Modelling of Nonthermal Dielectric Barrier Discharge Plasma at Atmospheric Pressure and Role of Produced Reactive Species in Surface Polymer Microbial Purification
Abstract
:1. Introduction
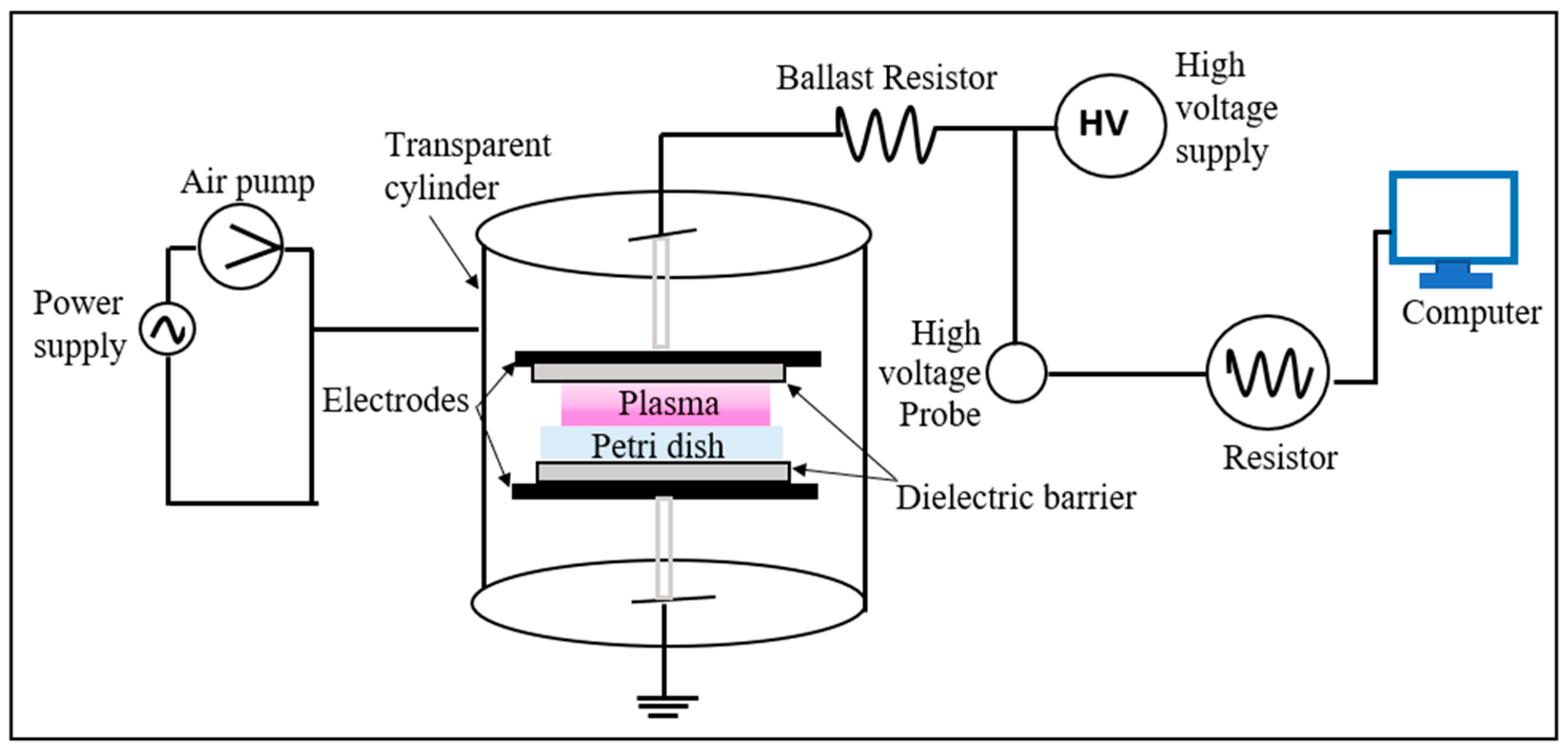
2. Microbial Inactivation Setup
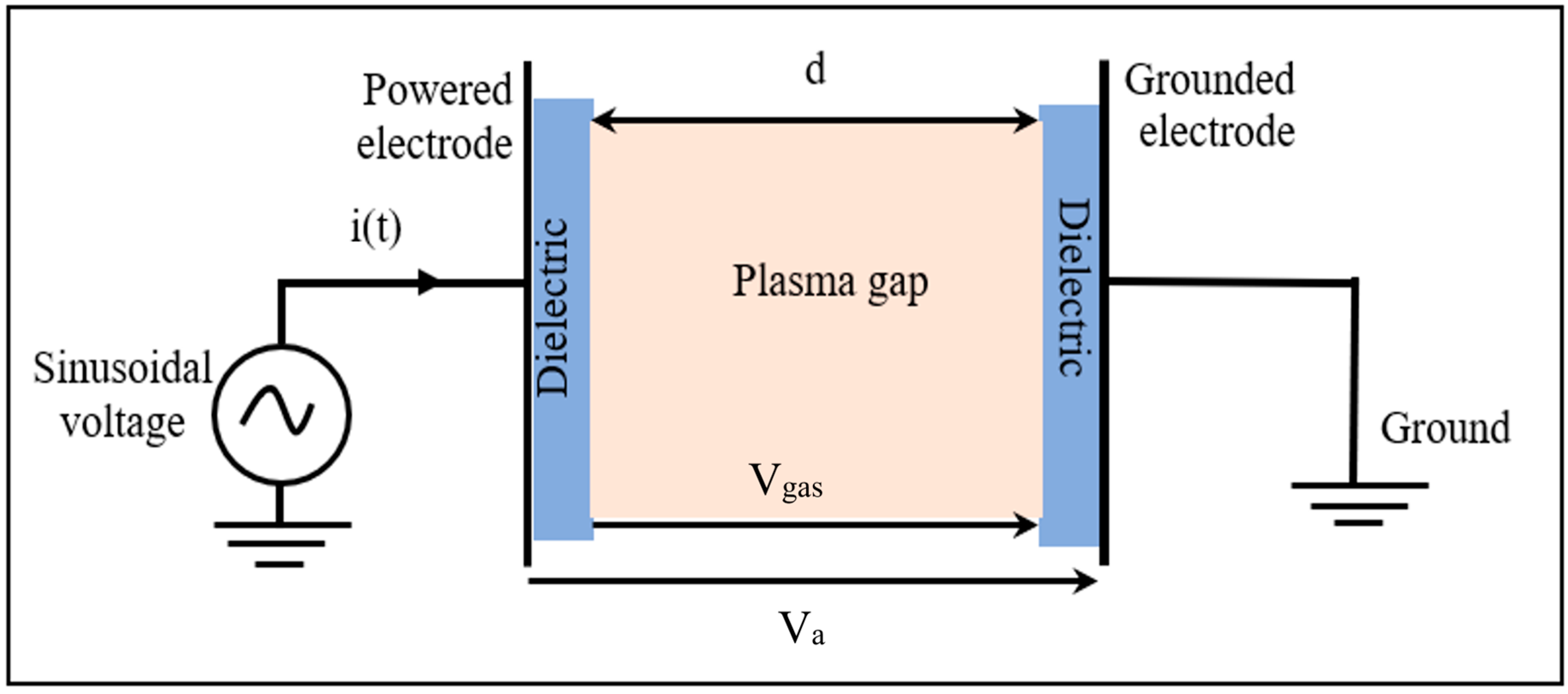
3. Model Description
3.1. Numerical Model Equations
3.2. Boundary and Initial Conditions
3.3. Computational Study
4. Results
4.1. Helium DBD Discharge
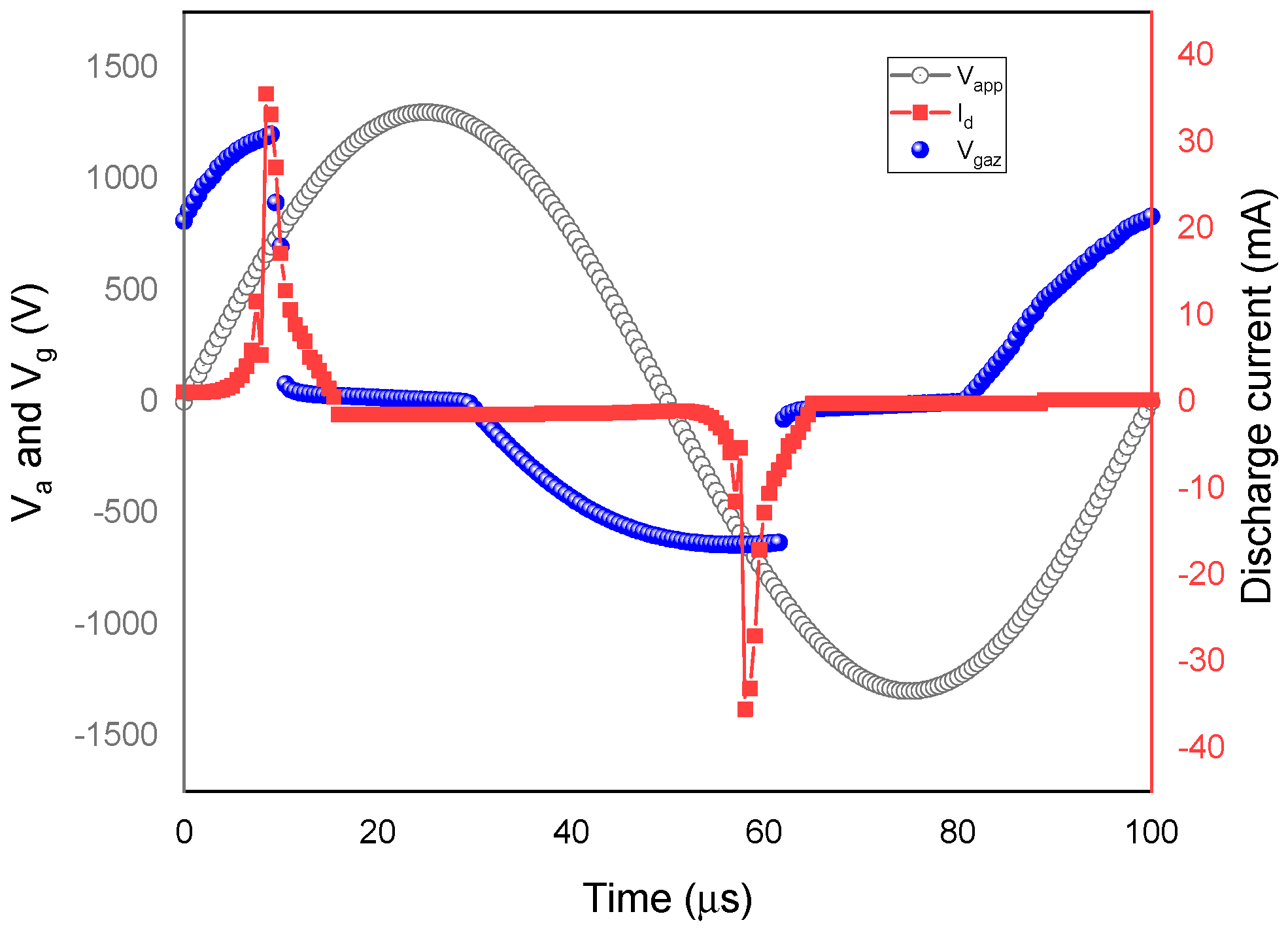
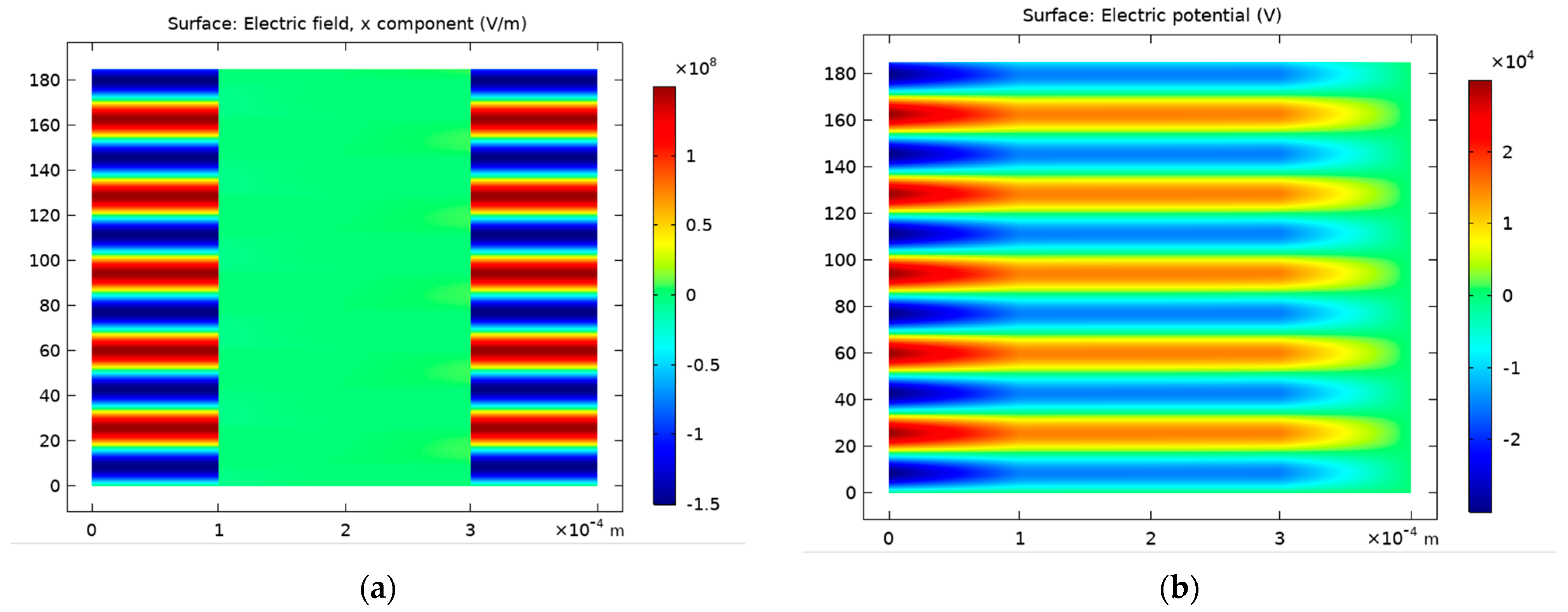
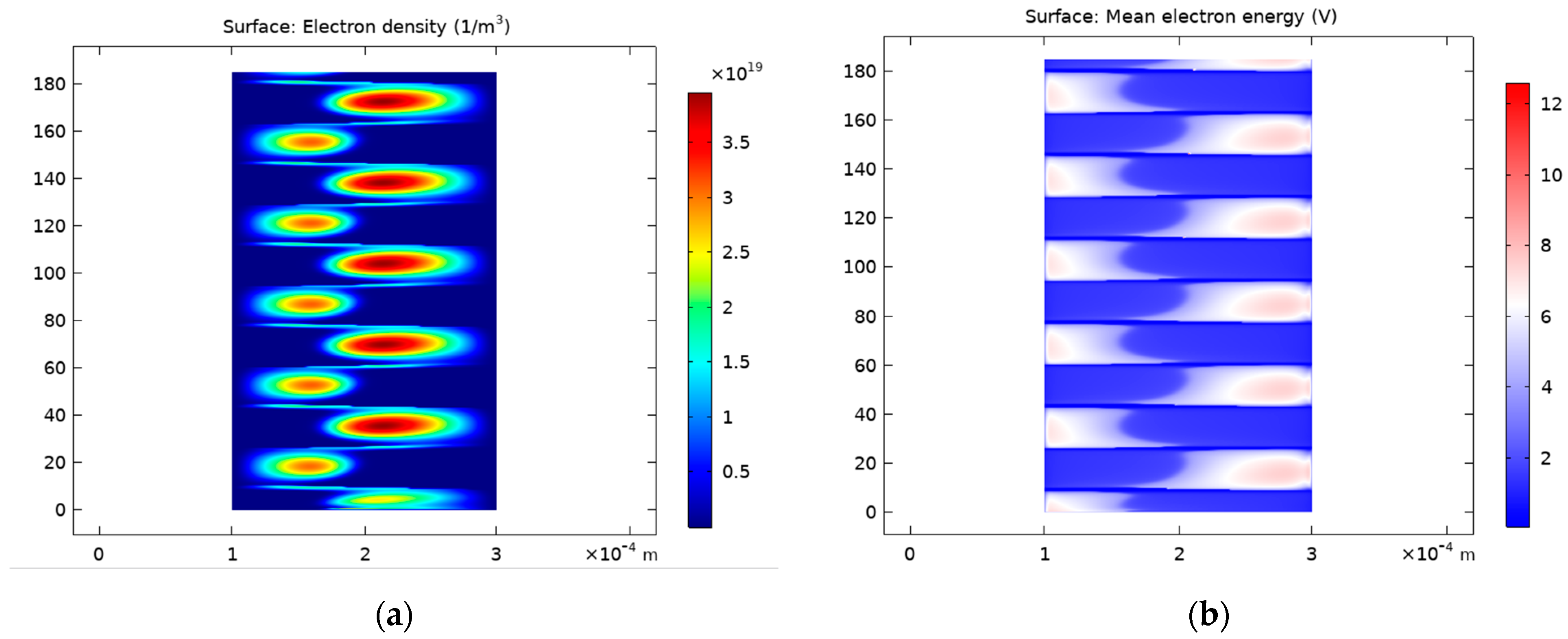
4.1.1. Discharge Structure
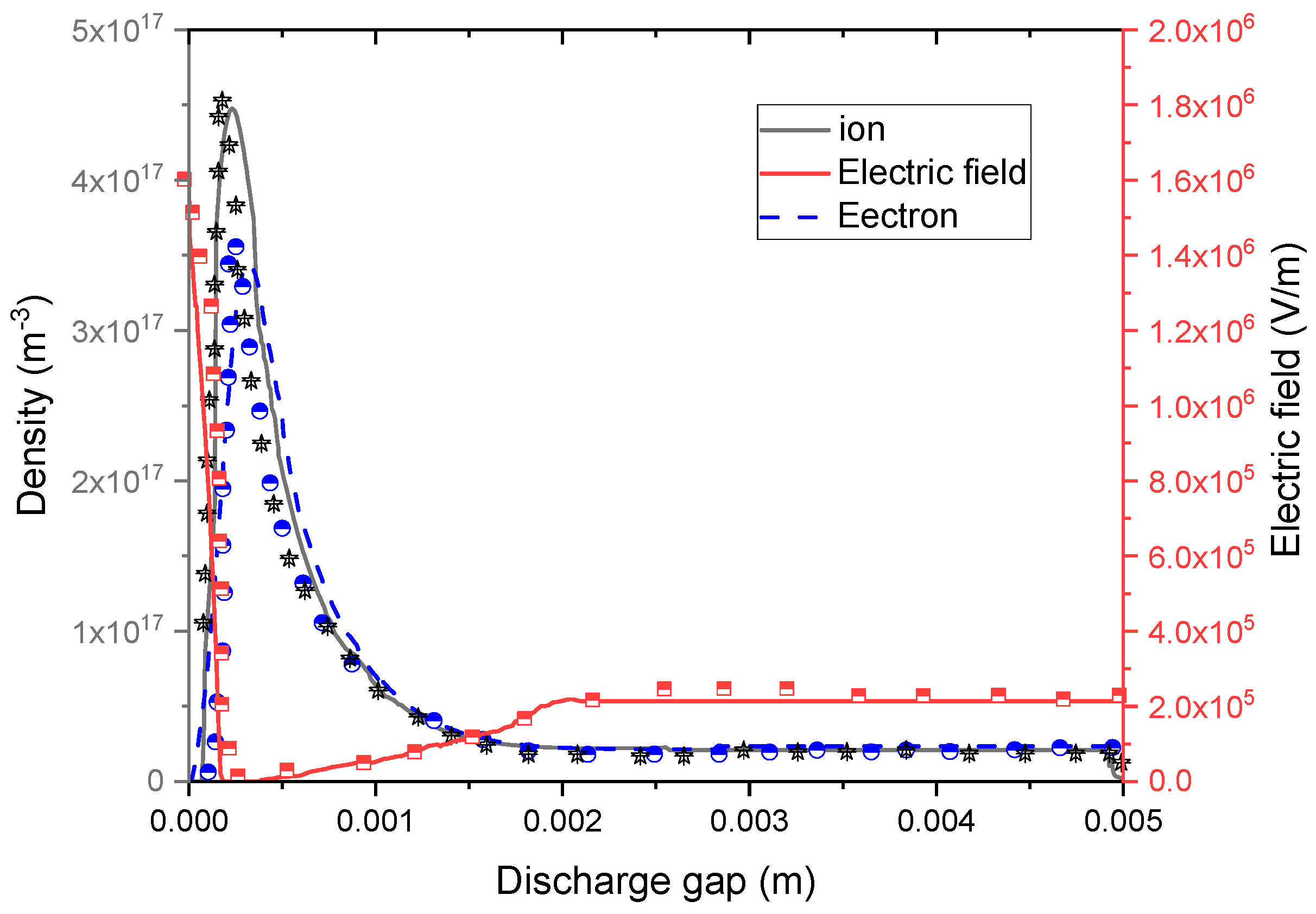
4.1.2. Predicted Electric Field and Species Distribution
4.2. Helium–Oxygen DBD Discharge
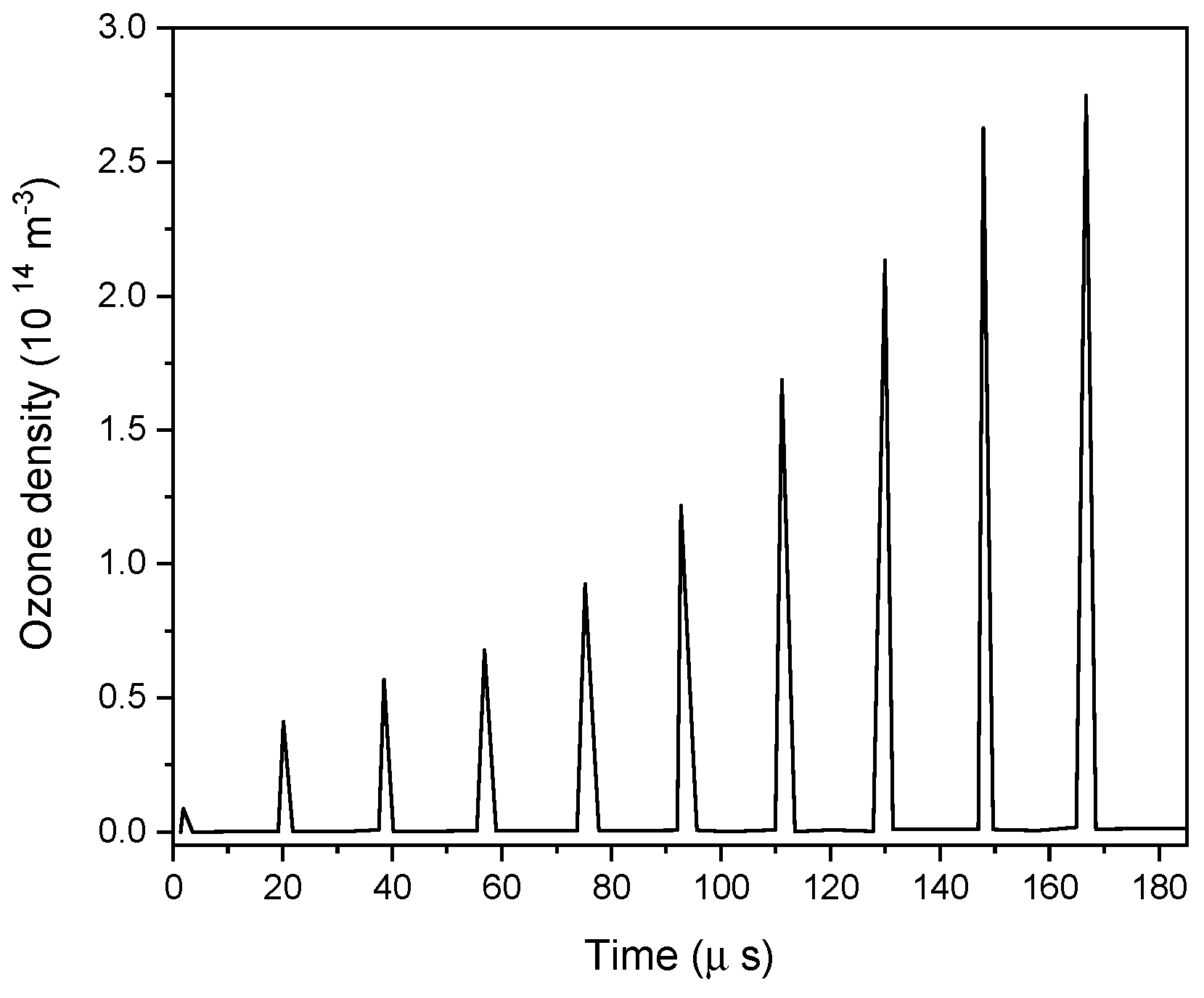
4.3. Role of Different Radical Species Produced by a Dielectric Barrier Discharge in Microbial Inactivation
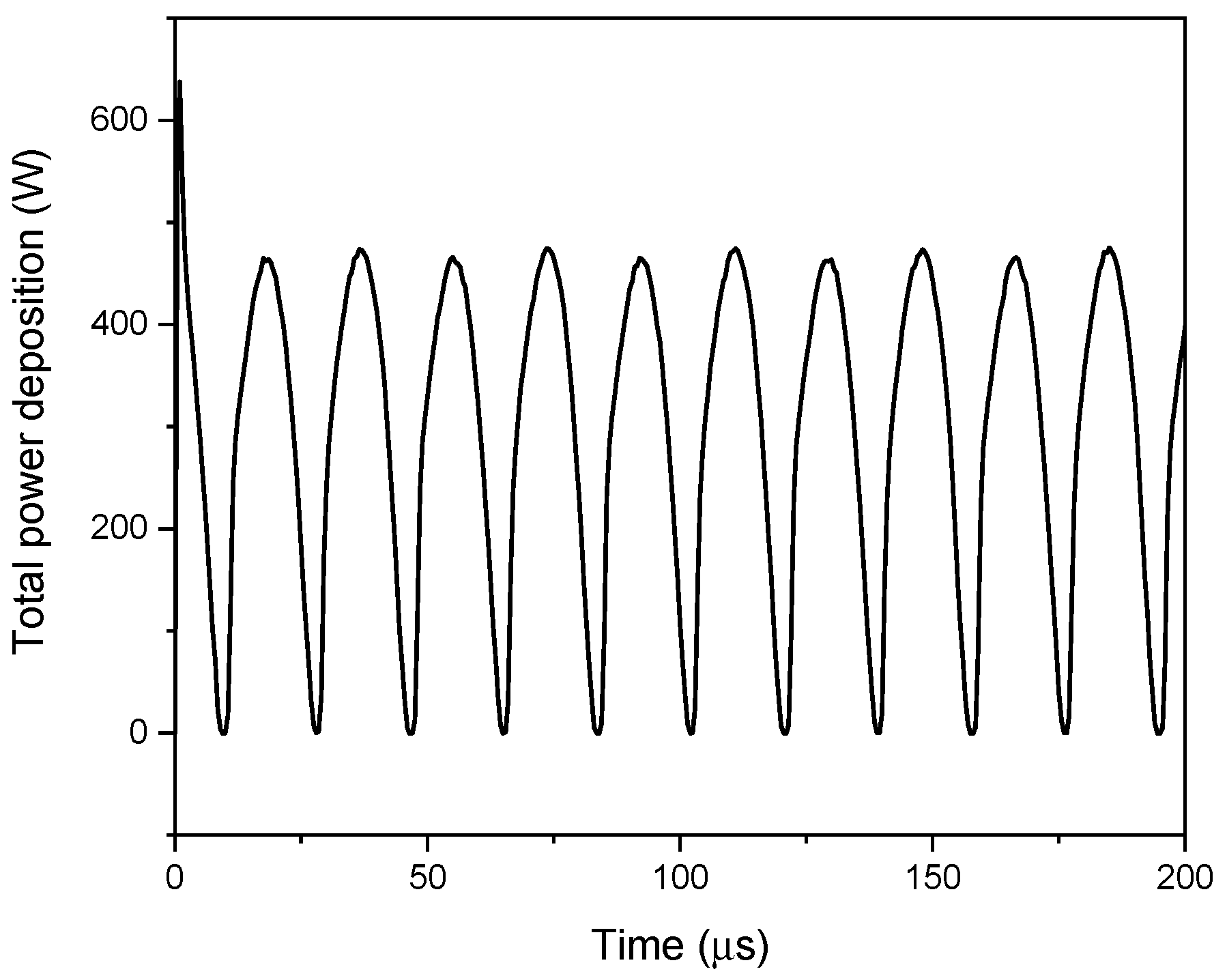
4.4. Power Distribution
4.5. Influence of the Operational Conditions
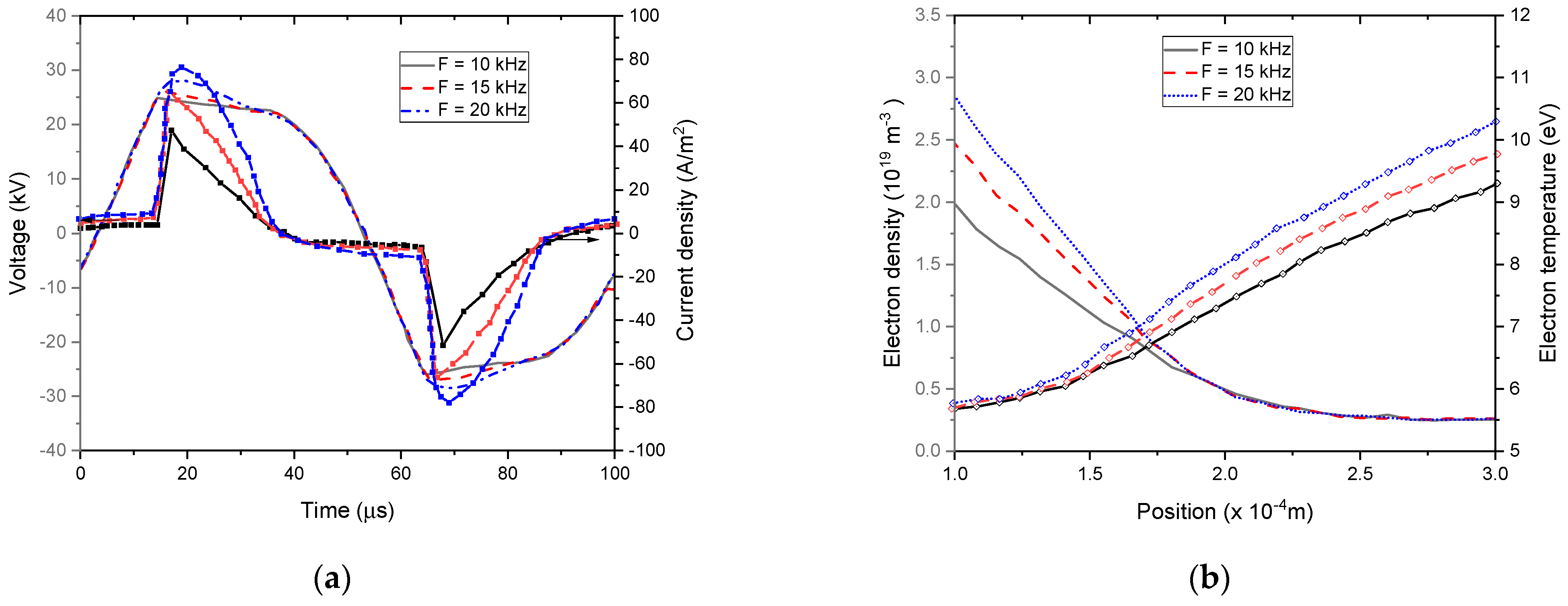
4.5.1. Influence of External Voltage and Frequency Modulation
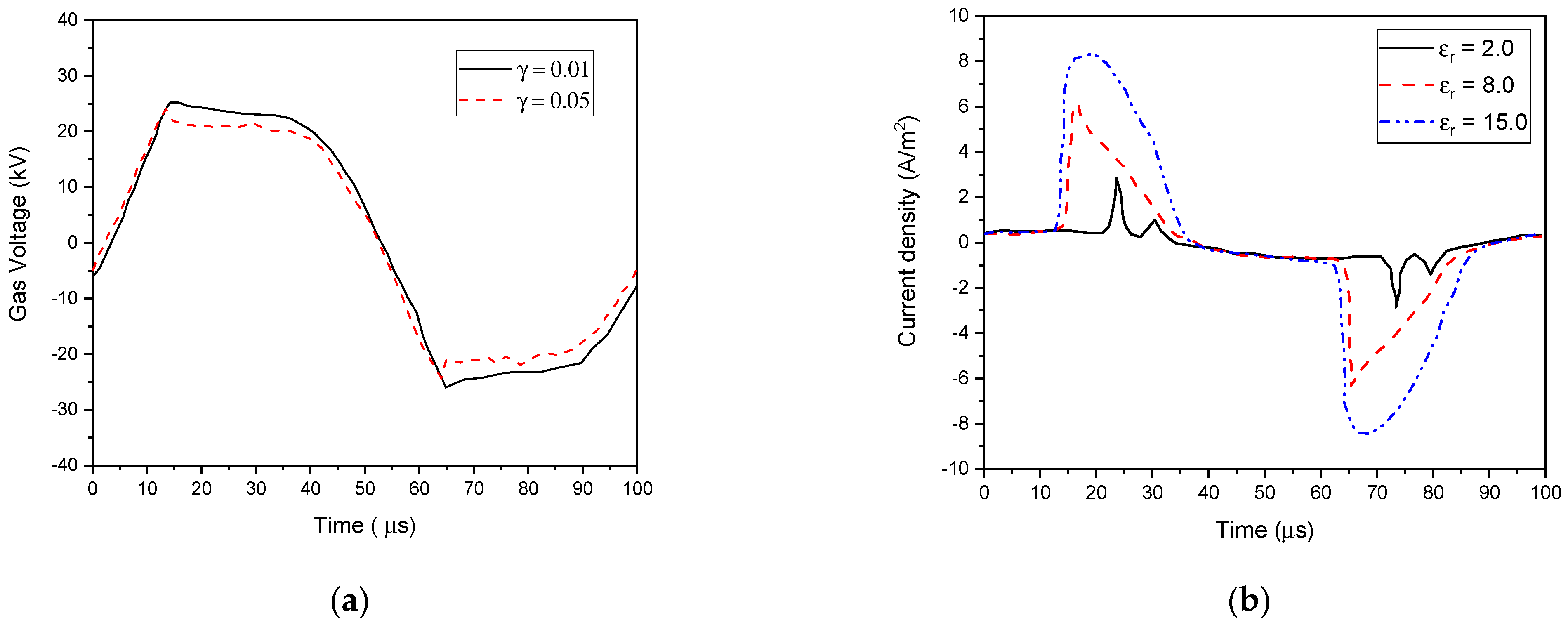
4.5.2. Effect of Secondary Electron Emission Coefficients and Dielectric Constant of the Barrier Material
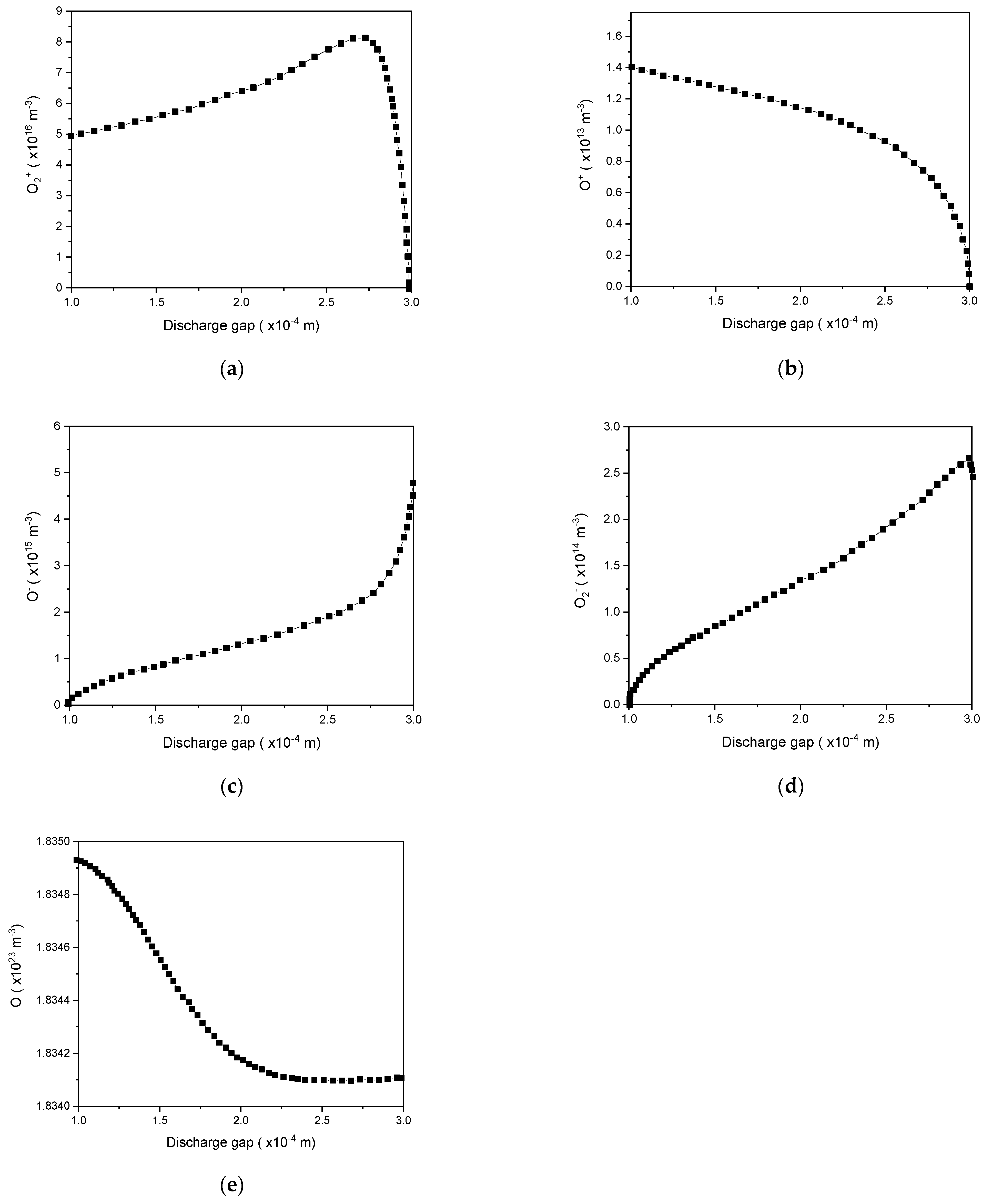
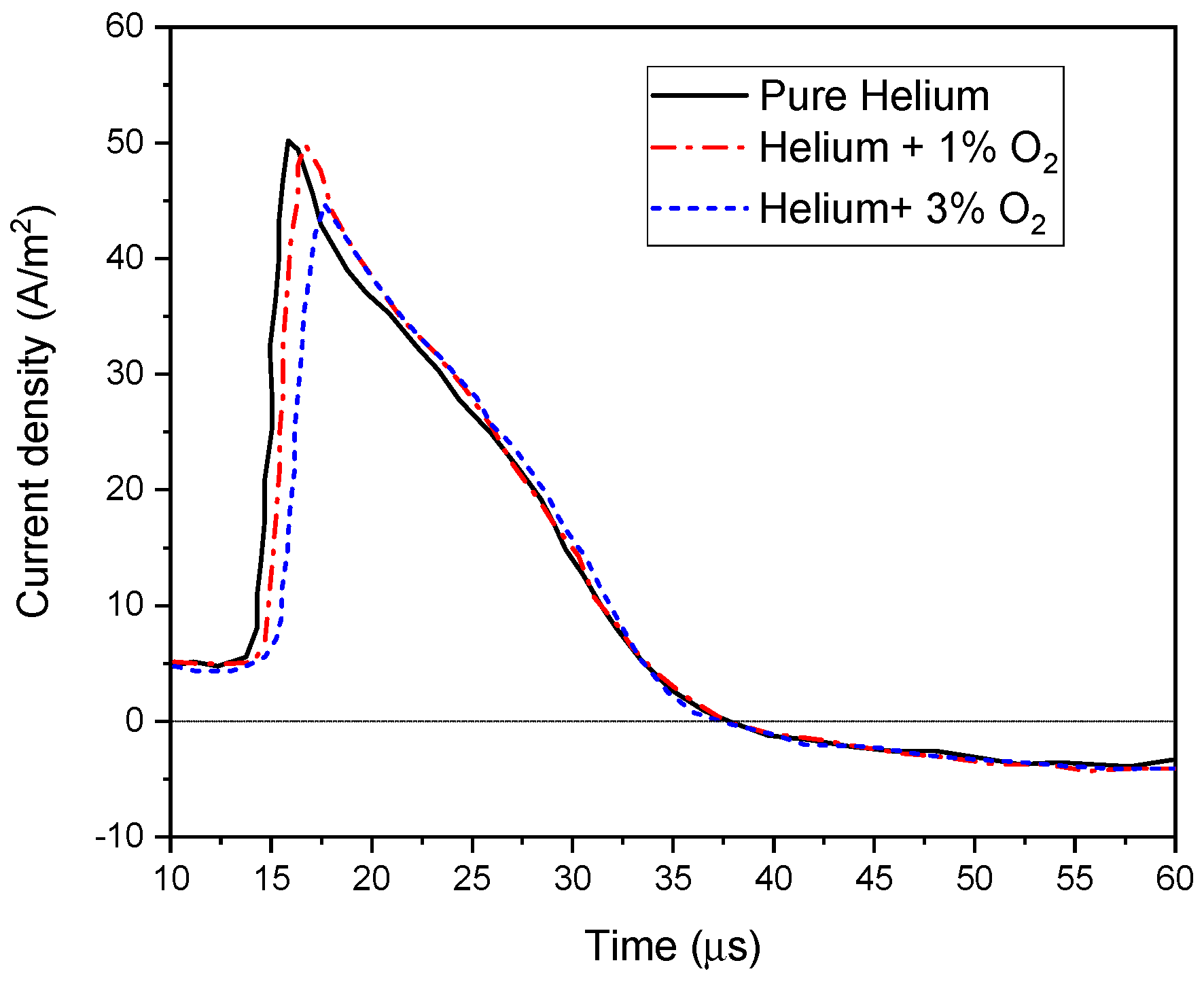
4.5.3. Influence of Oxygen Addition
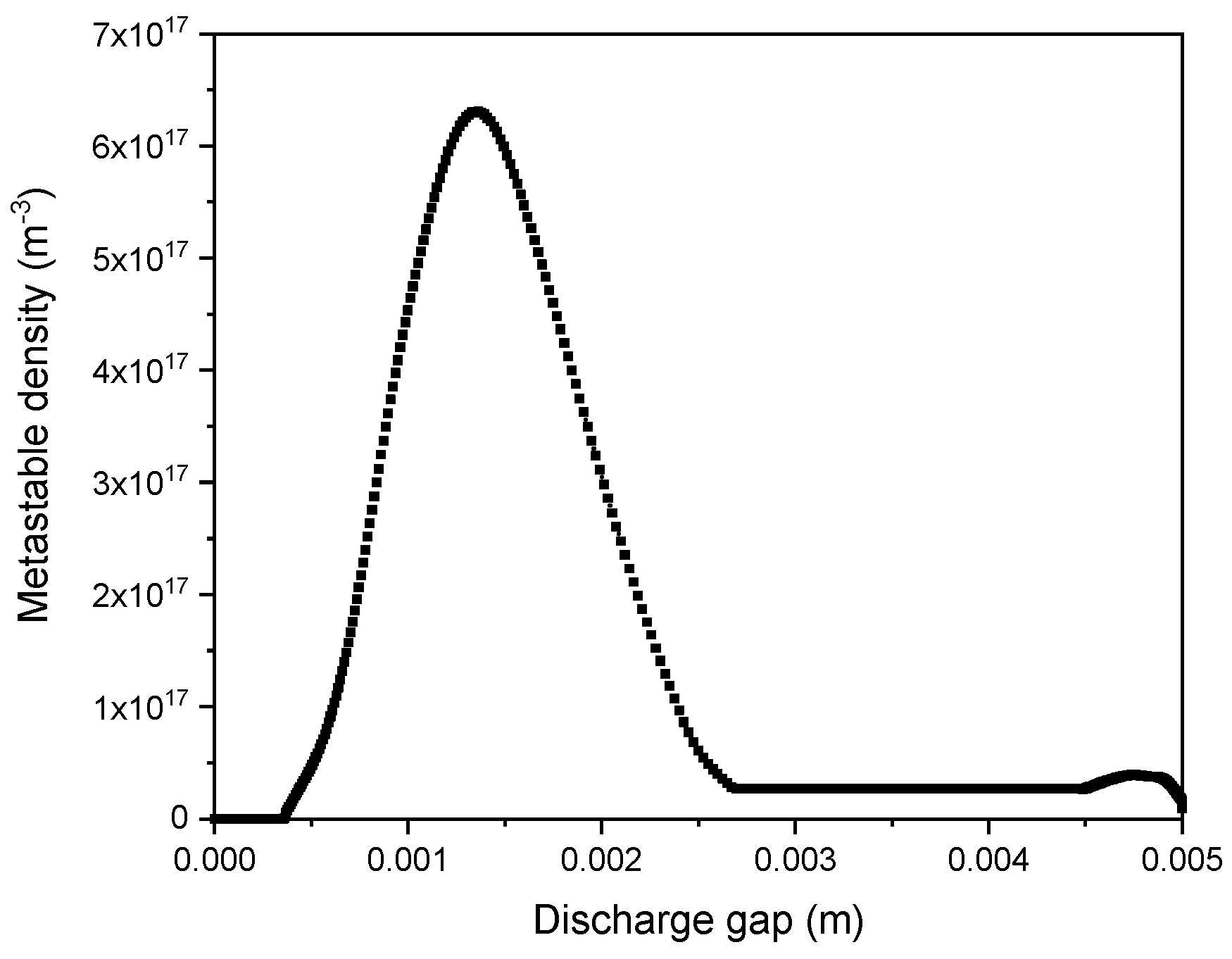
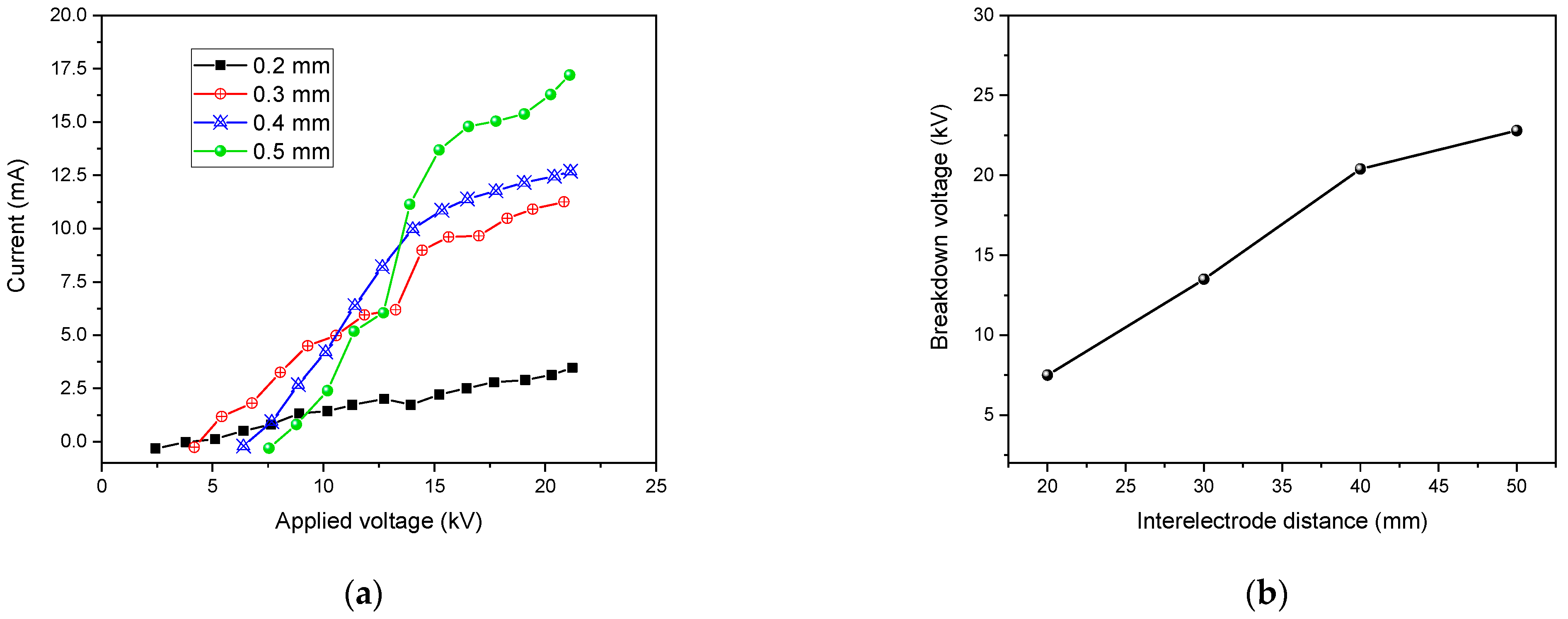
4.5.4. Influence of the Discharge Gap
