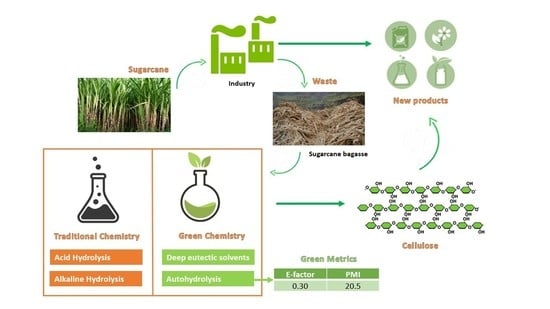
Comparative Study of Green and Traditional Routes for Cellulose Extraction from a Sugarcane By-Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
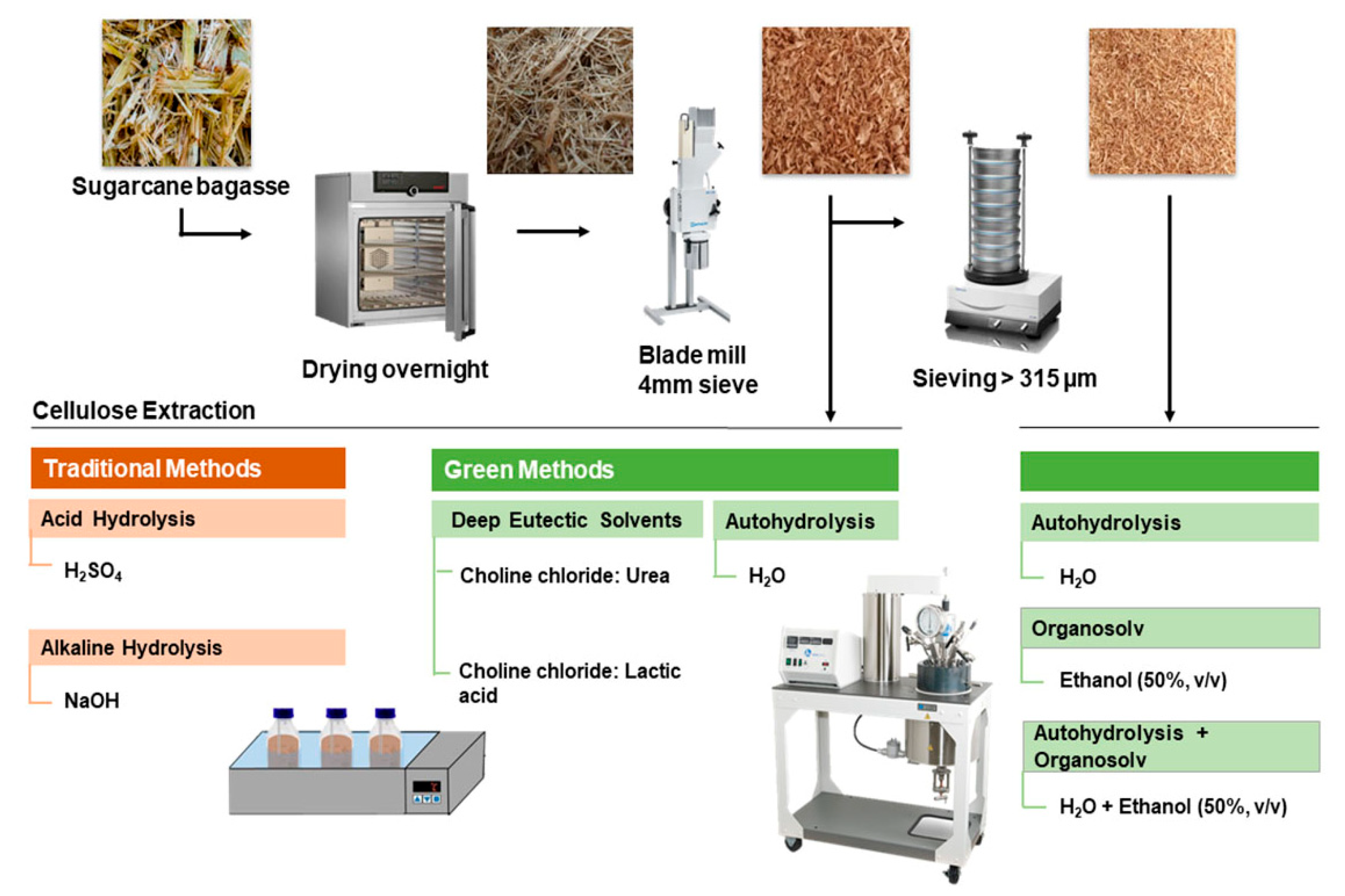
2.2. Raw Materials
2.3. Acid and Alkaline Hydrolyses
2.4. Deep Eutectic Solvents (DESs)
2.5. Hydrothermal Treatments
2.6. Chemical Composition
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Powder X-ray Diffraction (PXRD)
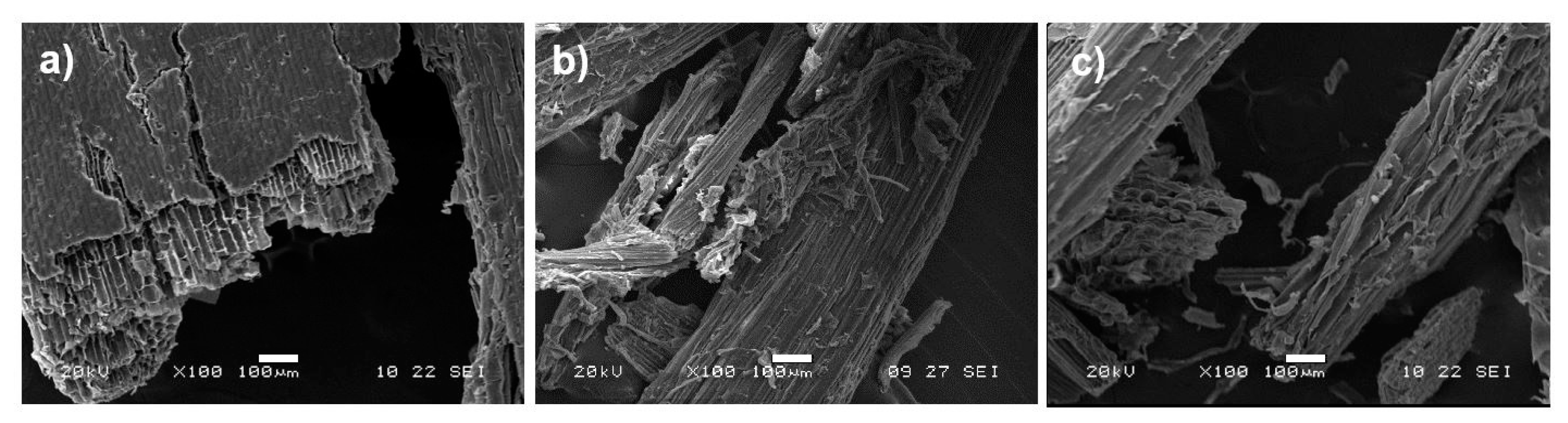
2.9. Scanning Electron Microscopy (SEM)
2.10. Sustainability Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Comparative Screening of Traditional and Green Methods for Cellulose Extraction
3.1.1. Chemical Composition
3.1.2. Structural Characterization
3.2. Optimization of the Hydrothermal Treatment
3.2.1. Chemical Composition
3.2.2. Structural Characterization
3.3. Sustainability and Cost Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.; Koschella, A. Cellulose Derivatives. Synthesis, Structure, and Properties; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-73167-4. [Google Scholar]
- Gao, J.; Li, Q.; Chen, W.; Liu, Y.; Yu, H. Self-Assembly of Nanocellulose and Indomethacin into Hierarchically Ordered Structures with High Encapsulation Efficiency for Sustained Release Applications. Chempluschem 2014, 79, 725–731. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Acidic Deep Eutectic Solvents As Hydrolytic Media for Cellulose Nanocrystal Production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- De Oliveira, F.B.; Bras, J.; Pimenta, M.T.B.; da Silva Curvelo, A.A.; Belgacem, M.N. Production of Cellulose Nanocrystals from Sugarcane Bagasse Fibers and Pith. Ind. Crops Prod. 2016, 93, 48–57. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water Barrier Properties of Starch Films Reinforced with Cellulose Nanocrystals Obtained from Sugarcane Bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; O’Connor, K.E. Carbon-Rich Wastes as Feedstocks for Biodegradable Polymer (Polyhydroxyalkanoate) Production Using Bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200. [Google Scholar] [CrossRef]
- Melati, R.B.; Schmatz, A.A.; Pagnocca, F.C.; Contiero, J.; Brienzo, M. Sugarcane Bagasse: Production, Composition, Properties, and Feedstock Potential. In Sugarcane: Production Systems, Uses and Economic Importance; Murphy, R., Ed.; Nova Science Pub Inc.: Hauppauge, NY, USA, 2017; pp. 1–38. [Google Scholar]
- Candido, R.G.; Gonçalves, A.R. Evaluation of Two Different Applications for Cellulose Isolated from Sugarcane Bagasse in a Biorefinery Concept. Ind. Crops Prod. 2019, 142, 111616. [Google Scholar] [CrossRef]
