee, M.; Kim, J.; et al. Therapeutic Potential of Stem Cells Strategy for Cardiovascular Diseases. Stem Cells Int. 2016, 2016, 4285938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunny, S.; Rajkumar, A.; Jyothidasan, A.; Namakkal Soorappan, R. Chapter 19–Cardiovascular regeneration. In Tissue Engineering; Sharma, C.P., Chandy, T., Thomas, V., Thankam, F.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 449–465. [Google Scholar]
- Bahardoust, M. Role of adipose-derived mesenchymal stem cells in the regeneration of cardiac tissue and improvement of cardiac function: A narrative review. Biointerface Res. Appl. Chem 2021, 11, 8446–8456. [Google Scholar]
- Luger, D.; Lipinski, M.J.; Westman, P.C.; Glover, D.K.; Dimastromatteo, J.; Frias, J.C.; Albelda, M.T.; Sikora, S.; Kharazi, A.; Vertelov, G. Intravenously delivered mesenchymal stem cells: Systemic anti-inflammatory effects improve left ventricular dysfunction in acute myocardial infarction and ischemic cardiomyopathy. Circ. Res. 2017, 120, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Miller, J.G.; Zhou, Y.; Robey, P.G.; Stroncek, D.F.; Arai, A.E.; Sachdev, V.; Horvath, K.A. Intramyocardial Bone Marrow Stem Cells in Patients Undergoing Cardiac Surgical Revascularization. Ann. Thorac. Surg. 2020, 109, 1142–1149. [Google Scholar] [CrossRef]
- Bao, L.; Meng, Q.; Li, Y.; Deng, S.; Yu, Z.; Liu, Z.; Zhang, L.; Fan, H. C-Kit Positive Cardiac Stem Cells and Bone Marrow–Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. J. Card. Fail. 2017, 23, 403–415. [Google Scholar] [CrossRef]
- Han, M.A.; Jeon, J.H.; Shin, J.Y.; Kim, H.J.; Lee, J.S.; Seo, C.W.; Yun, Y.J.; Yoon, M.Y.; Kim, J.T.; Yang, Y.I.; et al. Intramyocardial delivery of human cardiac stem cell spheroids with enhanced cell engraftment ability and cardiomyogenic potential for myocardial infarct repair. J. Control. Release 2021, 336, 499–509. [Google Scholar] [CrossRef]
- Tani, H.; Tohyama, S.; Kishino, Y.; Kanazawa, H.; Fukuda, K. Production of functional cardiomyocytes and cardiac tissue from human induced pluripotent stem cells for regenerative therapy. J. Mol. Cell. Cardiol. 2022, 164, 83–91. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tohyama, S.; Kanazawa, H.; Ichimura, H.; Chino, S.; Tanaka, Y.; Suzuki, Y.; Zhao, J.; Shiba, N.; Kadota, S.; et al. Intracoronary transplantation of pluripotent stem cell-derived cardiomyocytes: Inefficient procedure for cardiac regeneration. J. Mol. Cell. Cardiol. 2023, 174, 77–87. [Google Scholar] [CrossRef]
- Thavapalachandran, S.; Le, T.Y.L.; Romanazzo, S.; Rashid, F.N.; Ogawa, M.; Kilian, K.A.; Brown, P.; Pouliopoulos, J.; Barry, A.M.; Fahmy, P.; et al. Pluripotent stem cell-derived mesenchymal stromal cells improve cardiac function and vascularity after myocardial infarction. Cytotherapy 2021, 23, 1074–1084. [Google Scholar] [CrossRef]
- Nagai, T.; Takahashi, T.; Naito, A.T.; Ogura, T.; Nakaya, H.; Dong, H.; Kodama, I.; Lee, J.-K.; Komuro, I. Regeneration of Cardiac Conduction System by Adipose Tissue Derived-stem Cells. J. Card. Fail. 2009, 15, S149. [Google Scholar] [CrossRef]
- Kashiyama, N.; Kormos, R.L.; Matsumura, Y.; D’Amore, A.; Miyagawa, S.; Sawa, Y.; Wagner, W.R. Adipose-derived stem cell sheet under an elastic patch improves cardiac function in rats after myocardial infarction. J. Thorac. Cardiovasc. Surg. 2022, 163, e261–e272. [Google Scholar] [CrossRef]
- Porta-Sanchez, A.; Nayyar, S.; Magtibay, K.; Masse, S.; Bhaskaran, A.; Romagnuolo, R.; Masoudpoor, H.; Qiang, B.; Biswas, L.; Ghugre, N.; et al. Cardiac Regeneration with Human Embryonic Stem Cell-Derived Cardiomyocytes in Infarcted Swine is Associated with Ventricular Tachycardia That Has Focal Pattern of Activation. Can. J. Cardiol. 2018, 34, S187–S188. [Google Scholar] [CrossRef]
