Methods and Characteristics of Drug Extraction from Ion-Exchange-Resin-Mediated Preparations: Influences, Thermodynamics, and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chromatographic Conditions
2.2.2. Study of Extraction Method
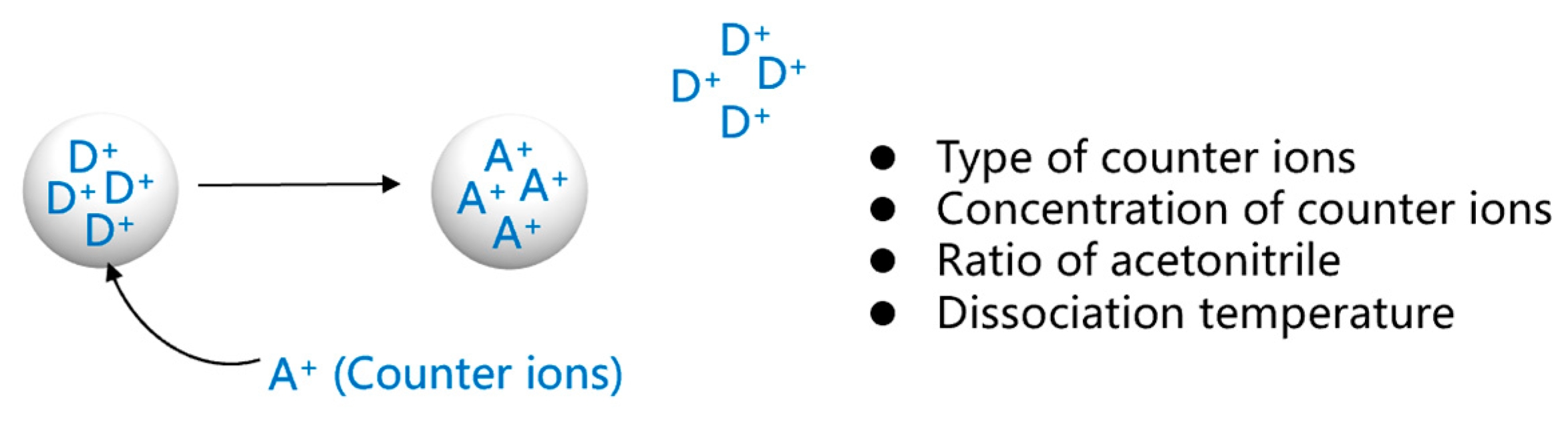
2.2.3. Study of Factors Influencing the Dissociation Process
2.2.4. Analytical Method Validation
2.2.5. Study of the Thermodynamics of Dissociation
2.2.6. Study of the Kinetics of Dissociation
Simple-Order Reactions
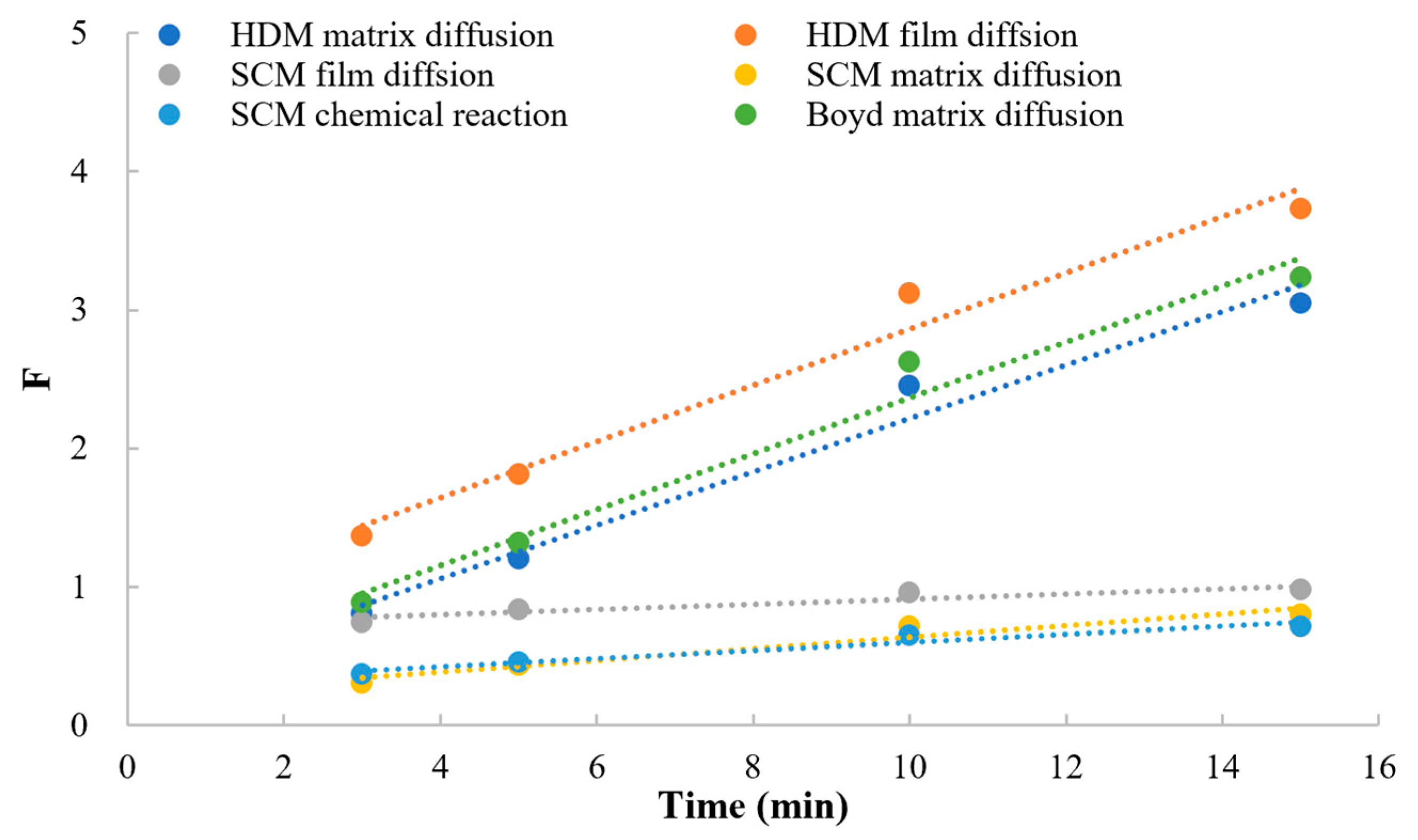
Model of Kinetics
- Shrinking core model (SCM)
- 2.