- Freitas, J.V.; Bilatto, S.; Squinca, P.; Pinto, A.S.S.; Brondi, M.G.; Bondancia, T.J.; Batista, G.; Klaic, R.; Farinas, C.S. Sugarcane Biorefineries: Potential Opportunities towards Shifting from Wastes to Products. Ind. Crops Prod. 2021, 172, 114057. [Google Scholar] [CrossRef]
- Linan, L.Z.; Cidreira, A.C.M.; da Rocha, C.Q.; de Menezes, F.F.; de Moraes Rocha, G.J.; Paiva, A.E.M. Utilization of Acai Berry Residual Biomass for Extraction of Lignocellulosic Byproducts. J. Bioresour. Bioprod. 2021, 6, 323–337. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Jeevith, K.; Geyandraprasath, K.; Roy, B.; Samraj, S. Extraction of Cellulosic Fibres from Agricultural Waste and Its Applications. AIP Conf. Proc. 2021, 2387, 40003. [Google Scholar] [CrossRef]
- Khan, R.; Jolly, R.; Fatima, T.; Shakir, M. Extraction Processes for Deriving Cellulose: A Comprehensive Review on Green Approaches. Polym. Adv. Technol. 2022, 33, 2069–2090. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose Processing from Biomass and Its Derivatization into Carboxymethylcellulose: A Review. Sci. Afr. 2022, 15, e01078. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Haldar, D.; Dey, P.; Patel, A.K.; Singhania, R.R.; Dong, C.-D.; Purkait, M.K. Sugarcane Bagasse into Value-Added Products: A Review. Environ. Sci. Pollut. Res. 2022, 29, 62785–62806. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Radotić, K.; Mićić, M. Methods for Extraction and Purification of Lignin and Cellulose from Plant Tissues; Humana Press: New York, NY, USA, 2016; pp. 365–376. [Google Scholar]
- Liao, Y.; de Beeck, B.O.; Thielemans, K.; Ennaert, T.; Snelders, J.; Dusselier, M.; Courtin, C.M.; Sels, B.F. The Role of Pretreatment in the Catalytic Valorization of Cellulose. Mol. Catal. 2020, 487, 110883. [Google Scholar] [CrossRef]
- Candido, R.G.; Godoy, G.G.; Gonçalves, A.R. Study of Sugarcane Bagasse Pretreatment with Sulfuric Acid as a Step of Cellulose Obtaining. Int. J. Nutr. Food Eng. 2012, 6, 6–10. [Google Scholar] [CrossRef]
- Canilha, L.; Santos, V.T.O.; Rocha, G.J.M.; Almeida, E.; Silva, J.B.; Giulietti, M.; Silva, S.S.; Felipe, M.G.A.; Ferraz, A.; Milagres, A.M.F.; et al. A Study on the Pretreatment of a Sugarcane Bagasse Sample with Dilute Sulfuric Acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Maryana, R.; Ma’rifatun, D.; Wheni, A.I.; Satriyo, K.W.; Rizal, W.A. Alkaline Pretreatment on Sugarcane Bagasse for Bioethanol Production. Energy Procedia 2014, 47, 250–254. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, D.M.; Sevastyanova, O.; de Queiroz, J.H.; Colodette, J.L. Cold Alkaline Extraction as a Pretreatment for Bioethanol Production from Eucalyptus, Sugarcane Bagasse and Sugarcane Straw. Energy Convers. Manag. 2016, 124, 315–324. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Microcrystalline Cellulose Production from Sugarcane Bagasse: Sustainable Process Development and Life Cycle Assessment. J. Clean. Prod. 2020, 249, 119342. [Google Scholar] [CrossRef]
- Chen, W.H.; Ye, S.C.; Sheen, H.K. Hydrolysis Characteristics of Sugarcane Bagasse Pretreated by Dilute Acid Solution in a Microwave Irradiation Environment. Appl. Energy 2012, 93, 237–244. [Google Scholar] [CrossRef]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of Dietary Fibers from Sugarcane Bagasse and Sugarcane Tops Using Microwave-Assisted Alkaline Treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Zhao, H.; Sun, R.C. Isolation and Characterization of Cellulose from Sugarcane Bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Kaar, W.E.; Gutierrez, C.V.; Kinoshita, C.M. Steam Explosion of Sugarcane Bagasse as a Pretreatment for Conversion to Ethanol. Biomass Bioenergy 1998, 14, 277–287. [Google Scholar] [CrossRef]
- Rocha, G.J.M.; Gonçalves, A.R.; Oliveira, B.R.; Olivares, E.G.; Rossell, C.E.V. Steam Explosion Pretreatment Reproduction and Alkaline Delignification Reactions Performed on a Pilot Scale with Sugarcane Bagasse for Bioethanol Production. Ind. Crops Prod. 2012, 35, 274–279. [Google Scholar] [CrossRef]
- De Aguiar, J.; Bondancia, T.J.; Claro, P.I.C.; Mattoso, L.H.C.; Farinas, C.S.; Marconcini, J.M. Enzymatic Deconstruction of Sugarcane Bagasse and Straw to Obtain Cellulose Nanomaterials. ACS Sustain. Chem. Eng. 2020, 8, 2287–2299. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep Eutectic Solvents (DESs) for Cellulose Dissolution: A Mini-Review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Li, J.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Extraction of Cellulose Nanocrystals Using a Recyclable Deep Eutectic Solvent. Cellulose 2019, 27, 1301–1314. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mu, T. Revisiting Greenness of Ionic Liquids and Deep Eutectic Solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Bjelić, A.; Hočevar, B.; Grilc, M.; Novak, U.; Likozar, B. A Review of Sustainable Lignocellulose Biorefining Applying (Natural) Deep Eutectic Solvents (DESs) for Separations, Catalysis and Enzymatic Biotransformation Processes. Rev. Chem. Eng. 2020, 38, 243–272. [Google Scholar] [CrossRef]
- Fauziah, S.; Draman, S.; Daik, R.; Mohd, N. Eco-Friendly Extraction and Characterization of Cellulose from Lignocellulosoic Fiber. ARPN J. Eng. Appl. Sci. 2016, 11, 9591–9595. [Google Scholar]
