Preparation and Characterization of Microalgae Styrene-Butadiene Composites Using Chlorella vulgaris and Arthrospira platensis Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Preparation of Polymer Composites
2.3. Polymer Testing Setup
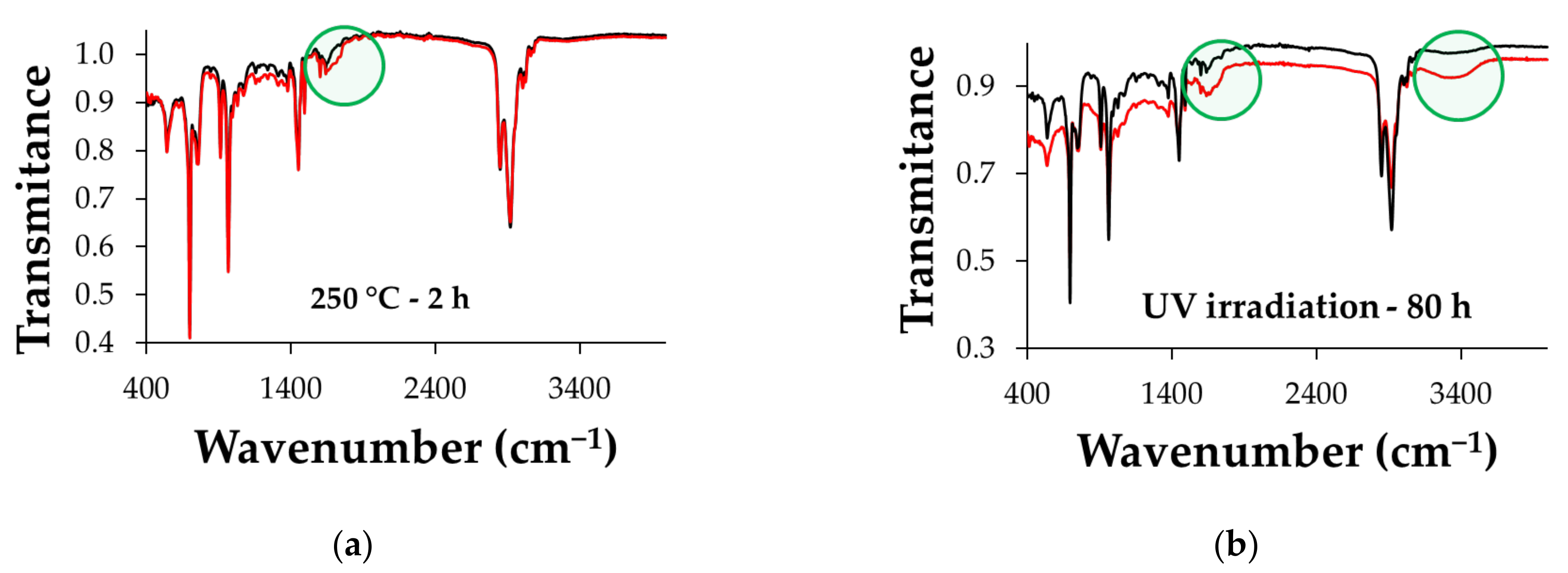
2.4. Characterization Techniques
3. Results and Discussion
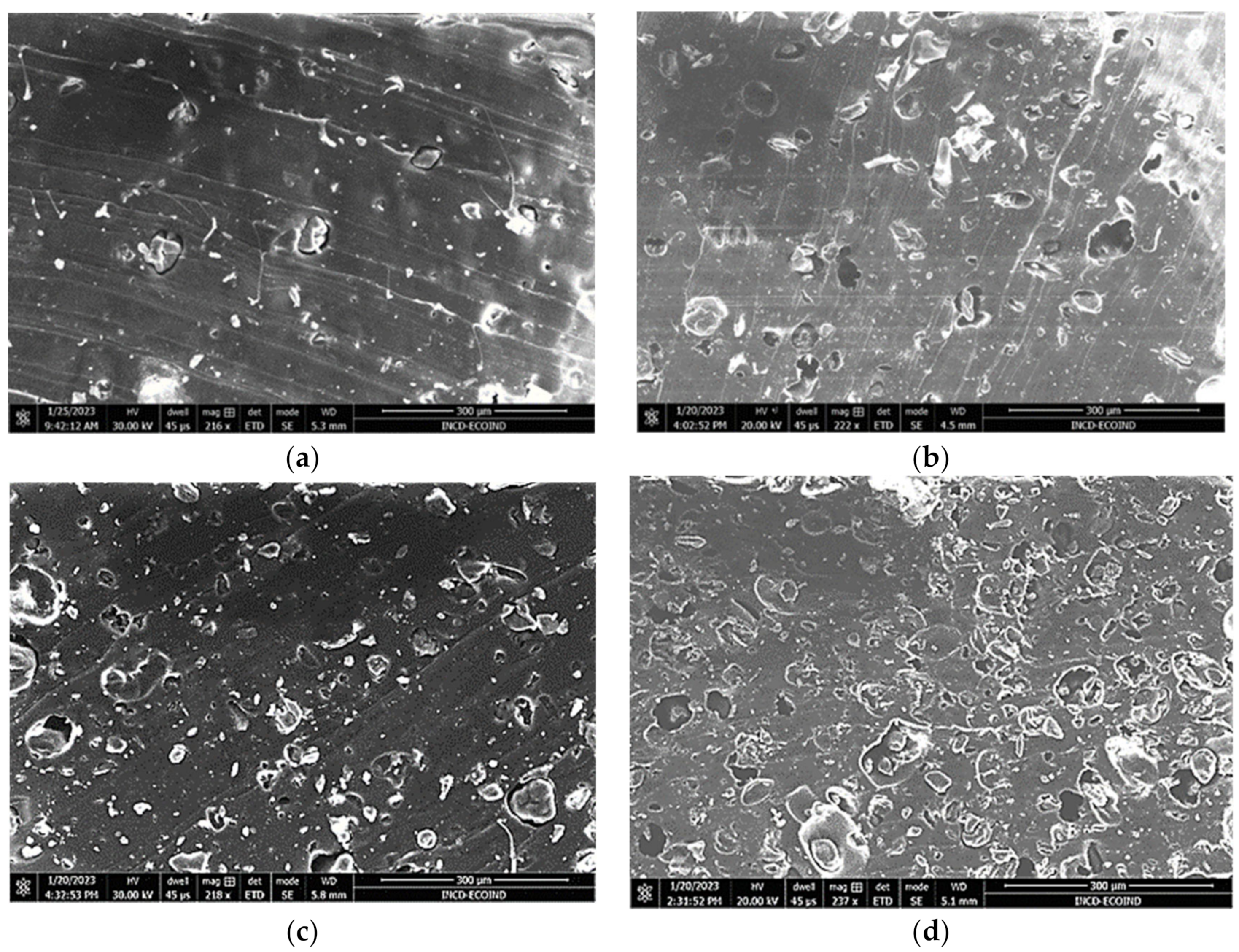
3.1. Microscopic Characterization and Particle-Size Distribution
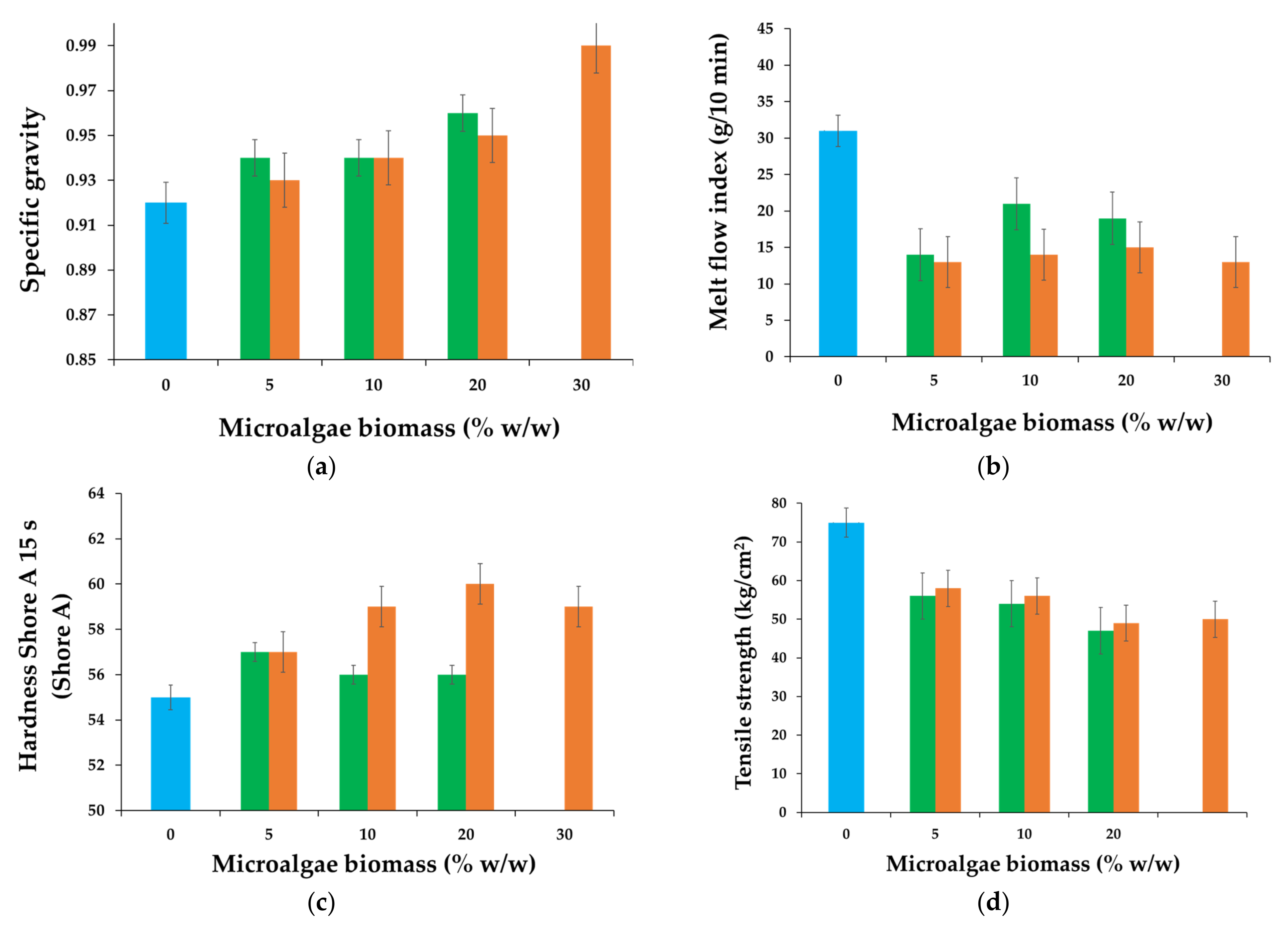
3.2. Physicomechanical Characterization of SBS Composite
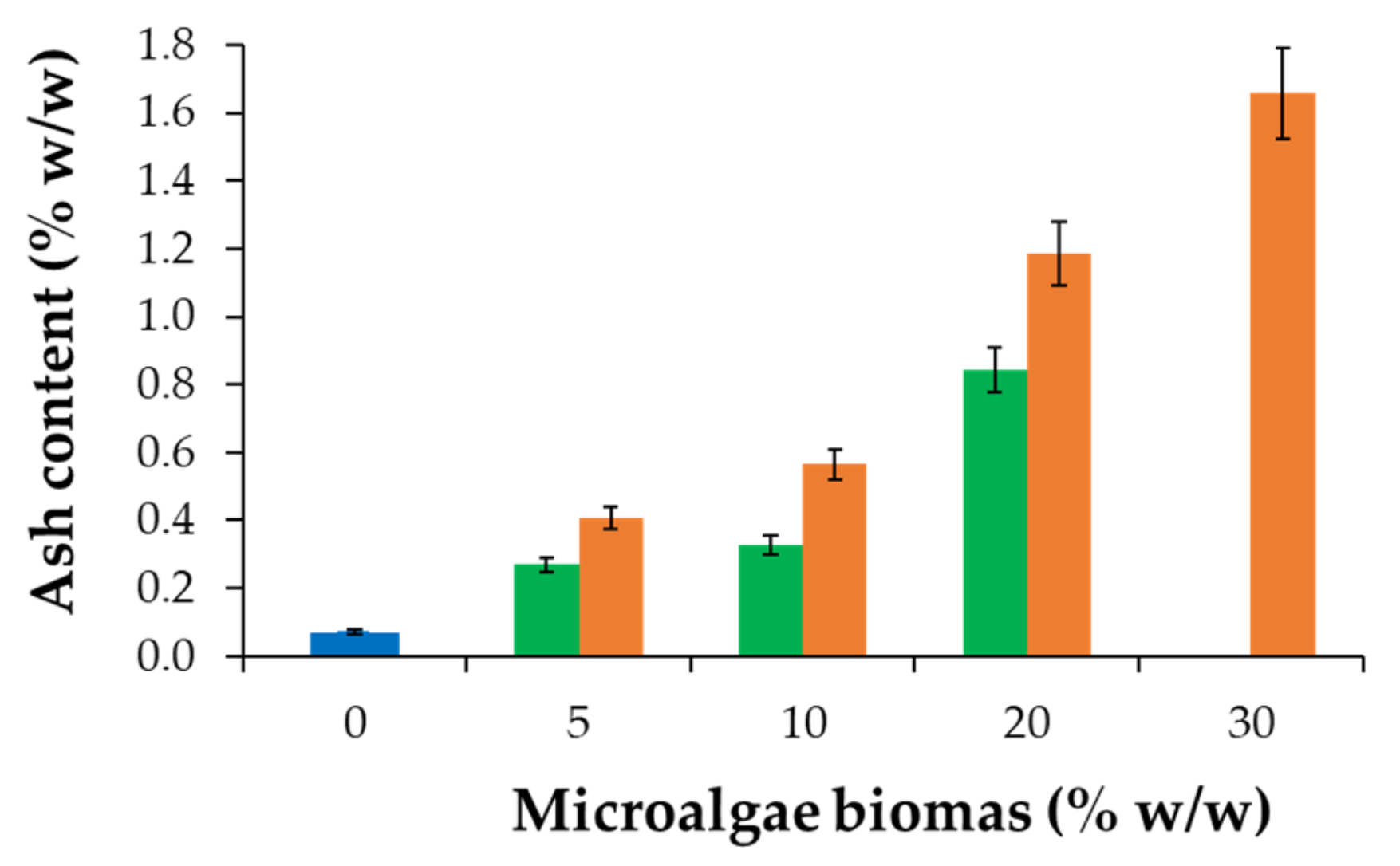
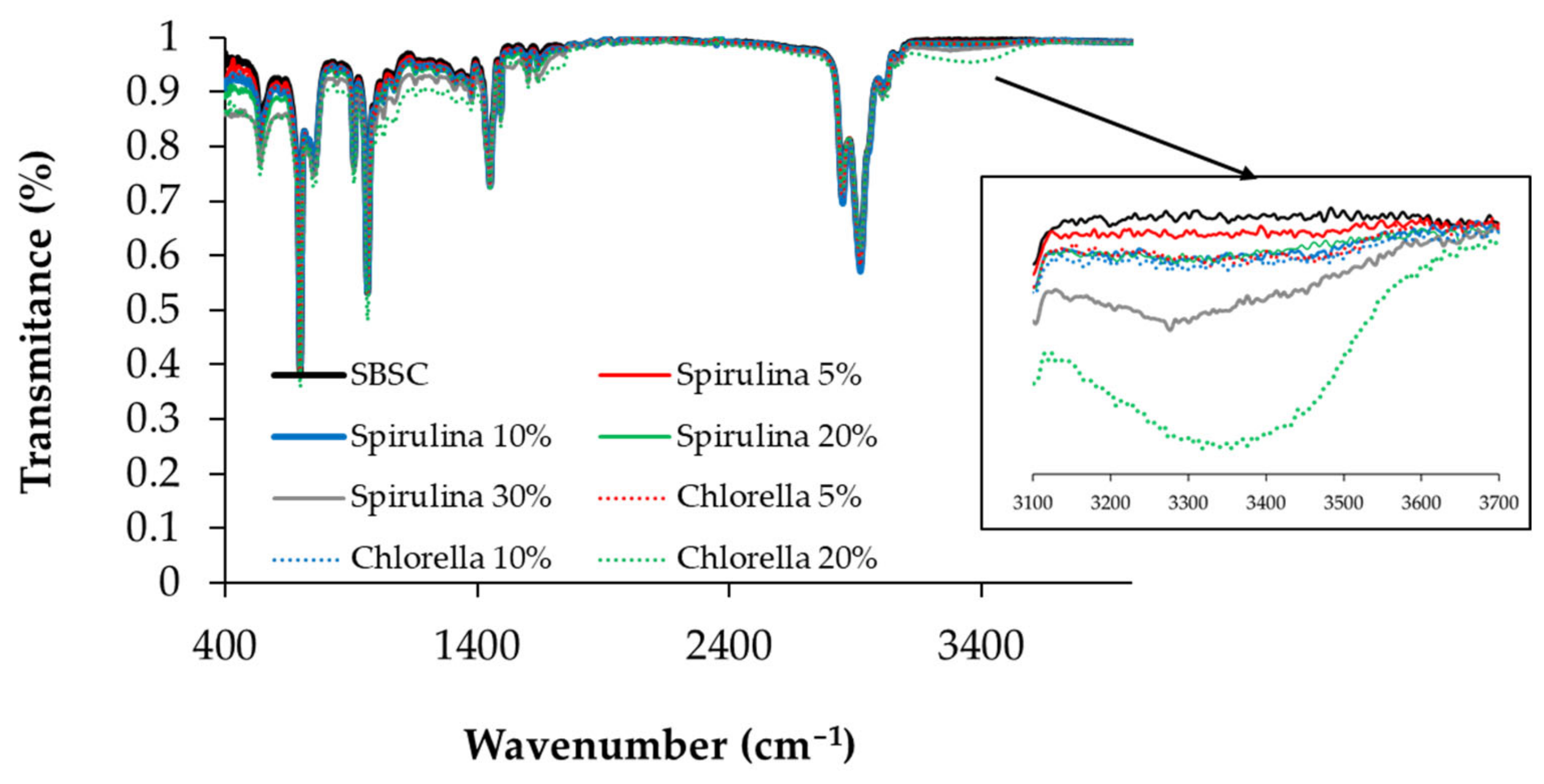