- Homogeneous diffusion model (HDM):
- 3.
- Boyd model (Boyd)
3. Results
3.1. Study of Extraction Method
3.2. Study of Factors Influencing the Dissociation Process
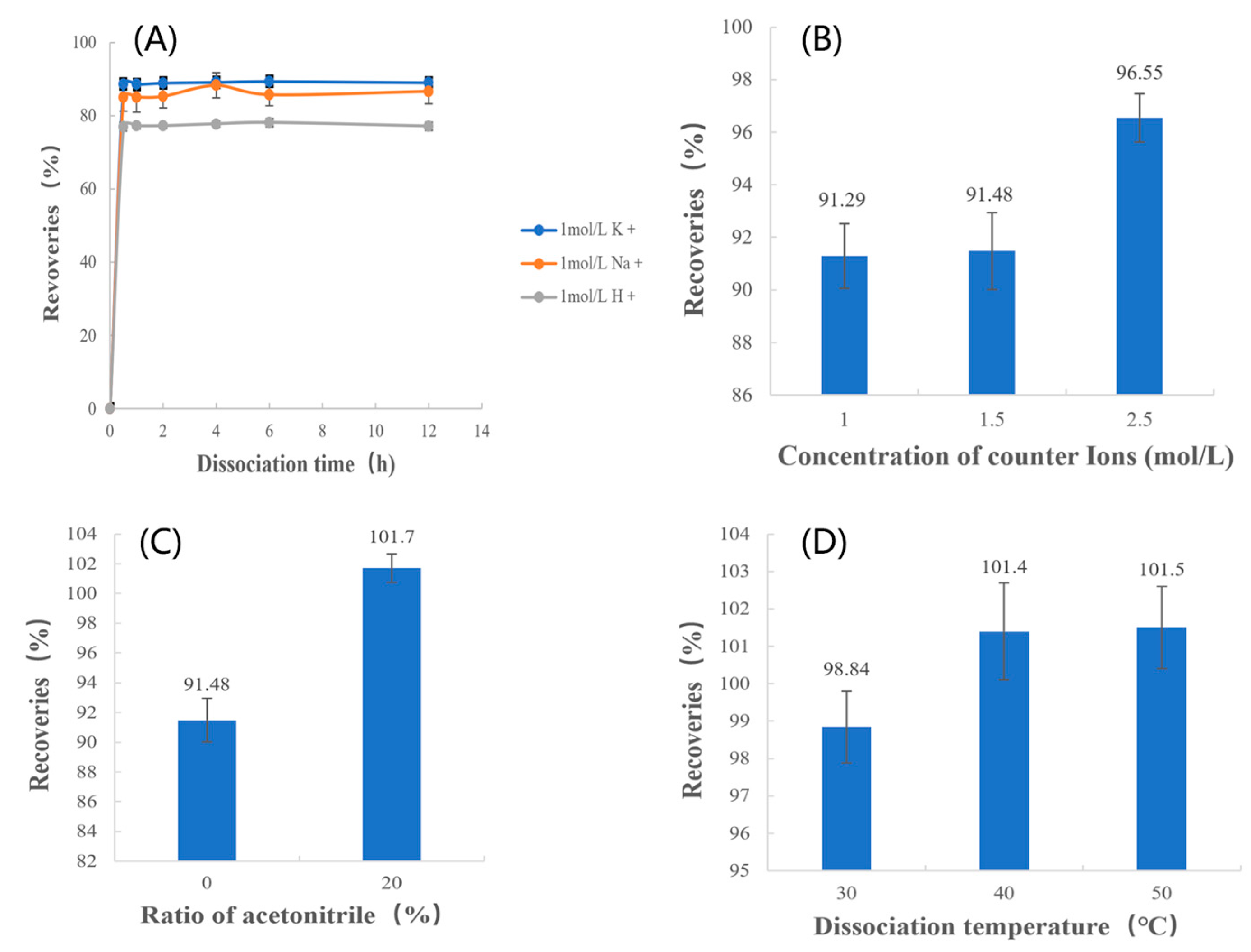
3.2.1. Type of Counterions
3.2.2. Concentration of Counterions
3.2.3. Ratio of Acetonitrile
3.2.4. Dissociation Temperature
3.3. Analytical Method Validation
3.4. Study of the Thermodynamics of Dissociation
3.5. Study of the Kinetics of Dissociation
3.5.1. Simple-Order Reactions
3.5.2. Model of Kinetics
3.6. Study of Factors Influencing the Dissociation Process Using the Boyd Model
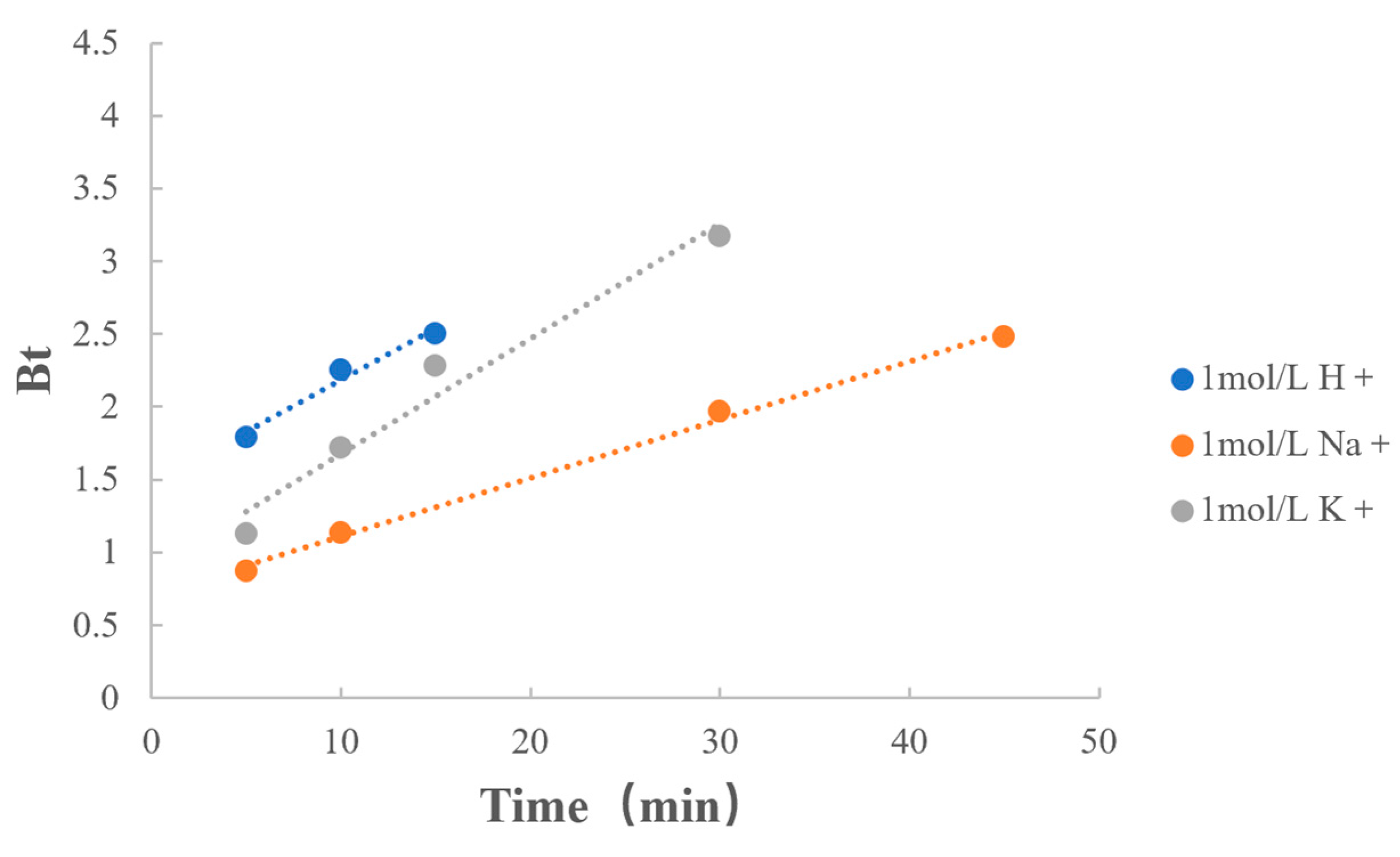
3.6.1. Type of Counterions
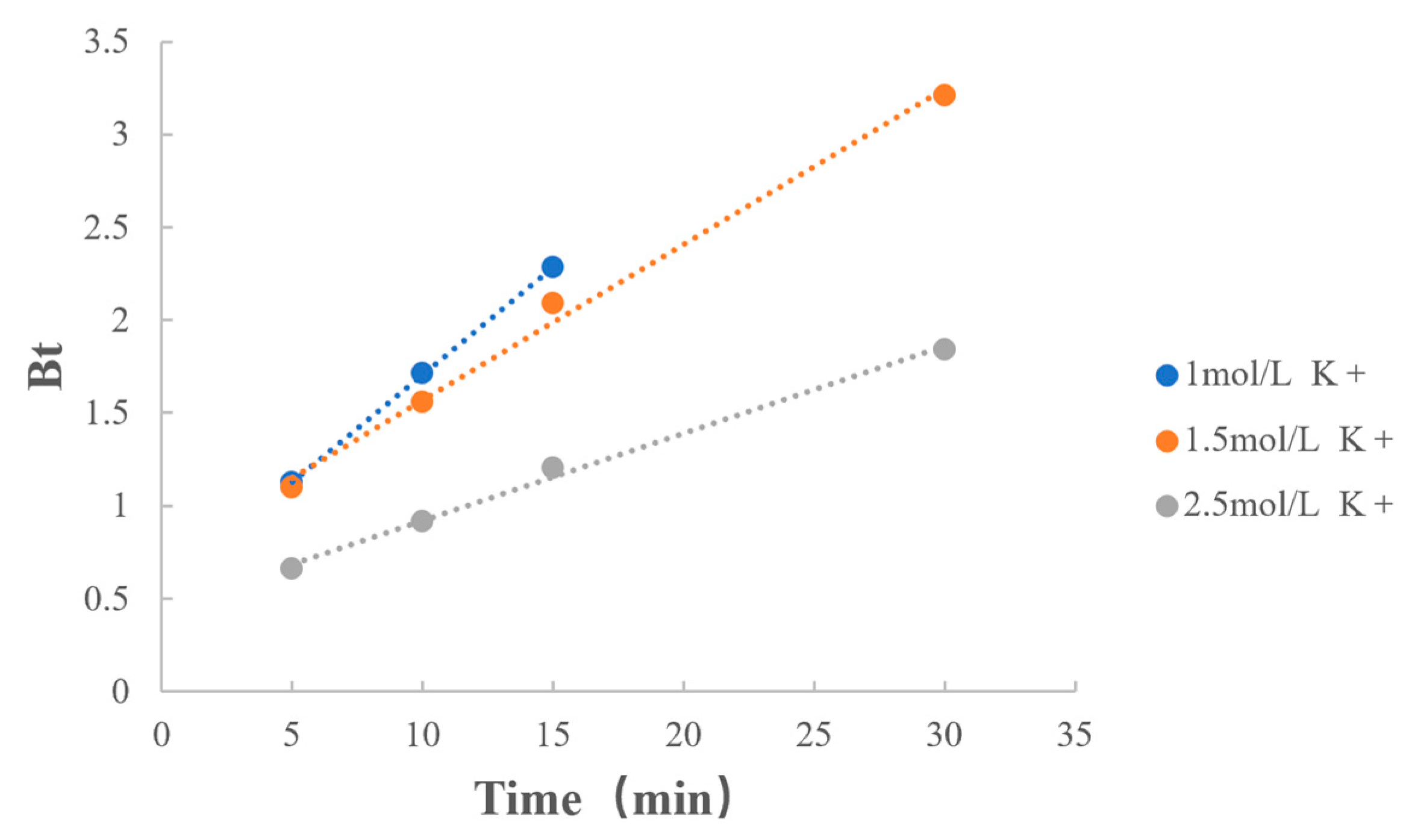
3.6.2. Concentration of Counterions
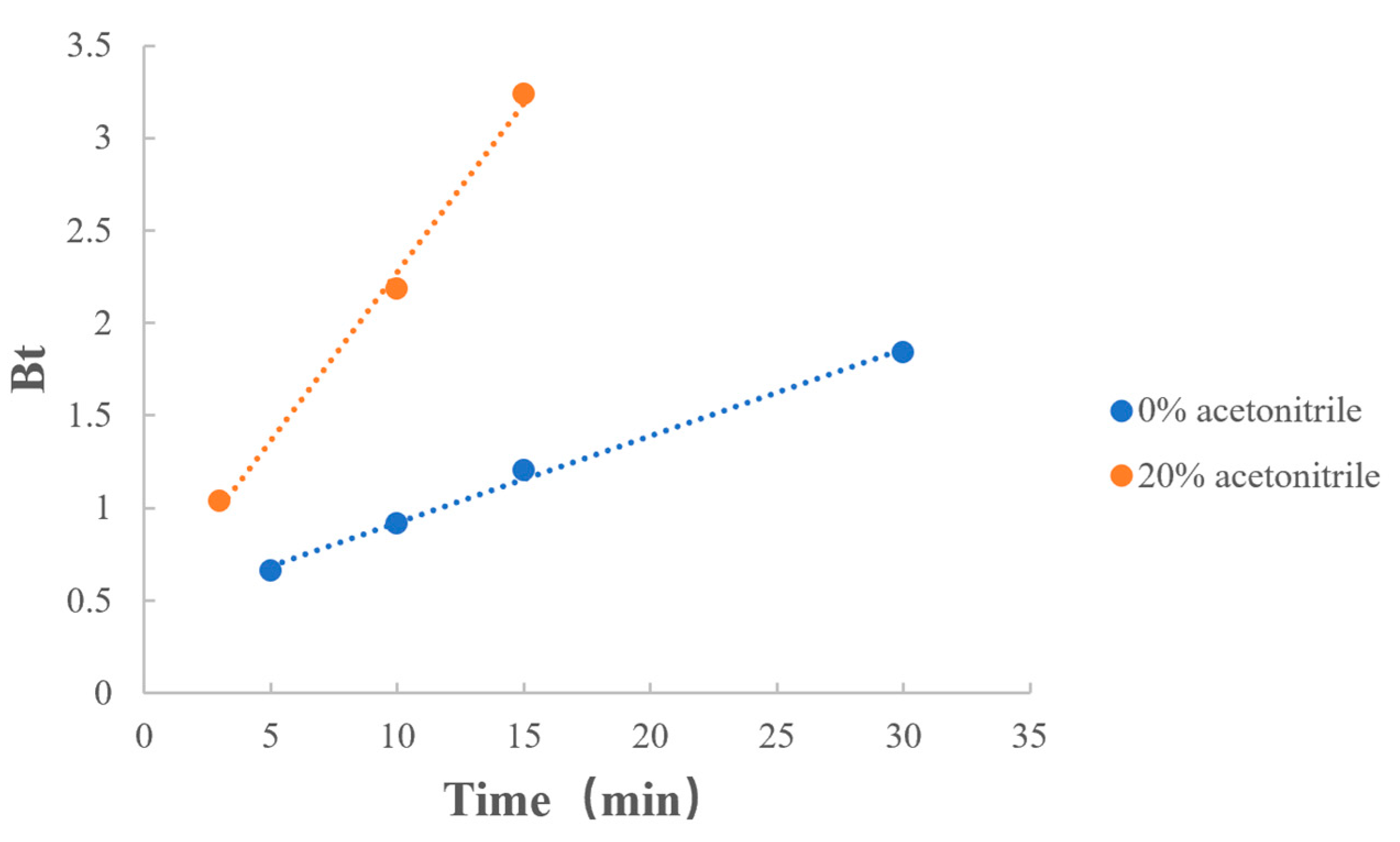
3.6.3. Ratio of Acetonitrile
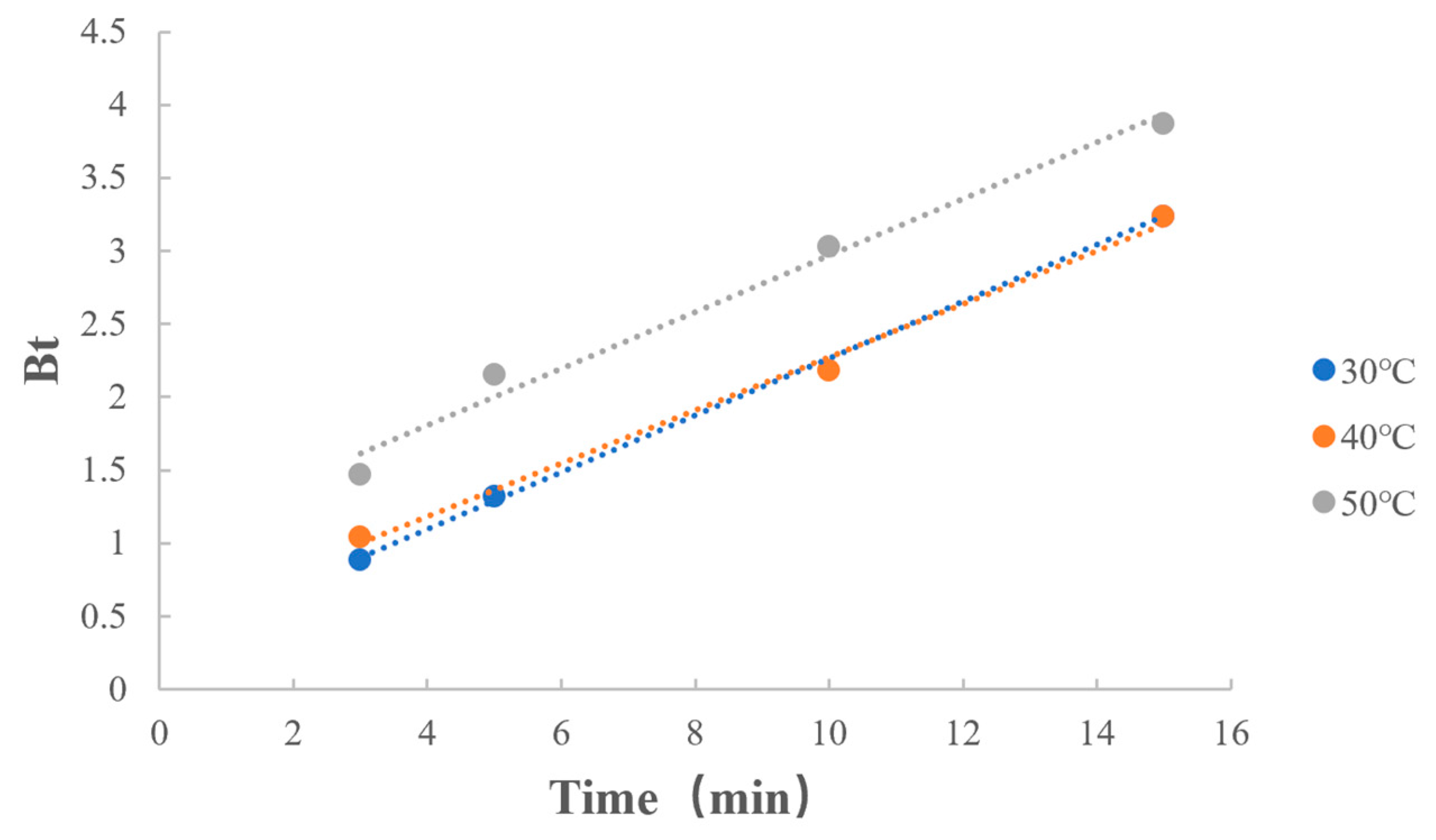
3.6.4. Dissociation Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.X.; Zhang, Z.P.; Li, A.M.; Pan, B.C.; Zhang, X.L. Advance in Ion Exchange and Adsorption Resins in China. Acta Polym. Sin. 2018, 7, 814–828. [Google Scholar]
- Suhagiya, V.K.; Goyani, A.N.; Gupta, R.N. Taste Masking by ion exchange resin and its new applications: A review. Int. J. Pharm. L Sci. Res. 2010, 1, 22–37. [Google Scholar]
- Zhang, T.-Y.; Du, R.-F.; Wang, Y.-J.; Hu, J.-L.; Wu, F.; Feng, Y. Research Progress of Preparation Technology of Ion-Exchange Resin Complexes. AAPS PharmSciTech 2022, 23, 105. [Google Scholar] [CrossRef]