- Chen, Z.; Wan, C. Ultrafast Fractionation of Lignocellulosic Biomass by Microwave-Assisted Deep Eutectic Solvent Pretreatment. Bioresour. Technol. 2018, 250, 532–537. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Kamenská, L.; Vrška, M.; Šíma, J. Deep Eutectic Solvents: Fractionation of Wheat Straw. BioResources 2015, 10, 8039–8047. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty, G.G.; Lynam, J.G. Secondary Agriculture Residues Pretreatment Using Deep Eutectic Solvents. Waste Biomass Valorization 2021, 12, 2259–2269. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.G.A.; Chen, L.; Fakayode, O.A.; Zhou, C. Synergism of Sweeping Frequency Ultrasound and Deep Eutectic Solvents Pretreatment for Fractionation of Sugarcane Bagasse and Enhancing Enzymatic Hydrolysis. Ultrason. Sonochem. 2021, 73, 105470. [Google Scholar] [CrossRef]
- Chourasia, V.R.; Pandey, A.; Pant, K.K.; Henry, R.J. Improving Enzymatic Digestibility of Sugarcane Bagasse from Different Varieties of Sugarcane Using Deep Eutectic Solvent Pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef] [PubMed]
- Ctor, H.; Ruiz, A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.; Teixeira, J.A. Hydrothermal Processing, as an Alternative for Upgrading Agriculture Residues and Marine Biomass According to the Biorefinery Concept: A Review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Moniz, P. Chapter 14—Hydrothermal/Liquid Hot Water Pretreatment (Autohydrolysis): A Multipurpose Process for Biomass Upgrading. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 315–347. ISBN 9780128023235. [Google Scholar]
- Sasaki, M.; Adschiri, T.; Arai, K. Fractionation of Sugarcane Bagasse by Hydrothermal Treatment. Bioresour. Technol. 2003, 86, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, W.; Song, B.; Yu, Q. Liquid Hot Water Pretreatment for Lignocellulosic Biomass Biorefinery. In Emerging Technologies for Biorefineries, Biofuels, and Value-Added Commodities; Springer International Publishing: Cham, Switzerland, 2021; pp. 81–109. [Google Scholar] [CrossRef]
- Yang, B.; Tao, L.; Wyman, C.E. Strengths, Challenges, and Opportunities for Hydrothermal Pretreatment in Lignocellulosic Biorefineries. Biofuels Bioprod. Biorefin. 2017, 12, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.M.; Alonso, M.V.; Oliet, M.; Domínguez, J.C.; Rigual, V.; Rodriguez, F. Effect of Autohydrolysis on Pinus Radiata Wood for Hemicellulose Extraction. Carbohydr. Polym. 2018, 194, 285–293. [Google Scholar] [CrossRef]