- Ishikane, S.; Hosoda, H.; Yamahara, K.; Akitake, Y.; Kyoungsook, J.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Miyazato, M.; Kangawa, K. Allogeneic transplantation of fetal membrane–derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation 2013, 96, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Niu, R.; Li, W.; Lin, J.; Stamm, C.; Steinhoff, G.; Ma, N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell. Mol. Life Sci. 2019, 76, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Hasan, A.; Khalaf, I.M.; Prasad, P.; Ghosal, K.; Gentile, C.; McClements, L.; Maureira, P. Stem cell-based approaches in cardiac tissue engineering: Controlling the microenvironment for autologous cells. Biomed. Pharmacother. 2021, 138, 111425. [Google Scholar] [CrossRef] [PubMed]
- Csöbönyeiová, M.; Beerová, N.; Klein, M.; Debreová-Čeháková, M.; Danišovič, Ľ. Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options? Int. J. Mol. Sci. 2022, 23, 10314. [Google Scholar] [CrossRef]
- Wolfien, M.; Klatt, D.; Salybekov, A.A.; Ii, M.; Komatsu-Horii, M.; Gaebel, R.; Philippou-Massier, J.; Schrinner, E.; Akimaru, H.; Akimaru, E.; et al. Hematopoietic stem-cell senescence and myocardial repair–Coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT Phase 3. EBioMedicine 2020, 57, 102862. [Google Scholar] [CrossRef]
- Huang, K.; Ozpinar, E.W.; Su, T.; Tang, J.; Shen, D.; Qiao, L.; Hu, S.; Li, Z.; Liang, H.; Mathews, K.; et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020, 12, eaat9683. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem cells and injectable hydrogels: Synergistic therapeutics in myocardial repair. Biotechnol. Adv. 2016, 34, 362–379. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.H.; Marsh, P.; Schmiess-Heine, L.; Burke, P.J.; Lee, A.; Lee, J.; Cao, H. Cardiac tissue engineering: State-of-the-art methods and outlook. J. Biol. Eng. 2019, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, F.; Thiha, A.; Wan Kamarul Zaman, W.S.; Kamarulzaman, Y.; Dahlan, N.A.; Jamaluddin, N.F.; Madou, M.J. Cardiac tissue engineering: A comparative analysis on microscaffold patterning. Mater. Today Commun. 2022, 33, 104285. [Google Scholar] [CrossRef]
- Farzamfar, S.; Aleahmad, M.; Gholamreza, S.K.; Majid, S.; Niloofar, N. Polycaprolactone/Gelatin Nanofibrous Scaffolds for Tissue Engineering. Biointerface Res. Appl. Chem 2021, 11, 11104–11115. [Google Scholar]
- Shadman, B.; Marzban, A.; Asadi, A.; Zahri, S.; Barzegar, A. Design of PLGA-based Scaffolds for Developing and Differentiating Mesenchymal Stem Cells (MSCs). Biointerface Res. Appl. Chem. 2021, 11, 12732–12742. [Google Scholar]
- Chansoria, P.; Etter, E.L.; Nguyen, J. Regenerating dynamic organs using biomimetic patches. Trends Biotechnol. 2022, 40, 338–353. [Google Scholar] [CrossRef]
- Feiner, R.; Fleischer, S.; Shapira, A.; Kalish, O.; Dvir, T. Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J. Control. Release 2018, 281, 189–195. [Google Scholar] [CrossRef]
- Li, Y.; Wei, L.; Lan, L.; Gao, Y.; Zhang, Q.; Dawit, H.; Mao, J.; Guo, L.; Shen, L.; Wang, L. Conductive biomaterials for cardiac repair: A review. Acta Biomater. 2022, 139, 157–178. [Google Scholar] [CrossRef]
- Ghofrani, A.; Taghavi, L.; Khalilivavdareh, B.; Rohani Shirvan, A.; Nouri, A. Additive manufacturing and advanced functionalities of cardiac patches: A review. Eur. Polym. J. 2022, 174, 111332. [Google Scholar] [CrossRef]
- Chang, T.; Liu, C.; Lu, K.; Wu, Y.; Xu, M.; Yu, Q.; Shen, Z.; Jiang, T.; Zhang, Y. Biomaterials based cardiac patches for the treatment of myocardial infarction. J. Mater. Sci. Technol. 2021, 94, 77–89. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef]
- Kapnisi, M.; Mansfield, C.; Marijon, C.; Guex, A.G.; Perbellini, F.; Bardi, I.; Humphrey, E.J.; Puetzer, J.L.; Mawad, D.; Koutsogeorgis, D.C.; et al. Auxetic Cardiac Patches with Tunable Mechanical and Conductive Properties toward Treating Myocardial Infarction. Adv. Funct. Mater. 2018, 28, 1800618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoyama, K.; Ahumada, M.; McTiernan, C.D.; Davis, D.R.; Variola, F.; Ruel, M.; Liang, W.; Suuronen, E.J.; Alarcon, E.I. Nanoengineered Electroconductive Collagen-Based Cardiac Patch for Infarcted Myocardium Repair. ACS Appl. Mater. Interfaces 2018, 10, 44668–44677. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, C.; Esworthy, T.; Huang, Y.; Yu, Z.-x.; Zhou, X.; San, H.; Lee, S.-j.; Hann, S.Y.; Boehm, M.; et al. 4D physiologically adaptable cardiac patch: A 4-month in vivo study for the treatment of myocardial infarction. Sci. Adv. 2020, 6, eabb5067. [Google Scholar] [CrossRef] [PubMed]
