Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of Elastin from Broiler Skin
2.2. Extraction and Purification of Collagen Type-I from Ovine Tendon
2.3. Fabrication of Collagen-Elastin (Col/Elas) Scaffolds
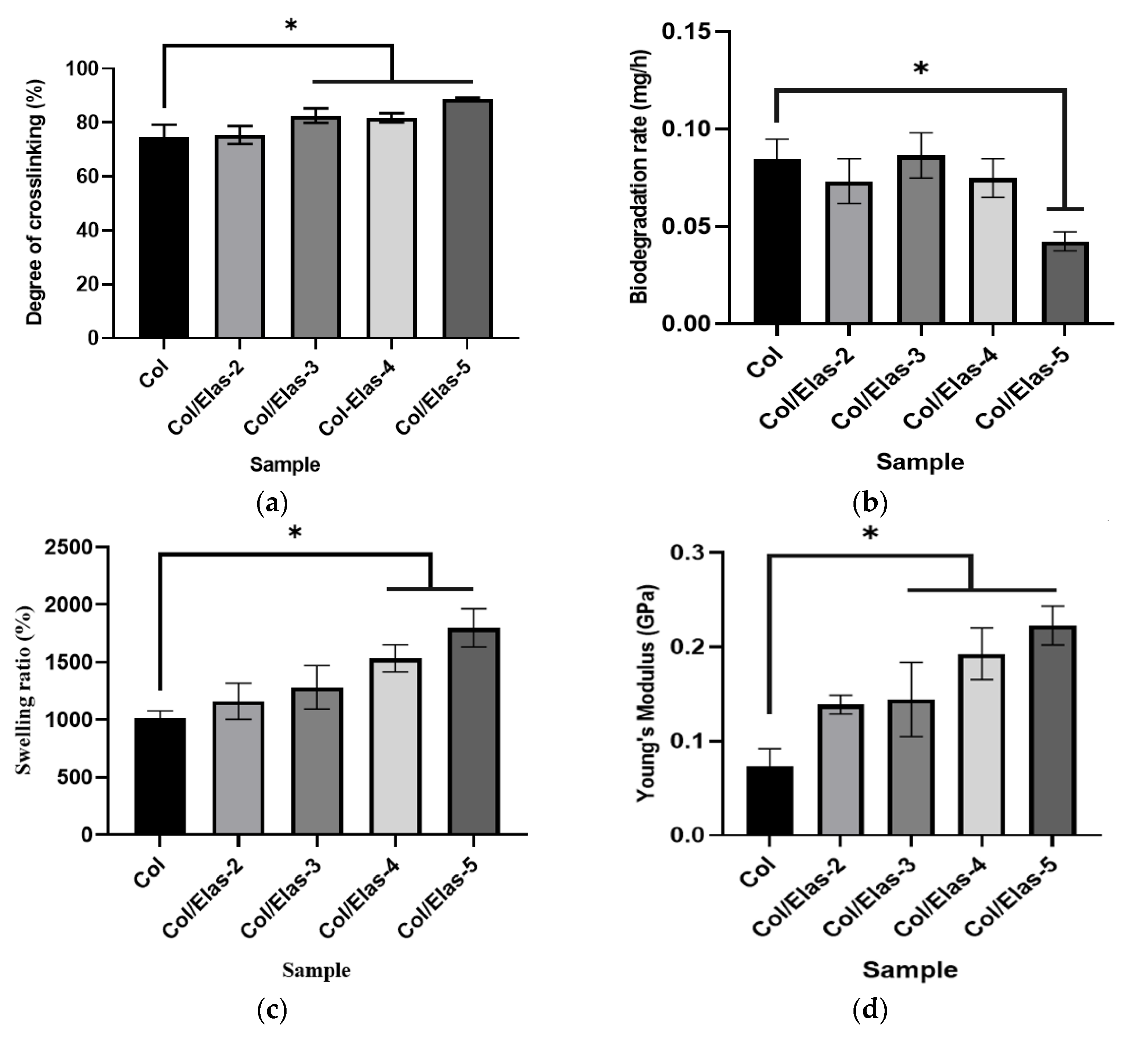
2.4. Degree of Crosslinking
2.5. Swelling Ratio
2.6. Porosity
2.7. Biodegradation
2.8. Chemical Characterisation of Scaffolds
2.9. Microstructure of Scaffolds
2.10. Tensile Strength
2.11. Human Skin Cell Harvest and Culture
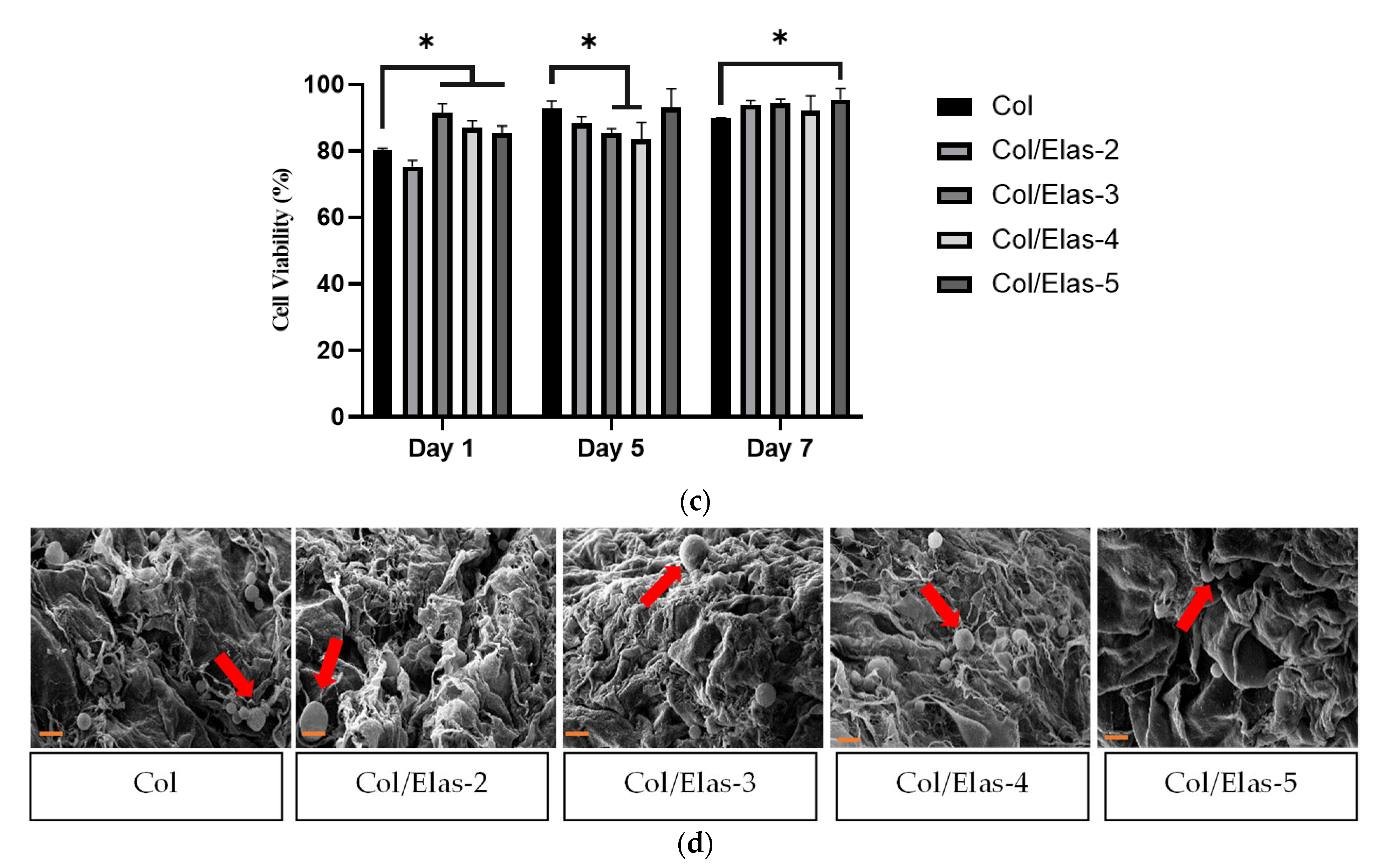
2.12. Live and Dead Assay
2.13. Cell Morphology
2.14. Cell Viability
2.15. Statistical Analysis
3. Results
3.1. Physical Characterisation of Hybrid Scaffold
3.2. Chemical Characterisation of Hybrid Bioscaffold
3.3. Biocompatibility of Elas/Col Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Rnjak, J.; Wise, S.G.; Mithieux, S.M.; Weiss, A.S. Severe burn injuries and the role of Elastin in the design of dermal substitutes. Tissue Eng. Part B Rev. 2011, 17, 81–91. [Google Scholar] [CrossRef]
- Coenen, A.M.J.; Bernaerts, K.V.; Harings, J.A.W.; Jockenhoevel, S.; Ghazanfari, S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018, 79, 60–82. [Google Scholar] [CrossRef]
- Prado-Audelo, M.L.D.; Mendoza-Muñoz, N.; Escutia-Guadarrama, L.; Giraldo-Gomez, D.M.; González-Torres, M.; Florán, B.; Cortes, H.; Leyva-Gómez, G. Recent advances in elastin-based biomaterials. J. Pharm. Pharm. Sci. 2020, 23, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Narani, N.; McCulloch, C.A.G. The compliance of collagen gels regulates transforming growth factor-β induction of α-smooth muscle actin in fibroblasts. Am. J. Pathol. 1999, 154, 871–882. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Benton, J.A.; Anseth, K.S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 2010, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Nillesen, S.T.; Wismans, R.; Reinhardt, D.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Depots of solubilised elastin promote the formation of blood vessels and elastic fibres in rat. J. Control. Release 2006, 116, e84–e85. [Google Scholar] [CrossRef]
- Daamen, W.F.; Nillesen, S.T.M.; Wismans, R.G.; Reinhardt, D.P.; Hafmans, T.; Veerkamp, J.H.; Van Kuppevelt, T.H. A biomaterial composed of collagen and solubilized elastin enhances angiogenesis and elastic fiber formation without calcification. Tissue Eng. Part A. 2008, 14, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Wang, Y.; Liu, K.; Mitts, T.F.; Jimenez, F. Proteolytic digest derived from bovine Ligamentum Nuchae stimulates deposition of new elastin-enriched matrix in cultures and transplants of human dermal fibroblasts. J. Dermatol. Sci. 2005, 39, 155–166. [Google Scholar] [CrossRef]
