High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogels
2.3. Characterization Methods
2.3.1. Visual Examination
2.3.2. Methods
2.3.3. Pharmacotechnical Evaluation
Tensile Strength and Elongation Ability
Moisture Content
pH Analysis
Swelling Ratio
Spreadability
3. Results and Discussion
3.1. FTIR Spectroscopy
3.2. XRD Analysis
3.3. Raman Analysis
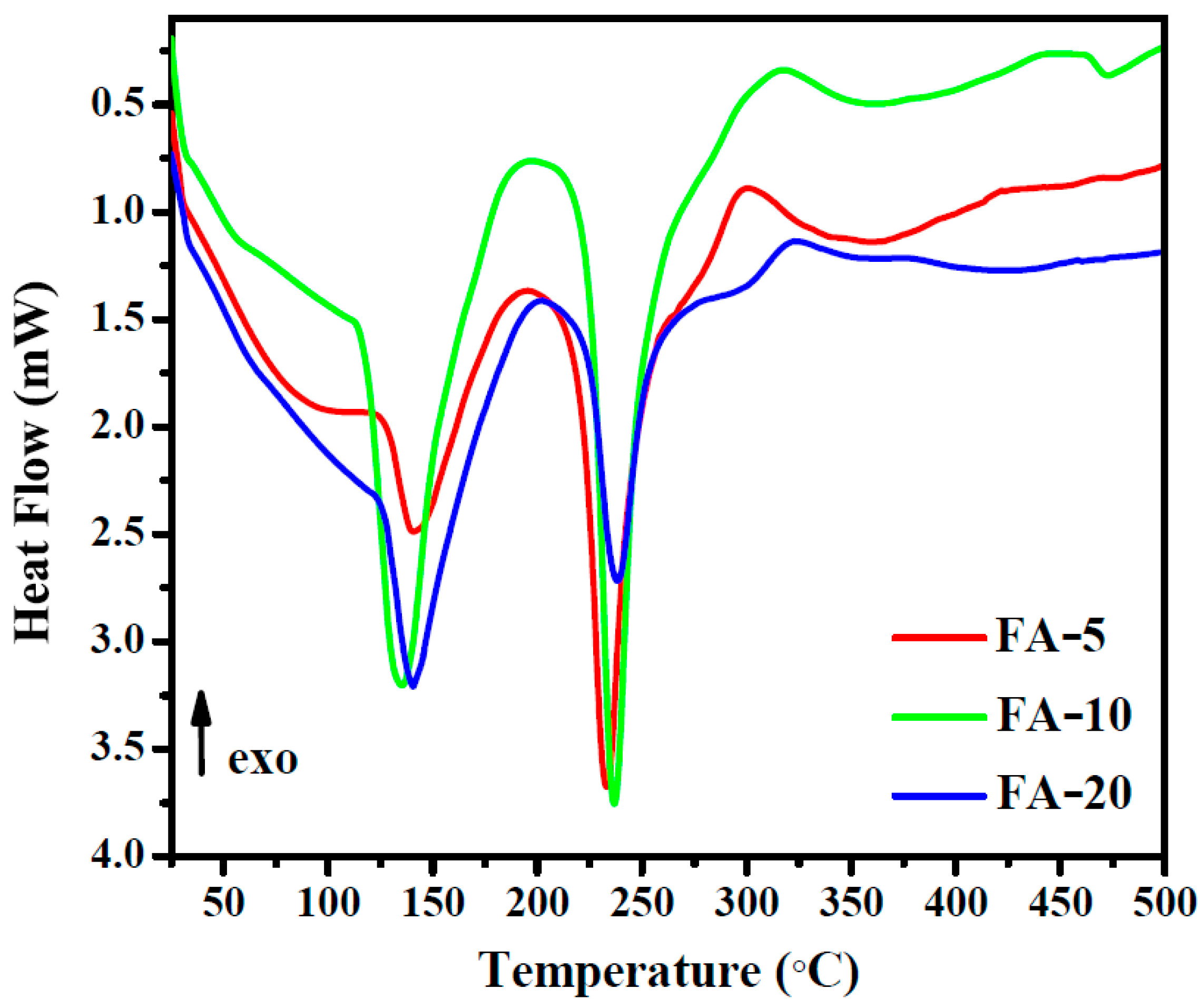
3.4. DSC Analysis
3.5. Thermal analysis
3.6. SEM Analysis
3.7. AFM Results




3.8. Pharmacotechnical Evaluation
Tensile Strength and Elongation Ability
| Tested Parameter * | Formulation Code | |||||
|---|---|---|---|---|---|---|
| FA-5 Wet | FA-5 Dry | FA-10 Wet | FA-10 Dry | FA-20 Wet | FA-20 Dry | |
| Tensile strength (kg/mm2) | - | 1.06 ± 0.12 | - | 1.97 ± 0.23 | - | 1.08 ± 0.25 |
| Elongation (%) | - | 26.52 ± 0.34 | - | 47.83 ± 0.85 | - | 29.22 ± 0.76 |
| Moisture content, % (w/w) | - | 4.39 ± 0.84 | - | 7.27 ± 0.55 | - | 8.81± 0.35 |
| pH | 5.81 ± 0.03 | 5.85 ± 0.11 | 5.93 ± 0.01 | 5.96 ± 0.03 | 6.04 ± 0.06 | 6.05 ± 0.02 |
| Swelling ratio (%, after 6 h) | - | 187 ± 3.11 | - | 231 ± 4.79 | - | 212 ± 3.77 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Agnes, C.J.; Murshed, M.; Takada, A.; Willie, B.M.; Tabrizian, M. A 6-bromoindirubin-3′-oxime incorporated chitosan-based hydrogel scaffold for potential osteogenic differentiation: Investigation of material properties in vitro. Int. J. Biol. Macromol. 2023, 227, 71–82. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Lamboni, L.; Ahmed, A.A.Q.; Zheng, R.; Boni, B.O.O.; Shi, Z.; Song, S.; Souho, T.; Mukole, B.M.; Qi, F.; et al. Antibacterial silk sericin/poly (vinyl alcohol) hydrogel with antifungal property for potential infected large burn wound healing: Systemic evaluation. Smart Mater. Med. 2023, 4, 37–58. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Zoetebier, B.; Dijkstra, P.J.; Karperien, M. Enzymatic co-crosslinking of star-shaped poly(ethylene glycol) tyramine and hyaluronic acid tyramine conjugates provides elastic biocompatible and biodegradable hydrogels. Bioact. Mater. 2023, 20, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, A.; Chen, W.; Lai, J.; Pei, Y.; Wen, J.; Yang, C.; Luo, J.; Zhang, J.; Lei, C.; et al. Fabrication of Biodegradable and Biocompatible Functional Polymers for Anti-Infection and Augmenting Wound Repair. Polymers 2023, 15, 120. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Jales, S.T.L.; Barbosa, R.d.M.; de Albuquerque, A.C.; Duarte, L.H.V.; da Silva, G.R.; Meirelles, L.M.A.; da Silva, T.M.S.; Alves, A.F.; Viseras, C.; Raffin, F.N.; et al. Development and Characterization of Aloe vera Mucilaginous-Based Hydrogels for Psoriasis Treatment. J. Compos. Sci. 2022, 6, 231. [Google Scholar] [CrossRef]
- Dewi, S.T.; Susanto, C. Effect of Aloe vera Hydrogel Application on Increasing the Number of Fibroblasts in Socket Wounds Post-Tooth Extraction: An In Vivo Study. Biosci. Med. J. Biomed. Transl. Res. 2021, 6, 1347–1352. [Google Scholar] [CrossRef]
- Balaji, A.; Vellayappan, M.V.; John, A.A.; Subramanian, A.P.; Jaganathan, S.K.; SelvaKumar, M.; Athif bin Mohd Faudzi, A.; Supriyanto, E.; Yusof, M. Biomaterials based nano-applications of Aloe vera and its perspective: A review. RSC Adv. 2015, 5, 86199–86213. [Google Scholar] [CrossRef]
- Spalding, L. The Pharmacopoeia of the United States of America: 1820; By the authority of the medical societies and colleges; Wells and Lilly: Boston, MA, USA, 1820. [Google Scholar]
- Sharma, P.; Kharkwal, A.C.; Kharkwal, H.; Abdin, M.Z.; Varma, A. A review on pharmacological properties of Aloe vera. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 31–37. [Google Scholar]
- Ryall, C.; Duarah, S.; Chen, S.; Yu, H.; Wen, J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics 2022, 14, 1072. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, K.; Paczkowska-Walendowska, M.; Osmałek, T.; Cielecka-Piontek, J. Towards the Preparation of a Hydrogel from Lyophilisates of the Aloe arborescens Aqueous Extract. Pharmaceutics 2022, 14, 1489. [Google Scholar] [CrossRef]
- Razia, S.; Park, H.; Shin, E.; Shim, K.S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef]
- Pounikar, Y.; Jain, P.; Khurana, N.; Omray, L.K.; Patil, S.; Gajbhiye, A. Formulation and characterization of Aloe vera cosmetic herbal hydrogel. Int. J. Pharm. Pharm. Sci. 2012, 4, 85–86. [Google Scholar]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants-A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Dinica, R.M.; Sandu, C.; Dediu Botezatu, A.V.; Cazanevscaia Busuioc, A.; Balanescu, F.; Ionica Mihaila, M.D.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from Valuable Romanian Animal and Plant Sources with Promising Anti-Inflammatory Activity as a Nutricosmetic Ingredient. Sustainability 2021, 13, 10170. [Google Scholar] [CrossRef]