- Abbasgholizadeh, R.; Islas, J.F.; Navran, S.; Potaman, V.N.; Schwartz, R.J.; Birla, R.K. A Highly Conductive 3D Cardiac Patch Fabricated Using Cardiac Myocytes Reprogrammed from Human Adipogenic Mesenchymal Stem Cells. Cardiovasc. Eng. Technol. 2020, 11, 205–218. [Google Scholar] [CrossRef]
- Pushp, P.; Bhaskar, R.; Kelkar, S.; Sharma, N.; Pathak, D.; Gupta, M.K. Plasticized poly(vinylalcohol) and poly(vinylpyrrolidone) based patches with tunable mechanical properties for cardiac tissue engineering applications. Biotechnol. Bioeng. 2021, 118, 2312–2325. [Google Scholar] [CrossRef]
- He, Y.; Hou, H.; Wang, S.; Lin, R.; Wang, L.; Yu, L.; Qiu, X. From waste of marine culture to natural patch in cardiac tissue engineering. Bioact. Mater. 2021, 6, 2000–2010. [Google Scholar] [CrossRef]
- Chen, W.L.; Kan, C.D. Using cell-Seeded Electrospun Patch for Myocardial Injury: In-vitro and in Rat Model. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5338–5341. [Google Scholar]
- O’Neill, H.S.; O’Sullivan, J.; Porteous, N.; Ruiz-Hernandez, E.; Kelly, H.M.; O’Brien, F.J.; Duffy, G.P. A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J. Tissue Eng. Regen. Med. 2018, 12, e384–e394. [Google Scholar] [CrossRef]
- Shah, M.; Kc, P.; Zhang, G. In Vivo Assessment of Decellularized Porcine Myocardial Slice as an Acellular Cardiac Patch. ACS Appl. Mater. Interfaces 2019, 11, 23893–23900. [Google Scholar] [CrossRef]
- Kazemi Asl, S.; Rahimzadegan, M.; Ostadrahimi, R. The recent advancement in the chitosan hybrid-based scaffolds for cardiac regeneration after myocardial infarction. Carbohydr. Polym. 2023, 300, 120266. [Google Scholar] [CrossRef]
- Qian, Z.; Sharma, D.; Jia, W.; Radke, D.; Kamp, T.; Zhao, F. Engineering stem cell cardiac patch with microvascular features representative of native myocardium. Theranostics 2019, 9, 2143–2157. [Google Scholar] [CrossRef]
- Su, T.; Huang, K.; Daniele, M.A.; Hensley, M.T.; Young, A.T.; Tang, J.; Allen, T.A.; Vandergriff, A.C.; Erb, P.D.; Ligler, F.S.; et al. Cardiac Stem Cell Patch Integrated with Microengineered Blood Vessels Promotes Cardiomyocyte Proliferation and Neovascularization after Acute Myocardial Infarction. ACS Appl. Mater. Interfaces 2018, 10, 33088–33096. [Google Scholar] [CrossRef] [PubMed]
- Hoeeg, C.; Dolatshahi-Pirouz, A.; Follin, B. Injectable Hydrogels for Improving Cardiac Cell Therapy—In Vivo Evidence and Translational Challenges. Gels 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaya, G.; Oytun, F. Rheological properties of injectable hyaluronic acid hydrogels for soft tissue engineering applications. Biointerface Res. Appl. Chem. 2021, 11, 8424–8430. [Google Scholar]
- Mallick, S.P.; Panda, S.P.; Gayatri, A.; Kunaal, Y.; Naresh, C.; Suman, D.K.; Samineni, J.; Siddiqui, N.; Singh, B.N. Chitosan Oligosaccharide Based Hydrogel: An Insight into the Mechanical, Drug Delivery, and Antimicrobial Studies. Biointerface Res. Appl. Chem. 2021, 11, 10293–10300. [Google Scholar]
- Peña, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, 1800079. [Google Scholar] [CrossRef]
- Liao, X.; Yang, X.; Deng, H.; Hao, Y.; Mao, L.; Zhang, R.; Liao, W.; Yuan, M. Injectable hydrogel-based nanocomposites for cardiovascular diseases. Front. Bioeng. Biotechnol. 2020, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Karvinen, J.; Kellomäki, M. Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Sanoh, N.C.; Salazar, G.M.; Penaloza, D.P. Magnetic Biopolymeric Hydrogel Composite Material with Self-healing Attribute. Biointerface Res. Appl. Chem 2021, 11, 14881–14888. [Google Scholar]