- Minardi, S.; Taraballi, F.; Wang, X.; Cabrera, F.J.; Van Eps, J.L.; Robbins, A.B.; Sandri, M.; Moreno, M.R.; Weiner, B.K.; Tasciotti, E. Biomimetic collagen/elastin meshes for ventral hernia repair in a rat model. Acta Biomater. 2017, 50, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Guo, M.; Zhang, X.; Luo, X. An elastin-based vasculogenic scaffold promotes marginal islet mass engraftment and function at an extrahepatic site. J. Immunol. Regen. Med. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, J.C.; González de Torre, I.; Ibañez-Fonseca, A.; Alonso, M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.B.; Lokanathan, Y.; Nadzir, M.M.; Aminuddin, S.; Ruszymah, B.H.I.; Chowdhury, S.R. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: A comparative study. Malays. J. Med. Sci. 2017, 24, 33–43. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171. [Google Scholar] [CrossRef]
- Ooi, K.S.; Haszman, S.; Wong, Y.N.; Soidin, E.; Hesham, N.; Mior, M.A.A.; Tabata, Y.; Ahmad, I.; Fauzi, M.B.; Yunus, M.H.M. Physicochemical characterization of bilayer hybrid nanocellulose-collagen as a potential wound dressing. Materials 2020, 13, 4352. [Google Scholar] [CrossRef]
- Nadalian, M.; Kamaruzaman, N.; Shakir, M.; Yusop, M. Isolation, purification and characterization of antioxidative bioactive elastin peptides from poultry skin. Food Sci. Anim. Resour. 2019, 39, 966–979. [Google Scholar] [CrossRef]
- Shigemura, Y.; Nakaba, M.; Shiratsuchi, E.; Suyama, M.; Yamada, M.; Kiyono, T.; Fukamizu, K.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of food-derived elastin peptide, prolyl-glycine (pro-gly), in human blood after ingestion of elastin hydrolysate. J. Agric. Food Chem. 2012, 60, 5128–5133. [Google Scholar] [CrossRef]
- Yoseph, Z.; Gladstone Christopher, J.; Assefa Demessie, B.; Tamil Selvi, A.; Sreeram, K.J.; Raghava Rao, J. Extraction of elastin from tannery wastes: A cleaner technology for tannery waste management. J. Clean. Prod. 2020, 243, 118471. [Google Scholar] [CrossRef]
- Shiratsuchi, E.; Nakaba, M.; Yamada, M. Elastin hydrolysate derived from fish enhances proliferation of human skin fibroblasts and elastin synthesis in human skin fibroblasts and improves the skin conditions. J. Sci. Food Agric. 2016, 96, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Nadalian, M.; Yusop, S.M.; Babji, A.S.; Mustapha, W.A.W.; Azman, M.A. Effects of enzymatic hydrolysis on the antioxidant activity of water- soluble elastin extracted from broiler and spent hen skin. Int. J. Appl. Biol. Pharm. Technol. 2015, 6, 1–10. [Google Scholar]