- Bakibaev, A.A.; Il’Yasov, S.G.; Tatarenko, O.V.; Tuguldurova, V.P.; Zorin, A.O.; Malkov, V.S.; Kasyanova, A.S. Allantoin: Synthesis and chemical properties. Becmнuк Kapaгaндuнcкoгo Унuвepcumema 2020, 1, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Bialik-Wąs, K.; Miastkowska, M.; Sapuła, P.; Pluta, K.; Malina, D.; Chwastowski, J.; Barczewski, M. Bio-Hybrid Hydrogels Incorporated into a System of Salicylic Acid-pH/Thermosensitive Nanocarriers Intended for Cutaneous Wound-Healing Processes. Pharmaceutics 2022, 14, 773. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Chelu, M.; Musuc, A.M.; Aricov, L.; Ozon, E.A.; Iosageanu, A.; Stefan, L.M.; Prelipcean, A.-M.; Popa, M.; Moreno, J.C. An-tibacterial Aloe vera Based Biocompatible Hydrogel for Use in Dermatological Applications. Int. J. Mol. Sci. 2023, 24, 3893. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Oliveira, M.B.; Mano, J.F.; Reis, R.L. Bio-inspired Aloe vera sponges for biomedical applications. Carbohydr. Polym. 2014, 112, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, B. Development and characterization of nanosoy-reinforced dextran nanocomposite membranes. J. Appl. Polym. Sci. 2016, 134, 44655. [Google Scholar] [CrossRef]
- Koga, A.Y.; Felix, J.C.; Silvestre, R.G.M.; Lipinski, L.C.; Carletto, B.; Kawahara, F.A.; Pereira, A.V. Evaluation of wound healing effect of alginate film containing Aloe vera gel and cross-linked with zinc chloride. Acta Cir. Bras. 2020, 35, e202000507. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Alves-Lima, D.; Lin, H.; Douglas, T.E.L.; Kuciel, S.; Zagórska, A.; Tyliszczak, B. Investigations on the impact of the introduction of the Aloe vera into the hydrogel matrix on cytotoxic and hydrophilic properties of these systems considered as potential wound dressings. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111977. [Google Scholar] [CrossRef]
- Galdorfini, B.; De Almeida, M.G.J.; Antonio, M.; Isaac, V.L.B. Cosmetics’ Quality Control. In Latest Research into Quality Control; Akyar, I., Ed.; InTech: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.who.int/docs/default-source/medicines/norms-and-standards/guidelines/quality-control/trs957-annex1-goodpractices-harmaceuticalqualitycontrol-laboratories.pdf?sfvrsn=ca0c211c_0 (accessed on 1 February 2023).