- Weerawan, N.; Chalitangkoon, J.; Monvisade, P. Self-Healing Hydrogels Based on Sodium Carboxymethyl Cellulose/Poly (vinyl alcohol) Reinforced with Montmorillonite. Biointerface Res. Appl. Chem 2022, 12, 4770–4779. [Google Scholar]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Diaz, M.D.; Christman, K.L. Injectable Hydrogels to Treat Myocardial Infarction. In Cardiovascular Regenerative Medicine: Tissue Engineering and Clinical Applications; Serpooshan, V., Wu, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 185–206. [Google Scholar]
- Traverse Jay, H.; Henry Timothy, D.; Dib, N.; Patel Amit, N.; Pepine, C.; Schaer Gary, L.; DeQuach Jessica, A.; Kinsey Adam, M.; Chamberlin, P.; Christman Karen, L. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Contessotto, P.; Orbanić, D.; Da Costa, M.; Jin, C.; Owens, P.; Chantepie, S.; Chinello, C.; Newell, J.; Magni, F.; Papy-Garcia, D.; et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 2021, 13, eaaz5380. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Tian, L.; Li, Y.; Zhang, J.; Wei, Y.; Jin, Z.; Liu, Z.; Liu, H. Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair. Stem Cells Int. 2019, 2019, 6708435. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, X.; Deng, C.; Teng, X.; Suuronen, E.J.; Shen, Z.; Zhong, Z. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)–poly(ethylene glycol)–oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Zhang, W.; He, S.; Lu, L.; Fan, H.; Yang, L.; Wang, Y. Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomaterials 2022, 290, 121849. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-based biohybrid injectable hydrogel for cardiac tissue engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.H.; Paul, A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater 2018, 69, 95–106. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 6, 68. [Google Scholar] [CrossRef]
- Cheow, E.S.H.; Cheng, W.C.; Lee, C.N.; De Kleijn, D.; Sorokin, V.; Sze, S.K. Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for myocardial ischemic (MI) injury. Mol. Cell. Proteom. 2016, 15, 2628–2640. [Google Scholar] [CrossRef] [Green Version]
- Stine, S.J.; Popowski, K.D.; Su, T.; Cheng, K. Exosome and Biomimetic Nanoparticle Therapies for Cardiac Regenerative Medicine. Curr. Stem Cell Res. Ther. 2020, 15, 674–684. [Google Scholar] [CrossRef]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.-J.; Yu, X.-Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. BioMed Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K.; et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, D.; Zakeri, N.; Zafar-Jafarzadeh, M.; Moroni, L.; Solati-Hashjin, M. Cardiovascular 3D bioprinting: A review on cardiac tissue development. Bioprinting 2022, 28, e00221. [Google Scholar] [CrossRef]
- Hayoun-Neeman, D.; Korover, N.; Etzion, S.; Ofir, R.; Lichtenstein, R.G.; Cohen, S. Exploring peptide-functionalized alginate scaffolds for engineering cardiac tissue from human embryonic stem cell-derived cardiomyocytes in serum-free medium. Polym. Adv. Technol. 2019, 30, 2493–2505. [Google Scholar] [CrossRef]
- Liang, Y.; Mitriashkin, A.; Lim, T.T.; Goh, J.C.-H. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. Biomaterials 2021, 276, 121008. [Google Scholar] [CrossRef]
- Li, X.-P.; Qu, K.-Y.; Zhang, F.; Jiang, H.-N.; Zhang, N.; Nihad, C.; Liu, C.-M.; Wu, K.-H.; Wang, X.-W.; Huang, N.-P. High-aspect-ratio water-dispersed gold nanowires incorporated within gelatin methacrylate hydrogels for constructing cardiac tissues in vitro. J. Mater. Chem. B 2020, 8, 7213–7224. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene Oxide-Gold Nanosheets Containing Chitosan Scaffold Improves Ventricular Contractility and Function After Implantation into Infarcted Heart. Sci. Rep. 2018, 8, 15069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventrix, I. A Study of VentriGel in Post-MI Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT02305602 (accessed on 8 December 2022).