- Anand, V.; Kandarapu, R.; Garg, S. Ion-exchange resins: Carrying drug delivery forward. Drug Discov. Today 2001, 6, 905–914. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.D.; Chang, R.K.; Hussain, M.A. Ion-Exchange Resins as Drug Delivery Carriers. J. Pharm. Sci. 2009, 98, 3886–3902. [Google Scholar] [CrossRef]
- Torre, M.; Marina, M.L. The State of the Art of Ligand-Loaded Complexing Resins. Characteristics and Applications. Crit. Rev. Anal. Chem. 1994, 24, 327–361. [Google Scholar] [CrossRef]
- Kammerer, J.; Carle, R.; Kammerer, D.R. Adsorption and Ion Exchange: Basic Principles and Their Application in Food Pro-cessing. J. Agric. Food Chem. 2011, 59, 22–42. [Google Scholar] [CrossRef]
- Yu, B.; Cong, H.; Yuan, H.; Liu, X.; Jiao, M.; Wang, D. Nano/Microstructured Ion Exchange Resins and Their Applications. J. Nanosci. Nanotechnol. 2014, 14, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.; Gupta, S.; Lam, A.; Shoulson, I.; Wigal, S.J.A.O.G.P. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: Proof-of-concept and proof-of-product studies. Arch. Gen. Psychiatry 2003, 60, 204. [Google Scholar] [CrossRef]
- Hicks, A.J.; Clay, F.J.; Hopwood, M.; James, A.C.; Jayaram, M.; Perry, L.A.; Batty, R.A.; Ponsford, J.L. The Efficacy and Harms of Pharmacological Interventions for Aggression After Traumatic Brain Injury—Systematic Review. Front. Neurol. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Wang, Z.; Zheng, A. Study on the Complexation and Release Mechanism of Methylphenidate Hydrochloride Ion Exchange Resin Complex. Polymers 2021, 13, 4394. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, T.; Li, J.; Sun, W.; He, H.; Gou, J.; Wang, J.; Yin, T.; Zhang, Y.; Tang, X. Studies on the in vitro ion exchange kinetics and thermodynamics and in vivo pharmacokinetics of the car-binoxamine-resin complex. Int. J. Pharm. 2020, 588, 119779. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.R.; Soliman, M.A.; Ali, A.H.; Othman, S.H. Kinetic studies on radio-selenium uptake by ion exchange resin. Sep. Sci. Technol. 2015, 51, 976–989. [Google Scholar] [CrossRef]
- Soliman, M.A.; Mahmoud, M.R.; Ali, A.H.; Othman, S.H. The sorption mechanism of Selenium-75 on Amberlite MB9L. J. Radioanal. Nucl. Chem. Int. J. Deal. All Asp. Appl. Nucl. Chem. 2016, 307, 567–575. [Google Scholar] [CrossRef]
- Aiouache, F.; Goto, S. Sorption effect on kinetics of etherification of tert-amyl alcohol and ethanol. Chem. Eng. Sci. 2003, 58, 2065–2077. [Google Scholar] [CrossRef]
- Morin, V.; Garrault, S.; Begarin, F.; Dubois-Brugger, I. The influence of an ion-exchange resin on the kinetics of hydration of tricalcium silicate. Cem. Concr. Res. 2010, 40, 1459–1464. [Google Scholar] [CrossRef]
- Khawassek, Y.M.; Eliwa, A.A.; Haggag, E.S.A.; Omar, S.A.; Abdel-Wahab, S.M. Adsorption of rare earth elements by strong acid cation exchange resin thermodynamics, characteristics and kinetics. SN Appl. Sci. 2019, 1, 51. [Google Scholar] [CrossRef] [Green Version]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef]
- Yu, Z.; Qi, T.; Qu, J.; Wang, L.; Chu, J. Removal of Ca(II) and Mg(II) from potassium chromate solution on Amberlite IRC 748 synthetic resin by ion exchange. J. Hazard. Mater. 2009, 167, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Kim, Y.; Joo, C.K.; Choi, K.; Yi, J. Batch adsorptive removal of copper ions in aqueous solutions by ion exchange resins: 1200H and IRN97H. Korean J. Chem. Eng. 2004, 21, 187–194. [Google Scholar] [CrossRef]