- Ertas, M.; Han, Q.; Jameel, H.; Chang, H. min Enzymatic Hydrolysis of Autohydrolyzed Wheat Straw Followed by Refining to Produce Fermentable Sugars. Bioresour. Technol. 2014, 152, 259–266. [Google Scholar] [CrossRef]
- Driemeier, C.; Mendes, F.M.; Santucci, B.S.; Pimenta, M.T.B. Cellulose Co-Crystallization and Related Phenomena Occurring in Hydrothermal Treatment of Sugarcane Bagasse. Cellulose 2015, 22, 2183–2195. [Google Scholar] [CrossRef]
- Mesa, L.; González, E.; Cara, C.; González, M.; Castro, E.; Mussatto, S.I. The Effect of Organosolv Pretreatment Variables on Enzymatic Hydrolysis of Sugarcane Bagasse. Chem. Eng. J. 2011, 168, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Vallejos, M.E.; Zambon, M.D.; Area, M.C.; da Silva Curvelo, A.A. Low Liquid-Solid Ratio Fractionation of Sugarcane Bagasse by Hot Water Autohydrolysis and Organosolv Delignification. Ind. Crops Prod. 2015, 65, 349–353. [Google Scholar] [CrossRef]
- Yu, H.; You, Y.; Lei, F.; Liu, Z.; Zhang, W.; Jiang, J. Comparative Study of Alkaline Hydrogen Peroxide and Organosolv Pretreatments of Sugarcane Bagasse to Improve the Overall Sugar Yield. Bioresour. Technol. 2015, 187, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.; Mendonça, R.; Da Silva, F.T. Organosolv Delignification of White-and Brown-Rotted Eucalyptus Grandis Hardwood. J. Chem. Technol. Biotechnol. 2000, 75, 18–24. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Kinetics of Organosolv Delignification of Fibre Crop Arundo donax L. Ind. Crops Prod. 2005, 21, 203–210. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Smit, A.T.; de Wild, P.J.; den Uil, H. Fractionation of Wheat Straw by Prehydrolysis, Organosolv Delignification and Enzymatic Hydrolysis for Production of Sugars and Lignin. Bioresour. Technol. 2012, 114, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cybulska, I.; Brudecki, G.P.; Zembrzuska, J.; Schmidt, J.E.; Lopez, C.G.B.; Thomsen, M.H. Organosolv Delignification of Agricultural Residues (Date Palm Fronds, Phoenix dactylifera L.) of the United Arab Emirates. Appl. Energy 2017, 185, 1040–1050. [Google Scholar] [CrossRef]
- Hilares, R.T.; Swerts, M.P.; Ahmed, M.A.; Ramos, L.; da Silva, S.S.; Santos, J.C. Organosolv Pretreatment of Sugar Cane Bagasse for Bioethanol Production. Ind. Eng. Chem. Res. 2017, 56, 3833–3838. [Google Scholar] [CrossRef]
- Garrote, G.; Eugenio, M.E.; Díaz, M.J.; Ariza, J.; López, F. Hydrothermal and Pulp Processing of Eucalyptus. Bioresour. Technol. 2003, 88, 61–68. [Google Scholar] [CrossRef]