- Fedak, D.P. Epicardial Infarct Repair Using CorMatrix®-ECM: Clinical Feasibility Study (EIR). Available online: https://clinicaltrials.gov/ct2/show/NCT02887768 (accessed on 8 December 2022).
- Pujol, F.I.G.T.I. Pericardial Matrix with Mesenchymal Stem Cells for the Treatment of Patients with Infarcted Myocardial Tissue (PERISCOPE). Available online: https://clinicaltrials.gov/ct2/show/NCT03798353 (accessed on 8 December 2022).
- Karjalainen, P.P. Atrial Appendage Micrograft Transplants to Assist Heart Repair After Cardiac Surgery (AAMS2). Available online: https://www.clinicaltrials.gov/ct2/show/NCT05632432 (accessed on 8 December 2022).
- Mewhort, H.E.; Turnbull, J.D.; Satriano, A.; Chow, K.; Flewitt, J.A.; Andrei, A.C.; Guzzardi, D.G.; Svystonyuk, D.A.; White, J.A.; Fedak, P.W. Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. Int. Soc. Heart Transplant 2016, 35, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mewhort, H.E.; Turnbull, J.D.; Meijndert, H.C.; Ngu, J.M.; Fedak, P.W. Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J. Thorac. Cardiovasc. Surg. 2014, 147, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- Yadid, M.; Oved, H.; Silberman, E.; Dvir, T. Bioengineering approaches to treat the failing heart: From cell biology to 3D printing. Nat. Rev. Cardiol. 2022, 19, 83–99. [Google Scholar] [CrossRef]
- Roy, A.; Saxena, V.; Pandey, L.M. 3D printing for cardiovascular tissue engineering: A review. Mater. Technol. 2018, 33, 433–442. [Google Scholar] [CrossRef]
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2021, 6, 27–47. [Google Scholar] [CrossRef]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2022, 139, 179–189. [Google Scholar] [CrossRef]
- Chingale, M.; Cheng, K.; Huang, K. 3D Bioprinting technology–one step closer towards cardiac tissue regeneration. Front. Mater. 2022, 8, 647. [Google Scholar] [CrossRef]
- Loerakker, S.; Humphrey, J.D. Computer Model-Driven Design in Cardiovascular Regenerative Medicine. Ann. Biomed. Eng. 2023, 51, 45–57. [Google Scholar] [CrossRef]
- Suwardi, A.; Wang, F.; Xue, K.; Han, M.-Y.; Teo, P.; Wang, P.; Wang, S.; Liu, Y.; Ye, E.; Li, Z.; et al. Machine Learning-Driven Biomaterials Evolution. Adv. Mater. 2022, 34, 2102703. [Google Scholar] [CrossRef] [PubMed]
- Busnatu, Ș.; Niculescu, A.-G.; Bolocan, A.; Petrescu, G.E.D.; Păduraru, D.N.; Năstasă, I.; Lupușoru, M.; Geantă, M.; Andronic, O.; Grumezescu, A.M.; et al. Clinical Applications of Artificial Intelligence–An Updated Overview. J. Clin. Med. 2022, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Ghanta Sai, N.; Clausen, A.; Saluja, P.; Sivakumar, K.; Dhar, G.; Chang, Q.; DeMazumder, D.; Rabbat Mark, G.; Greene Stephen, J.; et al. Contemporary Applications of Machine Learning for Device Therapy in Heart Failure. JACC: Heart Fail. 2022, 10, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.; Shah, R.; Yanamala, N.; Sengupta, P.P. Cardiovascular Imaging Databases: Building Machine Learning Algorithms for Regenerative Medicine. Curr. Stem Cell Rep. 2022, 8, 164–173. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Hernandez-Moreno, G.; Thomas, V. Chapter 10—Future of nanotechnology in tissue engineering. In Tissue Engineering; Sharma, C.P., Chandy, T., Thomas, V., Thankam, F.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 193–236. [Google Scholar]
- Benko, A.; Truong, L.B.; Medina-Cruz, D.; Mostafavi, E.; Cholula-Díaz, J.L.; Webster, T.J. Chapter 11–Green nanotechnology in cardiovascular tissue engineering. In Tissue Engineering; Sharma, C.P., Chandy, T., Thomas, V., Thankam, F.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 237–281. [Google Scholar]
- Zheng, Z.; Zhu, S.; Lv, M.; Gu, Z.; Hu, H. Harnessing nanotechnology for cardiovascular disease applications–A comprehensive review based on bibliometric analysis. Nano Today 2022, 44, 101453. [Google Scholar] [CrossRef]