- Yusop, S.M.; Nadalian, M.; Babji, A.S.; Mustapha, W.A.W.; Forghani, B.; Azman, M.A. Production of antihypertensive elastin peptides from waste poultry skin. ETP Int. J. Food Eng. 2016, 2, 21–25. [Google Scholar] [CrossRef]
- Kamaruzaman, N.; Yusop, S.M. Determination of stability of cosmetic formulations incorporated with water-soluble elastin isolated from poultry. J. King Saud Univ.-Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Kamaruzaman, N.; Fauzi, M.B.; Yusop, S.M. Characterization and toxicity evaluation of broiler skin elastin for potential functional biomaterial in tissue engineering. Polymers 2022, 14, 963. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Fei, L.; Cui, F.; Tang, C.; Yin, C. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 2007, 28, 1258–1266. [Google Scholar] [CrossRef]
- Cheheltani, R.; Cushla, M.; McGoverin, J.R.; Vorp, D.A.; Kiani, M.F.; Pleshko, N. Fourier transform infrared spectroscopy to quantify collagen and elastin in an in vitro model of extracellular matrix degradation in aorta. Analyst 2014, 139, 3039–3047. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Kuderko, J.; Bajek, A.; Maj, M.; Sionkowska, A.; Ziegler-Borowska, M. Collagen/elastin hydrogels cross-linked by squaric acid. Mater. Sci. Eng. C 2016, 60, 100–108. [Google Scholar] [CrossRef]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolym.-Biospectrosc. Sect. 2001, 62, 1–8. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Litowczenko, J.; Woźniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and current achievements in development of multifunctional bioscaffolds for medical application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Muhammad, M.A.; Mohd Yunus, M.H.; Mh Busra, F. Genipin-crosslinked gelatin scaffold in tissue engineering: A systematic review. Med. Health 2019, 14, 1–16. [Google Scholar] [CrossRef]
- Výborný, K.; Vallová, J.; Kočí, Z.; Kekulová, K.; Jiráková, K.; Jendelová, P.; Hodan, J.; Kubinová, Š. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. 2019, 9, 10674. [Google Scholar] [CrossRef]
- Kočí, Z.; Výborný, K.; Dubišová, J.; Vacková, I.; Jäger, A.; Lunov, O.; Jiráková, K.; Kubinová, Š. Extracellular matrix hydrogel derived from human umbilical cord as a scaffold for neural tissue repair and its comparison with extracellular matrix from porcine tissues. Tissue Eng. Part C Methods 2017, 23, 333–345. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Psunica-Panea, G.; Ficai, A.; Maria Marin, M.; Marin, T.; Georgiana Albu, M.; Denis Constantin, V.; Dinu-Pîrvu, C.; Vuluga, Z.; Cosmin Corobea, M.; Violeta Ghica, M. New collagen-dextran-zinc oxide composites for wound dressing. J. Nanomater. 2016, 2016, 5805034. [Google Scholar] [CrossRef]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, Z.R.; Chen, C.H.; Ho, M.L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2013, 8, e56330. [Google Scholar] [CrossRef]