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Y.; Reddivari, L.; Huang, J.Y. Development of cold plasma pre-treatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Jia, Y.; Li, M.; Dong, B.; Qian, G.; Zhouc, J.; Dai, X. Effects of chemical pre-treatments on microplastic extraction in sewage sludge and their physicochemical characteristics. Water Res. 2020, 171, 115379. [Google Scholar] [CrossRef]
- Malachová, K.; Novotný, Č. Ability of Trichoderma hamatum Isolated from Plastics-Polluted Environments to Attack Petroleum-Based, Synthetic Polymer Films. Processes 2020, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Herczeg, C.K.; Song, J. Sterilization of Polymeric Implants: Challenges and Opportunities. ACS Appl. Bio Mater. 2022, 5, 5077–5088. [Google Scholar] [CrossRef] [PubMed]
- Rivera, U.H.; Kadian, S.; Nejati, S.; White, J.; Sedaghat, S.; Mutlu, Z.; Rahimi, R. Printed Low-Cost PEDOT: PSS/PVA Polymer Composite for Radiation Sterilization Monitoring. ACS Sens. 2022, 7, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.A.A.; Fuster, M.G.; Martínez, T.M.; Montalbán, M.G.; Cenis, J.L.; Víllora, G.; Lozano-Pérez, A.A. The Effect of Sterilization on the Characteristics of Silk Fibroin Nanoparticles. Polymers 2022, 14, 498. [Google Scholar] [CrossRef]
- Juene Chong, W.; Shen, S.; Li, Y.; Trinchi, A.; Pejak, D.; Kyratzis, I.; Sola, A.; Wen, C. Additive manufacturing of antibacterial PLA-ZnO nanocomposites: Benefits, limitations and open challenges. J. Mater. Sci. Technol. 2022, 111, 120–151. [Google Scholar] [CrossRef]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere 2022, 308, 136404. [Google Scholar] [CrossRef]
- Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222. [Google Scholar] [CrossRef]
- Todorova, Y.; Benova, E.; Marinova, P.; Yotinov, I.; Bogdanov, T.; Topalova, Y. Non-Thermal Atmospheric Plasma for Microbial Decontamination and Removal of Hazardous Chemicals: An Overview in the Circular Economy Context with Data for Test Applications of Microwave Plasma Torch. Processes 2022, 10, 554. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Vaze, N.; Gangoli, S.; Vasilets, V.N.; Gutsol, A.F.; Milovanova, T.N.; Anandan, S.; Murasko, D.M.; Fridman, A.A. Rapid inactivation of Airborne Bacteria Using Atmospheric Pressure Dielectric Barrier Grating Discharge. IEEE Trans. Plasma Sci. 2007, 35, 1501. [Google Scholar] [CrossRef]
- Cooper, M.; Fridman, G.; Staack, D.; Gutsol, A.F.; Vasilets, V.N.; Anandan, S.; Cho, Y.I.; Fridman, A.; Tsapin, A. Decontamination of Surfaces from Extremophile Organisms Using Nonthermal Atmospheric-Pressure Plasmas. IEEE Trans. Plasma Sci. 2009, 37, 866–871. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, Y.; Lai, A.C.K.; Huang, G. Inactivation of airborne bacteria by cold plasma in air duct flow. Build Env. 2016, 106, 120–130. [Google Scholar] [CrossRef]