- Ichikawa, H.; Fujioka, K.; Adeyeye, M.C.; Fukumori, Y. Use of ion-exchange resins to prepare 100 microm sized microcapsules with prolonged drug-release by the Wurster process. Int. J. Pharm. 2001, 216, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, S.; Cui, P.; Wang, C.; Dai, J.; Zhou, L.; Huang, Y.; Hou, B.; Hao, H.; Zhou, L.; et al. Solvent Effects on Catechol Crystal Habits and Aspect Ratios: A Combination of Experiments and Molecular Dynamics Simulation Study. Crystals 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Nagy, P.I. Competing Intramolecular vs. Intermolecular Hydrogen Bonds in Solution. IJMS 2014, 15, 19562–19633. [Google Scholar] [CrossRef] [Green Version]

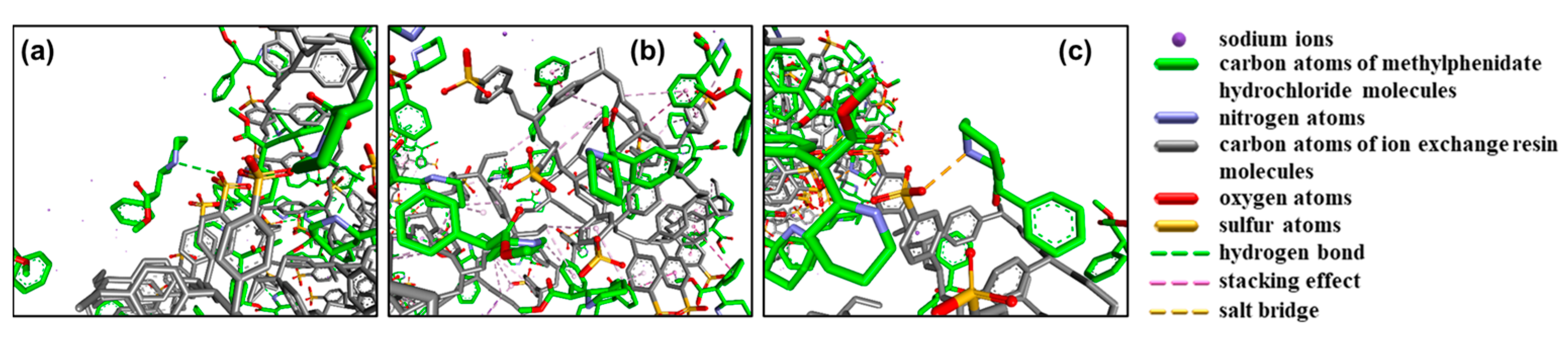



| Extraction Method | Diluent Type | Sample Extraction |
|---|---|---|
| Ultrasonic Extraction | Acetonitrile—pH 3.0 acidified water (25:75) | Ultrasound for 10 min |
| High-Pressure Homogenization | Acetonitrile–methanol (50:50) | Homogenize for 5 min |
| Ultrasonic Cell Crusher | Acetonitrile–methanol (50:50) | Process with ultrasonic cell crusher for 5 min |
| Dissociation by Counterions | 1 mol/L hydrochloric acid | Stir for 4 h |
| Factor | Type of Counterions | Dissociation Temperature (°C) | Concentration of Counterions (mol/L) | Ratio of Acetonitrile (%) | Concentration of MPH (mg/mL) |
|---|---|---|---|---|---|