- Cai, J.; Chen, X.; Wang, X.; Tan, Y.; Ye, D.; Jia, Y.; Liu, P.; Yu, H. High-water-absorbing calcium alginate fibrous scaffold fabricated by microfluidic spinning for use in chronic wound dressings. RSC Adv. 2018, 8, 39463–39469. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Perez-puyana, V.; Villanueva, P.; Jiménez-rosado, M.; de la Portilla, F.; Romero, A. Incorporation of elastin to improve polycaprolactone-based scaffolds for skeletal muscle via electrospinning. Polymers 2021, 13, 1501. [Google Scholar] [CrossRef]
- Tronci, G. The application of collagen in advanced wound dressings. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 363–389. [Google Scholar]
- Lim, M.M.; Sun, T.; Sultana, N. In Vitro Biological Evaluation of Electrospun Polycaprolactone/Gelatine Nanofibrous Scaffold for Tissue Engineering. J. Nanomater. 2015, 16, 416. [Google Scholar] [CrossRef]
- Suesca, E.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C.; Fontanilla, M.R. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater. Sci. Eng. C 2017, 77, 333–341. [Google Scholar] [CrossRef]
- Li Loh, Q.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Mour, M.; Das, D.; Winkler, T.; Hoenig, E.; Mielke, G.; Morlock, M.M.; Schilling, A.F. Advances in porous biomaterials for dental and orthopaedic applications. Materials 2010, 3, 2947–2974. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun composite mats of poly[(D, Llactide)-co-glycolide] and collagen with high porosity as potential scaffolds for skin tissue engineering. Macromol. Mater. Eng. 2009, 294, 611–619. [Google Scholar] [CrossRef]
- Tan, H.B.; Wang, F.Y.; Ding, W.; Zhang, Y.; Ding, J.; Cai, D.X.; Yu, K.F.; Yang, J.; Yang, L.; Xu, Y.Q. Fabrication and evaluation of porous keratin/chitosan (KCS) scaffolds for effectively accelerating wound healing. Biomed. Environ. Sci. 2015, 28, 178–189. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; Morais, H.A.; Silva, V.D.M.; Silva, M.R. Degree of hydrolysis and peptide profile of whey proteins using pancreatin. Braz. Soc. Food Nutr. 2013, 38, 278–290. [Google Scholar] [CrossRef]
- Su, H.; Fujiwara, T.; Bumgardner, J.D. A study of combining elastin in the chitosan electrospinning to increase the mechanical strength and bioactivity. Mar. Drugs 2021, 19, 169. [Google Scholar] [CrossRef]