- Delma, K.L.; Lechanteur, A.; Evrard, B.; Semd´e, R.; Piel, G. Sterilization methods of liposomes: Drawbacks of conventional methods and perspectives. Int. J. Pharm. 2021, 597, 120271. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hallab, N.J.; Samelko, L.; Hammond, D. Particulate Debris Released from Breast Implant Surfaces Is Highly Dependent on Implant Type. Aesthet. Surg. J. 2021, 41, NP782–NP793. [Google Scholar] [CrossRef] [PubMed]
- Yoganarasimha, S.; Trahan, W.R.; Best, A.M.; Bowlin, G.L.; Kitten, T.O.; Moon, P.C.; Madurantakam, P.A. Peracetic Acid: A Practical Agent for Sterilizing Heat-Labile Polymeric Tissue-Engineering Scaffolds. Tissue Eng. Pt C-Meth. 2014, 20, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Sohbatzadeh, F.; Mirzanejhad, S.; Ghasemi, M.; Talebzadeh, M. Characterization of a non-thermal plasma torch in streamer mode and its effect on polyvinyl chloride and silicone rubber surfaces. J. Electrostat. 2013, 71, 875–881. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Dhungana, S.; Gautam, S.; Pandey, B.P.; Joshi, U.M.; Subedi, D.P. Characterization Of Dielectric Barrier Discharge (DBD) Produced In Air At Atmospheric Pressure And Its Application In Surface Modification Of High-Density Polyethylene (HDPE). J. Technol. Space Plasmas 2020, 1, 27–35. [Google Scholar] [CrossRef]
- Bras, F.L.; Carré, G.; Aguemon, Y.; Colin, M.; Gellé, M.P. Inactivation of Enveloped Bovine Viral Diarrhea Virus and Non-Enveloped Porcine Parvovirus Using Low-Pressure Non-Thermal Plasma. Life 2021, 11, 1292. [Google Scholar] [CrossRef]