- Feldman, D. Polyurethane and polyurethane nanocomposites: Recent contributions to medicine. Biointerface Res. Appl. Chem 2021, 11, 8179–8189. [Google Scholar]
- Ojo, O.A.; Olayide, I.I.; Akalabu, M.C.; Ajiboye, B.O.; Ojo, A.B.; Oyinloye, B.E.; Ramalingam, M. Nanoparticles and their biomedical applications. Biointerface Res. Appl. Chem 2021, 11, 8431–8445. [Google Scholar]
- Tiplea, R.E.; Lemnaru, G.-M.; Trusca, R.D.; Holban, A.; Kaya, M.G.A.; Dragu, L.D.; Ficai, D.; Ficai, A.; Bleotu, C. Antimicrobial films based on chitosan, collagen, and zno for skin tissue regeneration. Biointerface Res. Appl. Chem 2021, 11, 11985–11995. [Google Scholar]
- Mousavi, A.; Vahdat, S.; Baheiraei, N.; Razavi, M.; Norahan, M.H.; Baharvand, H. Multifunctional Conductive Biomaterials as Promising Platforms for Cardiac Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 55–82. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, A.; Attar, F.; Sharifi, M.; Siddique, R.; Mraiche, F.; Falahati, M. Gold Nanoparticle-Based Platforms for Diagnosis and Treatment of Myocardial Infarction. ACS Biomater. Sci. Eng. 2020, 6, 6460–6477. [Google Scholar] [CrossRef]
- Zwi-Dantsis, L.; Wang, B.; Marijon, C.; Zonetti, S.; Ferrini, A.; Massi, L.; Stuckey, D.J.; Terracciano, C.M.; Stevens, M.M. Remote Magnetic Nanoparticle Manipulation Enables the Dynamic Patterning of Cardiac Tissues. Adv. Mater. 2020, 32, 1904598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoumi, B.; Abbasian, M.; Khalilzadeh, B.; Jahanban-Esfahlan, R.; Rezaei, A.; Samadian, H.; Derakhshankhah, H.; Jaymand, M. Gelatin-based nanofibrous electrically conductive scaffolds for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 693–702. [Google Scholar] [CrossRef]
- Agarwal, T.; Tan, S.-A.; Nie, L.; Zahmatkesh, E.; Ansari, A.; Rad, N.K.; Zarkesh, I.; Maiti, T.K.; Vosough, M. Chapter 11—Electroconductive nanofibrillar biocomposite platforms for cardiac tissue engineering. In Food, Medical, and Environmental Applications of Nanomaterials; Pal, K., Sarkar, A., Sarkar, P., Bandara, N., Jegatheesan, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 305–330. [Google Scholar]
- Kim, W.; Jang, C.H.; Kim, G.H. A Myoblast-Laden Collagen Bioink with Fully Aligned Au Nanowires for Muscle-Tissue Regeneration. Nano Lett. 2019, 19, 8612–8620. [Google Scholar] [CrossRef]
- Nazari, H.; Heirani-Tabasi, A.; Esmaeili, E.; Kajbafzadeh, A.-M.; Hassannejad, Z.; Boroomand, S.; Shahsavari Alavijeh, M.H.; Mishan, M.A.; Ahmadi Tafti, S.H.; Warkiani, M.E. Decellularized human amniotic membrane reinforced by MoS2-Polycaprolactone nanofibers, a novel conductive scaffold for cardiac tissue engineering. J. Biomater. Appl. 2022, 36, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Kearney, C.J.; Shen, N.; Khan, U.; Kelly, A.G.; Probst, C.; Brauchle, E.; Biccai, S.; Garciarena, C.D.; Vega-Mayoral, V.; et al. Electroconductive Biohybrid Collagen/Pristine Graphene Composite Biomaterials with Enhanced Biological Activity. Adv. Mater. 2018, 30, 1706442. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Anju, V.T.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int. J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Marei, I.; Crovella, S.; Abou-Saleh, H. Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1404. [Google Scholar] [CrossRef]
- Soumya, R.S.; Raghu, K.G. Recent advances on nanoparticle-based therapies for cardiovascular diseases. J. Cardiol. 2023, 81, 10–18. [Google Scholar] [CrossRef]
- Kitsara, M.; Kontziampasis, D.; Agbulut, O.; Chen, Y. Heart on a chip: Micro-nanofabrication and microfluidics steering the future of cardiac tissue engineering. Microelectron. Eng. 2019, 203–204, 44–62. [Google Scholar] [CrossRef]