| Type of Counterions | H+ | 30 | 1 | 0 | 0.1 |
| K+ | 30 | 1 | 0 | 0.1 | |
| Na+ | 30 | 1 | 0 | 0.1 | |
| Concentration of Counterions | K+ | 30 | 1 | 0 | 0.1 |
| K+ | 30 | 1.5 | 0 | 0.1 | |
| K+ | 30 | 2.5 | 0 | 0.1 | |
| Ratio of Acetonitrile | K+ | 30 | 2.5 | 0 | 0.1 |
| K+ | 30 | 2.5 | 20 | 0.1 | |
| Dissociation Temperature | K+ | 30 | 2.5 | 20 | 0.1 |
| K+ | 40 | 2.5 | 20 | 0.1 | |
| K+ | 50 | 2.5 | 20 | 0.1 |
| Items of Validation | Sample Preparation | Standards |
|---|---|---|
| Specificity | Blank solvent, blank excipients, reference solution, test solution, degradation products of acid (add 1.2 M HCl, 25 °C for 4 h), degradation products of base (add 1.2 M NaOH, 25 °C for 10 min), degradation products of oxygen (add 30% H2O2, 25 °C for 4 h) | Peak separation > 1.5, peak purity > 980 |
| Linearity and range | MPH reference solution of 0.06 mg/mL–0.32 mg/ml | r > 0.999 |
| Accuracy (n = 3) | MPH reference solutions of 0.16 mg/mL, 0.2 mg/mL, and 0.24 mg/mL were added to blank excipients | Recoveries in the range of 95.0%–105.0%, RSD ≤ 2.0% |
| Repeatability (n = 6) | Six reference solutions and test solutions of MPH | RSD ≤ 2.0% |
| Intermediate precision (n = 12) | Six reference solutions and test solution of MPH (2 analysts) | RSD ≤ 2.0% |
| Extraction Method | p-Values |
|---|---|
| Ultrasonic extraction | 0.0016 (<0.05) |
| High-pressure homogenization | 0.0017 (<0.05) |
| Ultrasonic cell crusher | 0.0015 (<0.05) |
| Dissociation | ----- |
| Factors Influencing the Dissociation Process | p-Values | |
|---|---|---|
| Concentration of counterions | 1 mol/L K+ | 0.0275 (<0.05) |