- Liu, Z.; Fatehi, P.; Jahan, M.S.; Ni, Y. Separation of Lignocellulosic Materials by Combined Processes of Pre-Hydrolysis and Ethanol Extraction. Bioresour. Technol. 2011, 102, 1264–1269. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Egües, I.; Serrano, L.; Labidi, J.; Spigno, G. Autohydrolysis and Organosolv Process for Recovery of Hemicelluloses, Phenolic Compounds and Lignin from Grape Stalks. Bioresour. Technol. 2012, 107, 267–274. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Vicente, A.A.; Teixeira, J.A. Kinetic Modeling of Enzymatic Saccharification Using Wheat Straw Pretreated under Autohydrolysis and Organosolv Process. Ind. Crops Prod. 2012, 36, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Meighan, B.N.; Lima, D.R.S.; Cardoso, W.J.; Baêta, B.E.L.; Adarme, O.F.H.; Santucci, B.S.; Pimenta, M.T.B.; de Aquino, S.F.; Gurgel, L.V.A. Two-Stage Fractionation of Sugarcane Bagasse by Autohydrolysis and Glycerol Organosolv Delignification in a Lignocellulosic Biorefinery Concept. Ind. Crops Prod. 2017, 108, 431–441. [Google Scholar] [CrossRef]
- Hu, M.; Yu, H.; Li, Y.; Li, A.; Cai, Q.; Liu, P.; Tu, Y.; Wang, Y.; Hu, R.; Hao, B.; et al. Distinct Polymer Extraction and Cellulose DP Reduction for Complete Cellulose Hydrolysis under Mild Chemical Pretreatments in Sugarcane. Carbohydr. Polym. 2018, 202, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Saelee, K.; Yingkamhaeng, N.; Nimchua, T.; Sukyai, P. Extraction and Characterization of Cellulose from Sugarcane Bagasse by Using Environmental Friendly Method. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Chiang Rai, Thailand, 26–29 November 2014. [Google Scholar]
- Jiang, Y.; Liu, X.; Yang, S.; Song, X.; Wang, S. Combining Organosolv Pretreatment with Mechanical Grinding of Sugarcane Bagasse for the Preparation of Nanofibrillated Cellulose in a Novel Green Approach. BioResources 2019, 14, 313–335. [Google Scholar] [CrossRef]
- Konde, K.S.; Nagarajan, S.; Kumar, V.; Patil, S.V.; Ranade, V.V. Sugarcane Bagasse Based Biorefineries in India: Potential and Challenges. Sustain. Energy Fuels 2021, 5, 52–78. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, E.; Portilla-Rivera, O.M.; Jarquín-Enríquez, L.; Ramírez, J.A.; Vázquez, M. Acid Hydrolysis of Wheat Straw: A Kinetic Study. Biomass Bioenergy 2012, 36, 346–355. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Tan, X.; Qi, W.; Yu, Q.; Zhou, G.; Zhuang, X.; Yuan, Z. High Conversion of Sugarcane Bagasse into Monosaccharides Based on Sodium Hydroxide Pretreatment at Low Water Consumption and Wastewater Generation. Bioresour. Technol. 2016, 218, 1230–1236. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Lab. (NREL): Golden, CO, USA, 2008; pp. 1–5.