- Liu, C.; Feng, X.; Li, G.; Gokulnath, P.; Xiao, J. Generating 3D human cardiac constructs from pluripotent stem cells. eBioMedicine 2022, 76, 103813. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsyst. Nanoeng. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, C.; Cao, S.; Chen, T.; Chen, G. Microfluidics for diagnosis and treatment of cardiovascular disease. J. Mater. Chem. B 2023, 11, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, J.; Chavan, R.; Moghadas, B.; Park, J.G.; Kodibagkar, V.D.; Migrino, R.Q.; Nikkhah, M. Cardiac ischemia on-a-chip to investigate cellular and molecular response of myocardial tissue under hypoxia. Biomaterials 2022, 281, 121336. [Google Scholar] [CrossRef]
- Pradeep, A.; Pillai, I.C.L.; Nair, B.; Satheesh Babu, T.G. Heart-on-a-Chip. In Microfluidics and Multi Organs on Chip; Mohanan, P.V., Ed.; Springer: Singapore, 2022; pp. 407–433. [Google Scholar]
- Tang, Y.; Tian, F.; Miao, X.; Wu, D.; Wang, Y.; Wang, H.; You, K.; Li, Q.; Zhao, S.; Wang, W. Heart-on-a-chip using human iPSC-derived cardiomyocytes with an integrated vascular endothelial layer based on a culture patch as a potential platform for drug evaluation. Biofabrication 2022, 15, 015010. [Google Scholar] [CrossRef]
- Criscione, J.; Rezaei, Z.; Hernandez Cantu, C.M.; Murphy, S.; Shin, S.R.; Kim, D.-H. Heart-on-a-chip platforms and biosensor integration for disease modeling and phenotypic drug screening. Biosens. Bioelectron. 2023, 220, 114840. [Google Scholar] [CrossRef]
- Veldhuizen, J.; Mann, H.F.; Karamanova, N.; Van Horn, W.D.; Migrino, R.Q.; Brafman, D.; Nikkhah, M. Modeling long QT syndrome type 2 on-a-chip via in-depth assessment of isogenic gene-edited 3D cardiac tissues. Sci. Adv. 2022, 8, eabq6720. [Google Scholar] [CrossRef]

| Substrate | Cells | Bioactive Molecules | Testing | Observations | Ref. |
|---|---|---|---|---|---|
| Decellularized porcine myocardial extracellular matrix | Synthetic cardiac stromal cells | - | Rat and porcine models of acute MI | Supports cardiac recovery Reduces scarring Promotes angiomyogenesis Boosts cardiac function The patch is clinically feasible and easy to store | [42] |
| Decellularized porcine myocardium slice | - | - | Rat model of acute MI | Firm attachment to host myocardium Prevents thinning of the left ventricular wall Allows infiltration of a large number of host cells Significant improvement of left ventricle wall contraction and cardiac functional parameters | [62] |
| Cellulose nanofibers modified with chitosan/silk fibroin (CS/SF) multilayers | Adipose tissue-derived mesenchymal stem cells | - | Rat model of acute MI | Less ventricular remodeling than direct cell injection Elevates left ventricular ejection fraction and fractional shortening Attenuates cardiac fibrosis and apoptosis Promotes local neovascularization | [53] |
| Chitosan films micropatterned with a re-entrant honeycomb (bowtie) pattern and coated with polyaniline and phytic acid | Neonatal rat ventricular myocytes and fibroblasts | - | Rat MI model | Conductive and cytocompatible patch No detrimental effect on the electrophysiology of both healthy and MI hearts Conform better to native heart movements than unpatterned patches No detrimental effect on cardiac function Negligible fibrotic response after two weeks | [54] |
| Collagen-based hybrid nanocomposite loaded with nanogold | Neonatal rat cardiomyocytes | - | Murine model 7 days post-MI | Increases connexin-43 expression in cells cultured under electrical stimulation Able to recover cardiac function Increased blood vessel density Reduces scar formation | [55] |
| Collagen patch incorporated with alginate microparticles | - | Hepatocyte growth factor Insulin-like growth factor-1 | Isolated myocardial tissue from rats | Extends the release of encapsulated proteins up to 15 days Increases motogenic and proliferative effect Favors the natural regenerative potential of cardiac stem cells | [61] |
| Gelatin methacrylate and polyethylene glycol diacrylate-based patch with myocardial fiber orientation | Cardiomyocytes, mesenchymal stromal cells, and endothelial cells | - | Mice model of chronic MI with ischemia-reperfusion | Increases cell density Reduces damaged tissue area Ensures high engraftment rates Strong integration within the epicardium Progressive implant vascularization | [56] |