- Balaci, T.; Velescu, B.; Karampelas, O.; Musuc, A.M.; Nițulescu, G.M.; Ozon, E.A.; Nițulescu, G.; Gîrd, C.E.; Fița, C.; Lupuliasa, D. Physico-Chemical and Pharmaco-Technical Characterization of Inclusion Complexes Formed by Rutoside with β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin Used to Develop Solid Dosage Forms. Processes 2021, 9, 26. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive buccal patches of miconazole nitrate: In vitro/in vivo performance and effect of ageing. Int. J Pharm. 2003, 264, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Huang, M.L.; Chiu, A.C.; Kuo, K.H.; Chiu, W.Y.; Chiu, L.H. Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater. Chem. Phys. 2008, 107, 266–273. [Google Scholar] [CrossRef]
- Derle, D.; Joshi, O.; Pawar, A.; Patel, J.; Perdeshi, V. Effect of tablet excipients on mucoadhesive properties of polyoxyethylene and Carbopol 971P. Int. J. Pharm. Pharm. Sci. 2009, 1, 198–205. [Google Scholar]
- Kulawik-Pióro, A.; Drabczyk, A.K.; Kruk, J.; Wróblewska, M.; Winnicka, K.; Tchórzewska, J. Thiolated Silicone Oils as New Components of Protective Creams in the Prevention of Skin Diseases. Materials 2021, 14, 4723. [Google Scholar] [CrossRef]
- Gore, E.; Picard, C.; Savary, G. Spreading behavior of cosmetic emulsions: Impact of the oil phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Del Grosso, V.; Ferrari, G. Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels 2022, 8, 152. [Google Scholar] [CrossRef]
- Djekic, L.; Krajišnik, D.; Mićic, Z.; Čalija, B. Formulation and physicochemical characterization of hydrogels with β-glycyrrhetinic acid/phospholipid complex phytosomes. J. Drug Deliv. Sci. Technol. 2016, 35, 81–90. [Google Scholar] [CrossRef]
- Pulgarín, O.; Larrea-Wachtendorff, D.; Ferrari, G. Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels 2023, 9, 87. [Google Scholar] [CrossRef]
- Bajer, D.; Janczak, K.; Bajer, K. Novel Starch/Chitosan/Aloe vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef] [Green Version]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C 2021, 120, 111667. [Google Scholar] [CrossRef]
- Beverina, M.; Sánchez-Cortés, S.; Schabes, F.I.; Zapata, J.; Cassará, M.A.; Tuttolomondo, M.E. Spectroscopic characterization (Raman and infrared) of Aloe maculata from the north Argentina region. Vib. Spectrosc. 2022, 122, 103423. [Google Scholar] [CrossRef]
- Kotcharat, P.; Chuysinuan, P.; Thanyacharoen, T.; Supanna, T.; Sarute, U. Enhanced Performance of Aloe vera-Incorporated Bacterial Cellulose/Polycaprolactone Composite Film for Wound Dressing Applications. J. Polym. Environ. 2022, 30, 1151–1161. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Medina Torres, L.; Gonzalez Laredo, R.F.; Rodriguez Gonzalez, V.M.; Eim, V.; Femenia, A. Influence of water deficit on the main polysaccharides and the rheological properties of Aloe vera (Aloe barbadensis Miller) mucilage. Ind. Crops Products 2017, 109, 644–653. [Google Scholar] [CrossRef]