| 1.5 mol/L K+ | 0.0306 (<0.05) | |
| 2.5 mol/L K+ | - | |
| Ratio of acetonitrile | 0% | 0.0030 (<0.05) |
| 20% | - | |
| Dissociation temperature | 30 °C | 0.0289 (<0.05) |
| 40 °C | 0.8509 (>0.05) | |
| 50 °C | - | |
| Items of Validation | Results | Standards |
|---|---|---|
| Linearity and range | Y = 0.6461X + 0.7529 (r = 0.9999) | r > 0.999 |
| Accuracy | Recoveries = 100.6% RSD = 1.56% | Recoveries in the range of 95.0–105.0%, RSD ≤ 2.0% |
| Repeatability | RSD = 0.17% | RSD ≤ 2.0% |
| Intermediate precision | RSD = 0.39% | RSD ≤ 2.0% |
| T(K) | 303 | 313 | 323 |
|---|---|---|---|
| Ke | 0.0280 | 0.0429 | 0.0504 |
| ΔG (kJ/mol) | 9.00 | 8.19 | 8.03 |
| ΔH (kJ/mol) | 2.7 × 10−4 | 2.7×10−4 | 2.7 × 10−4 |
| ΔS (J/mol) | −29.72 | −26.17 | −24.85 |
| Reaction Type | Kinetic Equation | Regression Equation | r |
|---|---|---|---|
| Zero-order | Qe − Qt = −kt | y = −1.9077x + 28.989 | 0.9347 |
| First-order | ln (Qe − Qt) = −kt | y = −0.1783x + 3.7309 | 0.9820 |
| Second-order | 1/Qe − Qt = kt | y = 0.0226x − 0.0417 | 0.9963 |
| Kinetic Model | Rate-Limiting Step | Y-Axis, F(x) | X-Axis | r |
|---|---|---|---|---|
| SCM | a. Film diffusion | F | t | 0.9347 |
| b. Matrix diffusion | t | 0.9695 | ||
| c. Chemical reaction | t | 0.9731 | ||
| HDM | a. Film diffusion | t | 0.9868 | |
| b. Matrix diffusion | t | 0.9881 | ||
| Boyd | a. Matrix diffusion | t | 0.9870 |
| Factors | Value | Regression Equation | r |
|---|---|---|---|
| Type of Counterions | 1 mol/L H+ | y = 0.0711x + 1.4717 | 0.9864 |
| 1 mol/L Na+ | y = 0.0401x + 0.7082 | 0.9982 | |