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor 25 Years on: The Rise of Green Chemistry and Sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Marć, M.; Gałuszka, A.; Namieśnik, J. Green Chemistry Metrics with Special Reference to Green Analytical Chemistry. Molecules 2015, 20, 10928–10946. [Google Scholar] [CrossRef]
- Mena-Cervantes, V.Y.; Hernández-Altamirano, R.; Tiscareño-Ferrer, A. Development of a Green One-Step Neutralization Process for Valorization of Crude Glycerol Obtained from Biodiesel. Environ. Sci. Pollut. Res. Int. 2020, 27, 28500–28509. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Tang, B.; Zeng, M.; Liang, Z.; Jiang, C.; Lin, J.; Xiao, W.; Liu, Z. Enhanced Saccharification of Sugarcane Bagasse by the Optimization of Low Concentration of NaOH and Ammonia Pretreatment. Ind. Crops Prod. 2021, 172, 114016. [Google Scholar] [CrossRef]
- Rabelo, S.C.; da Costa, A.C.; Vaz Rossel, C.E. Industrial Waste Recovery. Sugarcane Agric. Prod. Bioenergy Ethanol. 2015, 17, 365–381. [Google Scholar] [CrossRef]
- Rezende, C.A.; De Lima, M.; Maziero, P.; Deazevedo, E.; Garcia, W.; Polikarpov, I. Chemical and Morphological Characterization of Sugarcane Bagasse Submitted to a Delignification Process for Enhanced Enzymatic Digestibility. Biotechnol. Biofuels 2011, 4, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satlewal, A.; Agrawal, R.; Das, P.; Bhagia, S.; Pu, Y.; Puri, S.K.; Ramakumar, S.S.V.; Ragauskas, A.J. Assessing the Facile Pretreatments of Bagasse for Efficient Enzymatic Conversion and Their Impacts on Structural and Chemical Properties. ACS Sustain. Chem. Eng. 2019, 7, 1095–1104. [Google Scholar] [CrossRef]
- Santucci, B.S.; Maziero, P.; Rabelo, S.C.; Curvelo, A.A.S.; Pimenta, M.T.B. Autohydrolysis of Hemicelluloses from Sugarcane Bagasse During Hydrothermal Pretreatment: A Kinetic Assessment. Bioenergy Res. 2015, 8, 1778–1787. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose Prepared by Acid Hydrolysis of Isolated Cellulose from Sugarcane Bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Agnihotri, S.; Johnsen, I.A.; Bøe, M.S.; Øyaas, K.; Moe, S. Ethanol Organosolv Pretreatment of Softwood (Picea Abies) and Sugarcane Bagasse for Biofuel and Biorefinery Applications. Wood Sci. Technol. 2015, 49, 881–896. [Google Scholar] [CrossRef]
- Budzinski, K.; Blewis, M.; Dahlin, P.; D’Aquila, D.; Esparza, J.; Gavin, J.; Ho, S.V.; Hutchens, C.; Kahn, D.; Koenig, S.G.; et al. Introduction of a Process Mass Intensity Metric for Biologics. New Biotechnol. 2019, 49, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, S.; Sinha Roy, P.; Garnier, G.; Saito, K.; Allais, F. Are Lignin-Derived Monomers and Polymers Truly Sustainable? An in-Depth Green Metrics Calculations Approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Escobar, A.M.; Ruiz, D.M.; Autino, J.C.; Romanelli, G.P. Single-Step Synthesis of 4-Phenyl and 3,4-Dihydro-4-Phenyl Coumarins Using a Recyclable Preyssler Heteropolyacid Catalyst under Solvent-Free Reaction Conditions. Res. Chem. Intermed. 2015, 41, 10109–10123. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Manning, J.R.H.; Chiacchia, M. Bioinspired Synthesis as a Potential Green Method for the Preparation of Nanomaterials: Opportunities and Challenges. Curr. Opin. Green Sustain. Chem. 2018, 12, 110–116. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Vidmantiene, D.; Basinskiene, L.; Cernauskas, D.; Bartkiene, E.; Cizeikiene, D. Green Metrics for Sustainability of Biobased Lactic Acid from Starchy Biomass vs Chemical Synthesis. Catal. Today 2015, 239, 11–16. [Google Scholar] [CrossRef]