- Lim, Z.X.; Cheong, K.Y. Effects of drying temperature and ethanol concentration on bipolar switching characteristics of natural Aloe vera based memory devices. Phys. Chem. Chem. Phys. 2015, 17, 26833–26853. [Google Scholar] [CrossRef] [PubMed]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- McConaughy, S.D.; Stroud, P.A.; Boudreaux, B.; Hester, R.D.; McCormick, C.L. Structural characterization and solution properties of a galacturonate polysaccharide derived from Aloe vera capable of in situ gelation. Biomacromolecules 2008, 9, 472–480. [Google Scholar] [CrossRef]
- López, Z.; Zúñiga, M.N.S.; Femenia, A.; Acevedo-Hernández, G.J.; Flores, J.A.G.; Cano, M.E.; Knauth, P. Dry but Not Humid Thermal Processing of Aloe vera Gel Promotes Cytotoxicity on Human Intestinal Cells HT-29. Foods 2022, 11, 745. [Google Scholar] [CrossRef]
- Yi, Y.; Xu, W.; Wang, H.-X.; Huang, F.; Wang, L.-E. Natural polysaccharides experience physiochemical and functional changes during preparation: A review. Carbohydr. Polym. 2020, 234, 18. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Koga, A.Y.; Pereira, A.V.; Lipinski, L.C.; Oliveira, M.R.P. Evaluation of wound healing effect of alginate films containing Aloe vera (Aloe barbadensis Miller) gel. J. Biomater. Appl. 2018, 32, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Pereira, J.C.; Pais, A.A.C.C.; Sousa, J.S. Films based on chitosan polyelectrolyte complexes for skin drug: Development and characterization. J. Memb. Sci. 2008, 320, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Aman, R.M.; Zaghloul, R.A.; El-Dahhan, M.S. Formulation, optimization and characterization of allantoin-loaded chitosan nanoparticles to alleviate ethanol-induced gastric ulcer: In-vitro and in-vivo studies. Sci. Rep. 2021, 11, 2216. [Google Scholar] [CrossRef]
- Kang, Y.; Li, P.; Zeng, X.; Chen, X.; Xie, Y.; Zeng, Y.; Zhang, Y.; Xie, T. Biosynthesis, structure and antioxidant activities of xanthan gum from Xanthomonas campestris with additional furfural. Carbohydr. Polym. 2019, 216, 369–375. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Mohamed, R.R.; ElHafeez, E.A.; Sabaa, M.W. Synthesis of novel biodegradable antibacterial grafted xanthan gum. Carbohydr. Polym. 2017, 173, 305–311. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; Yu, M.; He, P.; Qiao, H.; Zhang, H.; Wang, Z. Characterization and Antioxidant Activity of a Low-Molecular-Weight Xanthan Gum. Biomolecules 2019, 9, 730. [Google Scholar] [CrossRef] [Green Version]
- Subramani, K.; Kolathupalayam Shanmugam, B.; Rangaraj, S.; Palanisamy, M.; Periasamy, P.; Venkatachalam, R. Screening the UV-blocking and antimicrobial properties of herbal nanoparticles prepared from Aloe vera leaves for textile applications. IET Nanobiotechnol. 2018, 12, 459–465. [Google Scholar] [CrossRef]