- Royintarat, T.; Seesuriyachan, P.; Boonyawan, D.; Choid, E.H.; Wattanutchariya, W. Mechanism and optimization of non-thermal plasma-activated water for bacterial inactivation by underwater plasma jet and delivery of reactive species underwater by cylindrical DBD plasma. Curr. Appl. Phys. 2019, 19, 1006–1014. [Google Scholar] [CrossRef]
- Keidar, M.; Beilis, I. Plasma Engineering: Applications from Aerospace to Bio and Nanotechnology, 1st ed.; Academic Press Oxford: London, UK, 2013. [Google Scholar]
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric pressure plasma of dielectric barrier discharges. Pure Appl. Chem. 2005, 77, 487–495. [Google Scholar] [CrossRef]
- Arserim, E.H.; Salvi, D.; Fridman, G. Microbial Inactivation by Non-equilibrium Short-Pulsed Atmospheric Pressure Dielectric Barrier Discharge (Cold Plasma): Numerical and Experimental Studies. Food Eng. Rev. 2021, 13, 136–147. [Google Scholar] [CrossRef]
- Kang, W.S.; Park, J.M.; Kim, Y.; Hong, S.H. Numerical Study on Influences of Barrier Arrangements on Dielectric Barrier Discharge Characteristics. IEEE Trans. Plasma Sci. 2003, 31, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Gadkari, S.; Tu, X.; Gu, S. Fluid model for a partially packed dielectric barrier discharge plasma reactor. Phys. Plasmas 2017, 24, 093510. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Li, L.; Hen, C.B.; Song, Y.; Zhao, Y.; Xiu, X. Numerical simulation of evolution features of the atmospheric-pressure CF4 plasma generated by the pulsed dielectric barrier discharge. Eur. Phys. J. D 2016, 70, 136. [Google Scholar] [CrossRef]