- Purcel, G.; Meliţă, D.; Andronescu, E.; Grumezescu, A.M. Collagen-based nanobiomaterials: Challenges in soft tissue engineering. In Nanobiomaterials in Soft Tissue Engineering: Applications of Nanobiomaterials; Grumezescu, A.M., Ed.; Elsevier Inc.: Oxford, UK, 2016; pp. 173–200. ISBN 9780323428651. [Google Scholar]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Grabska-Zielí Nska, S.; Skopínska-Wiśniewska, J.; Jakubowska, E. Examining the impact of squaric acid as a crosslinking agent on the properties of chitosan-based films. Int. J. Mol. Sci. Artic. 2021, 22, 3329. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Xu, Y.; Li, X.; Yuan, L.; Tan, H.; Li, D.; Mu, C. Fabrication of antibacterial collagen-based composite wound dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166. [Google Scholar] [CrossRef]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ɛ-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ganesan, K.; Simionescu, D.T.; Vyavahare, N.R. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 2004, 25, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, A.B.; Geyik, C. From viability to cell death: Claims with insufficient evidence in high-impact cell culture studies. PLoS ONE 2022, 17, e0250754. [Google Scholar] [CrossRef]
- Sallehuddin, N.; Fadilah, N.I.M.; Hwei, N.M.; Wen, A.P.Y.; Yusop, S.M.; Rajab, N.F.; Hiraoka, Y.; Tabata, Y.; Fauzi, M.B. Characterization and cytocompatibility of Collagen–Gelatin–Elastin (CollaGee) acellular skin substitute towards human dermal fibroblasts: In Vitro assessment. Biomedicines 2022, 10, 1327. [Google Scholar] [CrossRef]



| Sample | Element Weight (%) | ||
|---|---|---|---|
| C | O | N | |
| Col | 75.51 ± 2.48 | 23.37 ± 0.69 | 8.56 ± 0.83 |
| Col/Elas-2 | 70.66 ± 2.89 * | 23.79 ± 0.65 | 6.70 ± 0.62 * |
| Col/Elas-3 | 64.91 ± 2.07 * | 29.06 ± 0.60 * | 7.04 ± 0.18 * |
| Col/Elas-4 | 72.50 ± 2.00 | 24.07 ± 0.99 | 6.02 ± 0.20 * |
| Col/Elas-5 | 59.06 ± 1.36 * | 32.93 ± 0.98 * | 7.09 ± 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruzaman, N.; Fauzi, M.B.; Tabata, Y.; Yusop, S.M. Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications. Polymers 2023, 15, 1929. https://doi.org/10.3390/polym15081929
Kamaruzaman N, Fauzi MB, Tabata Y, Yusop SM. Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications. Polymers. 2023; 15(8):1929. https://doi.org/10.3390/polym15081929
Chicago/Turabian StyleKamaruzaman, Nurkhuzaiah, Mh Busra Fauzi, Yasuhiko Tabata, and Salma Mohamad Yusop. 2023. "Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications" Polymers 15, no. 8: 1929. https://doi.org/10.3390/polym15081929
APA StyleKamaruzaman, N., Fauzi, M. B., Tabata, Y., & Yusop, S. M. (2023). Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications. Polymers, 15(8), 1929. https://doi.org/10.3390/polym15081929