| Fibrin gel-based 3D patch | Cardiac myocytes reprogrammed from human adipogenic mesenchymal stem cells | Isoproterenol Epinephrine | In vitro | Increases the expression of mTOR, KCNV1, GJA5, KCNJ16, CTNNT2, KCNV2, MYO3, FOXO1 and KCND2 Restores the electrical activity of infarcted hearts Improves cardiac functions | [57] |
| Polycaprolactone nanoscale-to-microscale fibers | Skeletal myoblast stem cells | - | Rat MI model | Presents strong compliance and survival after transplantation Release VEGF | [60] |
| Polyvinyl alcohol and polyvinyl pyrrolidone-based patch | Neonatal mouse cardiomyocytes | - | Rat model | Biocompatible and biodegradable No signs of tissue damage or necrosis at the implantation site, no detectable wound complications, inflammatory response, or adverse tissue reactions | [58] |
| Fibrin gel-based patch with microengineered blood vessels | Human umbilical vein endothelial cells and human cardiac stem cells | - | Rat model of acute MI | Induces profound mitotic activities of cardiomyocytes Significantly enhances myocardial capillary density | [65] |
| Porous self-conductive cellulose hydrogel | Cardiomyocytes | - | Rat model of acute MI | Significantly promotes the maturation and spontaneous contraction of cardiomyocytes Enhances cardiac function of animal models | [59] |
| ClinicalTrials.gov Identifier | Official Title | Intervention/ Treatment | Phase | Reference |
|---|---|---|---|---|
| NCT02305602 | A Phase I, Open-label Study of the Effects of Percutaneous Administration of an Extracellular Matrix Hydrogel, VentriGel, Following Myocardial Infarction | Biological: VentriGel | Phase 1 | [98] |
| NCT02887768 | Cardiac Infarct Repair Using CorMatrix®-ECM: Clinical Feasibility Study | Device: Epicardial Infarct Repair with CorMatrix-ECM Procedure: Coronary Artery Bypass Grafting Surgery | Early Phase 1 | [99] |
| NCT03798353 | Pericardial Matrix with Mesenchymal Stem Cells for the Treatment of Patients With Infarcted Myocardial Tissue (The PERISCOPE Trial) | Combination Product: PeriCord: Expanded and cryopreserved allogeneic umbilical cord Wharton’s jelly-derived adult mesenchymal stem cells colonized on human pericardial matrix. Procedure: Surgery by sternotomy | Phase 1 | [100] |
| NCT05632432 | Atrial Appendage Micrograft Transplantation in Conjunction with Cardiac Surgery—the AAMS2 Randomized Controlled Trial | Procedure: Epicardial AAMs-patch transplantation Diagnostic Test: RNA-stabilized whole blood sampling Diagnostic Test: Plasma sampling Diagnostic Test: Transthoracic echocardiography Diagnostic Test: Late-gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMRI) Other: Symptom-scaling Other: 6 min walking test (6MWT) Diagnostic Test: Blood sampling (NT-proBNP) Diagnostic Test: Transesophageal echocardiography | Not applicable | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scafa Udriște, A.; Niculescu, A.-G.; Iliuță, L.; Bajeu, T.; Georgescu, A.; Grumezescu, A.M.; Bădilă, E. Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration. Polymers 2023, 15, 1177. https://doi.org/10.3390/polym15051177
Scafa Udriște A, Niculescu A-G, Iliuță L, Bajeu T, Georgescu A, Grumezescu AM, Bădilă E. Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration. Polymers. 2023; 15(5):1177. https://doi.org/10.3390/polym15051177
Chicago/Turabian StyleScafa Udriște, Alexandru, Adelina-Gabriela Niculescu, Luminița Iliuță, Teodor Bajeu, Adriana Georgescu, Alexandru Mihai Grumezescu, and Elisabeta Bădilă. 2023. "Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration" Polymers 15, no. 5: 1177. https://doi.org/10.3390/polym15051177
APA StyleScafa Udriște, A., Niculescu, A.-G., Iliuță, L., Bajeu, T., Georgescu, A., Grumezescu, A. M., & Bădilă, E. (2023). Progress in Biomaterials for Cardiac Tissue Engineering and Regeneration. Polymers, 15(5), 1177. https://doi.org/10.3390/polym15051177