- Golubovskii, Y.B.; Maiorov, V.A.; Behnke, J.; Behnke, J.F. Modelling of the homogeneous barrier discharge in helium at atmospheric pressure. J. Phys. D Appl. Phys. 2002, 36, 39. [Google Scholar] [CrossRef]
- Shivkumar, G.; Alrefae, M.A.; Tholeti, S.S.; Macheret, S.O.; Fisher, T.S.; Alexeenko, A.A. Discharge regimes and emission characteristics of capacitively coupled radio frequency argon plasma with a square wave input. J. Appl. Phys. 2019, 125, 223301. [Google Scholar] [CrossRef]
- COMSOL Multiphysics® v. 5.1. CFD Module User’s Guide; COMSOL AB: Stockholm, Sweden, 2015; Available online: http://www.comsol.com (accessed on 1 November 2021).
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; Dodet, B.; Odic, E. Atmospheric pressure humid argon DBD plasma for the application of sterilization measurement and simulation of hydrogen, oxygen, and hydrogen peroxide formation. Int. J. Plasma Env. Sc. Technol. 2007, 1, 96–101. [Google Scholar]
- Abidat, R.; Rebiai, S.; Benterrouche, L. Numerical simulation of atmospheric dielectric barrier discharge in helium gas using COMSOL Multiphysics. In Proceedings of the 3rd International Conference on Systems and Control, Algiers, Algeria, 29–31 October 2013. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, J.H.; Hwang, Y.S. One-dimensional discharge simulation of nitrogen DBD atmospheric pressure plasma. Thin Solid Film. 2006, 506–507, 389–395. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Soltani, H. Time-dependent one-dimensional simulation of atmospheric dielectric barrier discharge in N2/O2/H2O using COMSOL Multiphysics. J. Theor. Appl. Phys. 2018, 12, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Georghiou, G.E.; Papadakis, A.P.; Morrow, R.; Metaxas, A.C. Numerical modelling of atmospheric pressure gas discharges leading to plasma production. J. Phys. D Appl. Phys. 2005, 38, R303–R328. [Google Scholar] [CrossRef]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G.A. Review on microbiological decontamination of fresh produce with nonthermal plasma. J. Appl. Microbiol. 2017, 122, 1438–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portugal, S.; Roy, S.; Lin, J. Functional relationship between material property, applied frequency and ozone generation for surface dielectric barrier discharges in atmospheric air. Sci. Rep. 2017, 6388, 1–11. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.S.; Lee, J.H.; Jeong, B.H. Space Sterilization Effect Through High Density Plasma Ozone Using DBD Device. J. Eelectr. Eng. Technol. 2022, 17, 2771–2778. [Google Scholar] [CrossRef]
- Shrestha, P.; Subedi, D.; Joshi, U. Electrical characterization of atmospheric pressure dielectric barrier discharge in air. In Proceedings of the 5th International Conference on the Frontiers of Plasma Physics and Technology, Singapore, 18–22 April 2011; Available online: https://www-pub.iaea.org/MTCD/publications/PDF/TE-1713-CD/talks/posters/Shrestha-paper.pdf (accessed on 7 December 2022).