| 1 mol/L K+ | y = 0.0792x + 0.8858 | 0.9831 |
| Factors | Value | Regression Equation | r |
|---|---|---|---|
| Quantity of Counterions | 1 mol/L K+ | y = 0.1153x + 0.5547 | 0.9999 |
| 1.5 mol/L K+ | y = 0.0838x + 0.7327 | 0.9970 | |
| 2.5 mol/L K+ | y = 0.0469x + 0.4513 | 0.9977 |
| Factors | Value | Regression Equation | r |
|---|---|---|---|
| Ratio of acetonitrile | 0% | y = 0.0469x + 0.4513 | 0.9977 |
| 20% | y = 0.1817x + 0.4575 | 0.9974 |
| Factors | Value | Regression Equation | r |
|---|---|---|---|
| Dissociation temperature | 30 °C | y = 0.1944x + 0.3198 | 0.9998 |
| 40 °C | y = 0.1817x + 0.4575 | 0.9974 | |
| 50 °C | y = 0.1931x + 1.0388 | 0.9920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Li, C.; Wang, S.; Zhang, H.; Wang, Z.; Zheng, A.; Gao, X. Methods and Characteristics of Drug Extraction from Ion-Exchange-Resin-Mediated Preparations: Influences, Thermodynamics, and Kinetics. Polymers 2023, 15, 1191. https://doi.org/10.3390/polym15051191
Yuan J, Li C, Wang S, Zhang H, Wang Z, Zheng A, Gao X. Methods and Characteristics of Drug Extraction from Ion-Exchange-Resin-Mediated Preparations: Influences, Thermodynamics, and Kinetics. Polymers. 2023; 15(5):1191. https://doi.org/10.3390/polym15051191
Chicago/Turabian StyleYuan, Junlin, Conghui Li, Shanshan Wang, Hui Zhang, Zengming Wang, Aiping Zheng, and Xiuli Gao. 2023. "Methods and Characteristics of Drug Extraction from Ion-Exchange-Resin-Mediated Preparations: Influences, Thermodynamics, and Kinetics" Polymers 15, no. 5: 1191. https://doi.org/10.3390/polym15051191