- Andonegi, M.; Irastorza, A.; Izeta, A.; de la Caba, K.; Guerrero, P. Physicochemical and Biological Performance of Aloe vera-Incorporated Native Collagen Films. Pharmaceutics 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and Fructans from Aloe vera (Aloe barbadensis Miller) Plants as Novel Prebiotic. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Pacheco, M.S.; de Moraes, M.A.; Lopes, P.S.; Severino, P.; Souto, E.B.; Da Silva, C.F. Effect of chitosan and Aloe vera extract concentrations on the physicochemical properties of chitosan biofilms. Polymers 2021, 13, 1187. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. In. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Avella, M.; Di Pace, E.; Immirzi, B.; Impallomeni, G.; Malinconico, M.; Santagata, G. Addition of glycerol plasticizer to seaweeds derived alginates: Influence of microstructure on chemical–physical properties. Carbohydr. Polym. 2007, 69, 503–511. [Google Scholar] [CrossRef]
- Londhe, V.; Shirsat, R. Formulation and characterization of fast-dissolving sublingual film of iloperidone using Box–Behnken design for enhancement of oral bioavailability. AAPS PharmSciTech 2018, 19, 1392–1400. [Google Scholar] [CrossRef]
- Ahlawat, K.S.; Khatkar, B.S. Processing, food applications and safety of Aloe vera product indicating the interactions of collagen with the additives incorporated in the formulations: A review. J. Food Sci. Technol. 2011, 48, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, P.; Adarsh, P.P.; Sujit, D. Pharmaceutical Creams and their use in wound healing: A Review. J. Drug Deliv. Ther. 2019, 9, 907–912. [Google Scholar] [CrossRef]
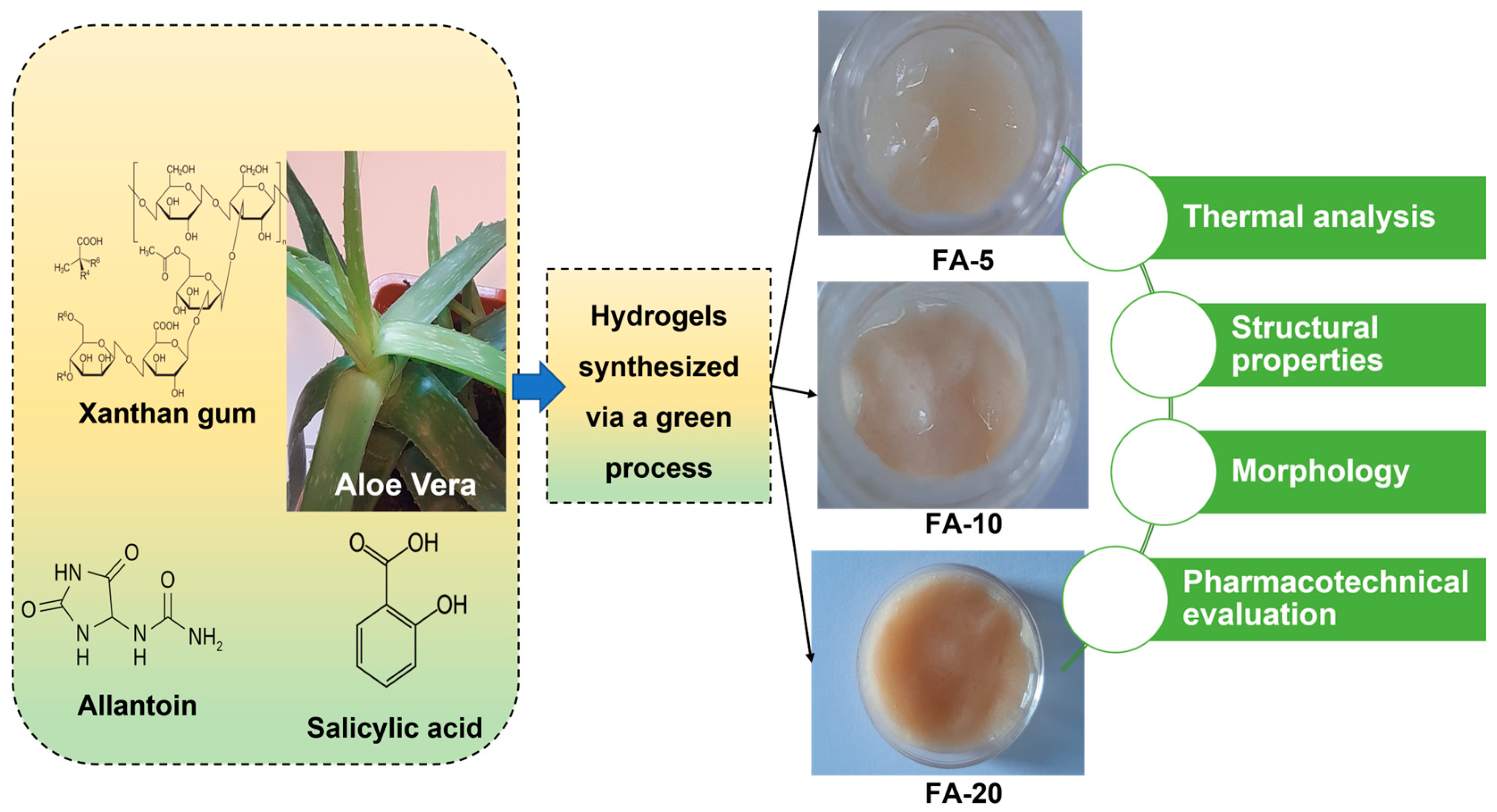






| Formulae | Appearances | Color | Homogeneity [42,43] | Consistency | Aloe vera Content | Phase Separation | |
|---|---|---|---|---|---|---|---|
| (w/v) % | Dried (wt%) | ||||||
| FA-5 | Homogeneous | Translucent, pale beige, neutral | Very good | Good | 5 | 38 | No phase separation |
| FA-10 | Homogeneous | Opaque, natural beige | Very good | Good | 10 | 55 | No phase separation |
| FA-20 | Homogeneous | Opaque, intense natural beige | Very good | Good | 20 | 71 | No phase separation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelu, M.; Popa, M.; Ozon, E.A.; Pandele Cusu, J.; Anastasescu, M.; Surdu, V.A.; Calderon Moreno, J.; Musuc, A.M. High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers 2023, 15, 1312. https://doi.org/10.3390/polym15051312
Chelu M, Popa M, Ozon EA, Pandele Cusu J, Anastasescu M, Surdu VA, Calderon Moreno J, Musuc AM. High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers. 2023; 15(5):1312. https://doi.org/10.3390/polym15051312
Chicago/Turabian StyleChelu, Mariana, Monica Popa, Emma Adriana Ozon, Jeanina Pandele Cusu, Mihai Anastasescu, Vasile Adrian Surdu, Jose Calderon Moreno, and Adina Magdalena Musuc. 2023. "High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties" Polymers 15, no. 5: 1312. https://doi.org/10.3390/polym15051312
APA StyleChelu, M., Popa, M., Ozon, E. A., Pandele Cusu, J., Anastasescu, M., Surdu, V. A., Calderon Moreno, J., & Musuc, A. M. (2023). High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers, 15(5), 1312. https://doi.org/10.3390/polym15051312