- Bol’shakov, A.; Cruden, B.A.; Mogul, R.; Rao, M.V.V.S.; Sharma, S.P.; Khare, B.N.; Meyyappan, M. Radio-Frequency Oxygen Plasma as a Sterilization Source. AIAA J. 2004, 42, 823–827. [Google Scholar] [CrossRef]
- Xu, G.M.; Ma, Y.; Zhang, G.J. DBD Plasma Jet in Atmospheric Pressure Argon. IEEE Trans. Plasma Sci. 2008, 36, 1352–1353. [Google Scholar] [CrossRef]
- Chirokov, A.; Khot, S.N.; Gangoli, S.P.; Fridman, A.; Henderson, P.; Gutsol, A.F.; Dolgopolsky, A. Numerical and experimental investigation of the stability of radiofrequency (RF) discharges at atmospheric pressure. Plasma Sour. Sci. Technol. 2009, 18, 025025. [Google Scholar] [CrossRef]
- BOLSIG, Electron Boltzmann Equation Solver. Available online: http://www.bolsig.laplace.univ-tlse.fr/ (accessed on 1 November 2022).
- LXCAT. Electron Scattering Database; University of Toulouse: Toulouse, France, 2022; Available online: https://www.lxcat.net (accessed on 1 November 2022).
- Dickinson, E.J.F.; Ekstrom, H.; Fontes, E. COMSOL Multiphysics®: Finite element software for electrochemical analysis. A mini review. Electrochem. Commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Zhang, Z. Numerical Simulation of Surface Dielectric Barrier Discharge with Functionally Graded Material. Front. Phys. 2022, 10, 874–887. [Google Scholar] [CrossRef]
- Massines, F.; Rabehi, A.; Decomps, P.; Ben Gadri, R.; Segur, P.; Mayoux, C. Experimental and theoretical study of a glow discharge at atmospheric pressure controlled by dielectric barrier. J. Appl. Phys. 1998, 83, 2950. [Google Scholar] [CrossRef]
- Saridj, A.; Belarbi, A.W. Numerical modelling of a DBD in glow mode at atmospheric pressure. J. Theor. Appl. Phys. 2019, 1, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Balcon, N.; Hagelaar, G.J.M.; Boeuf, J.P. Numerical model of an argon atmospheric pressure RF discharge. IEEE Trans. Plasma Sci. 2008, 36, 2782–2787. [Google Scholar] [CrossRef]
- Shrestha, R.; Pradhan, S.P.; Guragain, R.P.; Subedi, D.P.; Pandey, B.P. Investigating the Effects of Atmospheric Pressure Air DBD Plasma on Physio-Chemical and Microbial Parameters of Groundwater. Open Access Libr. J. 2020, 7, e6144. [Google Scholar] [CrossRef]
- Shkurenkov, A.; Mankelevich, Y.A.; Rakhimova, T.V. Two-dimensional simulation of an atmospheric-pressure RF DBD in a H2:O2 mixture: Discharge structures and plasma chemistry. Plasma Sources Sci. T 2013, 22, 015021. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; He, J.; Cheng, G.; Xu, J.; Lu, M.; Shangguan, Y.; Zhang, A. Mechanism of dielectric barrier discharge plasma technology to improve the quantity of short-chain fatty acids in anaerobic fermentation of waste active sludge. Front. Microbiol. 2022, 13, 963260. [Google Scholar] [CrossRef]
- Goossensa, O.; Dekempeneer, E.; Vangeneugden, D.; Van de Leest, R.; Leys, C. Application of atmospheric pressure dielectric barrier discharges in deposition, cleaning and activation. Surf. Coat. Tech. 2001, 142–144, 474–481. [Google Scholar] [CrossRef]
- Helmke, A.; Hoffmeister, D.; Berge, F.; Emmert, S.; Laspe, P.; Mertens, N.; Weltmann, K.D. Physical and Microbiological Characterisation of Staphylococcus epidermidis Inactivation by Dielectric Barrier Discharge Plasma. Plasma Process. Polym. 2011, 8, 278–286. [Google Scholar] [CrossRef]
- Papageorghiou, L.; Panousis, E.; Loiseau, J.F.; Spyrou, N.; Held, B. Two-dimensional modelling of a nitrogen dielectric barrier discharge (DBD) at atmospheric pressure: Filament dynamics with the dielectric barrier on the cathode. J. Phys. D Appl. Phys. 2009, 42, 105201. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Samir, T.; Ma, Z. Numerical study of effect of secondary electron emission on discharge characteristics in low pressure capacitive RF argon discharge. Phys. Plasmas 2014, 21, 083511. [Google Scholar] [CrossRef]
- Tristant, P.; Desmaison, J.; Leprince, P.; Tendero, C.; Tixier, C. Atmospheric pressure plasmas: A review. Spectrochim. Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Oliveiraa, G.; Jensenb, J.L.; Keenerc, K.M.; Campanella, O.H. Modelling the inactivation of Bacillus subtilis spores during cold plasma sterilization. Innov. Food Sci. Emerg. 2019, 52, 334–342. [Google Scholar] [CrossRef]
- Fang, Z.; Ji, S.; Pan, J.; Shao, T.; Zhang, C. Electrical Model and Experimental Analysis of the Atmospheric-Pressure Homogeneous Dielectric Barrier Discharge in He. IEEE Trans. Plasma Sci. 2012, 40, 883–891. [Google Scholar] [CrossRef]
- Fricke, K.; Tresp, H.; Bussiahn, R.; Schroder, K.; Woedtke, T.V.; Weltmann, K.D. On the Use of Atmospheric Pressure Plasma for the Bio-Decontamination of Polymers and Its Impact on Their Chemical and Morphological Surface Properties. Plasma Chem. Plasma P 2012, 32, 801–816. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma: A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33 (Pt 2), 1108–1119. [Google Scholar] [CrossRef]
- Luo, H.; Liang, Z.; Wang, X.; Guan, Z.; Wang, L. Homogeneous dielectric barrier discharge in nitrogen at atmospheric pressure. J. Phys. D Appl. Phys. 2010, 43, 155201. [Google Scholar] [CrossRef]
- Feizollahia, E.; Misrab, N.N.; Roopesh, M.S. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689. [Google Scholar] [CrossRef]
- Leipold, F.; Schultz-Jensen, N.; Kusano, Y.; Bindslev, H.; Jacobsen, T. Decontamination of objects in a sealed container by means of atmospheric pressure plasmas. Food Control 2011, 22, 1296–1301. [Google Scholar] [CrossRef]
- Xian, Y.; Lu, X.; Tang, Z.; Xiong, Q.; Gong, W.; Liu, D.; Jiang, Z.; Pan, Y. Optical and electrical diagnostics of an atmospheric pressure room-temperature plasma plume. J. Appl. Phys. 2010, 107, 063308. [Google Scholar] [CrossRef]
- Panousis, E.; Clement, F.; Loiseau, J.F.; Spyrou, N.; Held, B.; Thomachot, M.; Marlin, L. An electrical comparative study of two atmospheric pressure dielectric barrier discharge reactors. Plasma Sources Sci. T 2006, 15, 828–839. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Meng, W.; Zhao, Z.; Cui, X. Influence of N2 pressure on surface discharge characteristics of PEEK under positive repetitive square voltage. High Volt. 2021, 6, 793–804. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elaissi, S.; Alsaif, N.A.M. Modelling of Nonthermal Dielectric Barrier Discharge Plasma at Atmospheric Pressure and Role of Produced Reactive Species in Surface Polymer Microbial Purification. Polymers 2023, 15, 1235. https://doi.org/10.3390/polym15051235
Elaissi S, Alsaif NAM. Modelling of Nonthermal Dielectric Barrier Discharge Plasma at Atmospheric Pressure and Role of Produced Reactive Species in Surface Polymer Microbial Purification. Polymers. 2023; 15(5):1235. https://doi.org/10.3390/polym15051235
Chicago/Turabian StyleElaissi, Samira, and Norah A. M. Alsaif. 2023. "Modelling of Nonthermal Dielectric Barrier Discharge Plasma at Atmospheric Pressure and Role of Produced Reactive Species in Surface Polymer Microbial Purification" Polymers 15, no. 5: 1235. https://doi.org/10.3390/polym15051235