- Hsien, C.; Low, J.S.C.; Chung, S.Y.; Tan, D.Z.L. Quality-Based Water and Wastewater Classification for Waste-to-Resource Matching. Resour. Conserv. Recycl. 2019, 151, 104477. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated Cellulose and New Nanocomposite Materials: A Review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Indran, S.; Raj, R.E.; Sreenivasan, V. Characterization of new natural cellulosic fiber from Cissus quadrangularis root. Carbohydr. Polym. 2014, 110, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohydr. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef] [PubMed]


| Sample | HBA | HBD | Ratio HBA:HBD |
|---|---|---|---|
| EU1:1 | Choline chloride | Urea | 1:1 |
| EU1:2 | 1:2 | ||
| ELa1:2 | Lactic acid | 1:2 |
| Yield (%) | Chemical Composition (%) | ||||
|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Soluble Lignin | Klason Lignin | ||
| SCB—all fractions * | - | 36.2 ± 1.1 a | 22.6 ± 0.6 a,b | 4.1 ± 0.2 a | 20.0 ± 0.3 a |
| AcH | 84.5 | 42.5 ± 0.4 b | 23.9 ± 0.3 a,b | 3.8 ± 0.0 a,e | 21.4 ± 0.3 a |
| AlkH | 63.9 | 57.1 ± 0.5 c | 19.9 ± 0.1 a,c | 3.0 ± 0.0 a,e | 8.6 ± 0.6 b |
| EU1:1 | 90.5 | 27.8 ± 0.3 d | 20.6 ± 0.4 a,c | 9.2 ± 0.2 b | 10.9 ± 0.4 b |
| EU1:2 | 92.7 | 34.3 ± 1.0 a | 25.9 ± 1.8 b | 11.4 ± 0.3 c | 15.5 ± 1.0 c |
| ELa1:2 | 90.7 | 34.7 ± 0.9 a | 17.7 ± 0.3 c | 5.9 ± 0.2 d | 15.8 ± 0.1 c |
| AuH | 56.2 | 59.1 ± 0.9 c | 11.4 ± 0.2 d | 2.3 ± 0.6 e | 26.0 ± 0.0 d |
| Yield (%) | Chemical Composition (%) | ||||
|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Soluble Lignin | Klason Lignin | ||
| SCB > 315 µm * | - | 44.8 ± 2.1 a | 34.9 ± 7.8 a | 5.3 ± 0.1 a | 23.5 ± 0.0 a |
| AuH_170 | 63.5 | 69.4 ± 0.4 b,c | 8.8 ± 1.6 b | 2.4 ± 0.3 b | 24.9 ± 2.3 a,b,c |
| AuH_180 | 53.2 | 63.0 ± 1.6 b | 11.3 ± 1.4 b | 2.0 ± 0.0 c | 30.8 ± 0.0 a,b |
| AuH_190 | 49.4 | 64.4 ± 2.1 b | 7.9 ± 1.5 b | 2.3 ± 0.0 b, c | 32.0 ± 0.0 b |
| EtOH | 66.3 | 61.1 ± 3.1 b | 13.2 ± 2.3 b | 4.8 ± 0.0 a | 21.3 ± 2.6 a,c |
| AuH_EtOH | 42.4 | 80.3 ± 4.2 c | 0.00 ± 0.0 b | 1.9 ± 0.1 c | 17.0 ± 1.2 c |
| Process | E-factor | PMI | Energy Consumption (kWh/kg) |
|---|---|---|---|
| AuH_170 | 0.30 | 20.5 | 15.2 |
| AuH_EtOH | 0.56 | 49.33 | 23.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, F.; Freixo, R.; Pereira, C.F.; Ribeiro, A.B.; Costa, E.M.; Pintado, M.E.; Ramos, Ó.L. Comparative Study of Green and Traditional Routes for Cellulose Extraction from a Sugarcane By-Product. Polymers 2023, 15, 1251. https://doi.org/10.3390/polym15051251
Casanova F, Freixo R, Pereira CF, Ribeiro AB, Costa EM, Pintado ME, Ramos ÓL. Comparative Study of Green and Traditional Routes for Cellulose Extraction from a Sugarcane By-Product. Polymers. 2023; 15(5):1251. https://doi.org/10.3390/polym15051251
Chicago/Turabian StyleCasanova, Francisca, Ricardo Freixo, Carla F. Pereira, Alessandra B. Ribeiro, Eduardo M. Costa, Manuela E. Pintado, and Óscar L. Ramos. 2023. "Comparative Study of Green and Traditional Routes for Cellulose Extraction from a Sugarcane By-Product" Polymers 15, no. 5: 1251. https://doi.org/10.3390/polym15051251
APA StyleCasanova, F., Freixo, R., Pereira, C. F., Ribeiro, A. B., Costa, E. M., Pintado, M. E., & Ramos, Ó. L. (2023). Comparative Study of Green and Traditional Routes for Cellulose Extraction from a Sugarcane By-Product. Polymers, 15(5), 1251. https://doi.org/10.3390/polym15051251