3.3. Physicochemical Characterization of SBS Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grand View Research. Food Packaging Market Size, Share & Trends Analysis Report by Type (Rigid, Flexible, Semi-Rigid), by Material (Paper & Paper-based, Plastic, Metal, Glass), by Application, by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/food-packaging-market (accessed on 5 December 2022).
- Piergiovanni, L.; Limbo, S. Introduction to food packaging materials. In Food Packaging Materials; Springer: Cham, Switzerland, 2016; pp. 1–3. [Google Scholar]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packaging Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Gilbert, M. Plastics materials: Introduction and historical development. In Brydson’s Plastics Materials; Butterworth-Heinemann: Oxford, UK, 2017; pp. 1–18. [Google Scholar]
- Margolis, J.M. Part 2: Engineering Plastics in Engineering Plastics Handbook; McGraw-Hill Education: New York, NY, USA, 2006. [Google Scholar]
- Kühne, F.; Biedermann, M.; Eicher, A.; Felder, F.; Sander, S.; Schmidt, R.; Lehmann, S.; McCombie, G.; Merkel, S.; Kappenstein, O.; et al. Characterisation of Elastomers as Food Contact Materials–Part 1: Quantification of Extractable Compounds, Swelling of Elastomers in Food Simulants and Release of Elements. Molecules 2021, 26, 509. [Google Scholar] [CrossRef]
- Obrecht, W.; Lambert, J.-P.; Happ, M.; Oppenheimer-Stix, C.; Dunn, J.; Krüger, R. Rubber, 4. Emulsion Rubbers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Vian, W.D.; Denton, N.L. Hardness Comparison of Polymer Specimens Produced with Different Processes; Purdue University Purdue e-Pubs: West Lafayette, IN, USA, 2018. [Google Scholar]
- Cai, W.; Xu, D.; Qian, L.; Wei, J.; Xiao, C.; Qian, L.; Lu, Z.Y.; Cui, S. Force-induced Transition of π–π Stacking in a Single Polystyrene Chain. J. Am. Chem. Soc. 2019, 141, 9500–9503. [Google Scholar] [CrossRef]
- Polacco, G.; Stastna, J.; Biondi, D.; Zanzotto, L. Relation between polymer architecture and nonlinear viscoelastic behavior of modified asphalts. Curr. Opin. Colloid Interface Sci. 2006, 11, 230–245. [Google Scholar] [CrossRef]
- De, S.K.; White, J.R. (Eds.) Rubber Technologist’s Handbook; Smithers Rapra Publishing: Shrewsbury, UK, 2001; Volume 1. [Google Scholar]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between microplastics and soil fauna: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Wang, S.C.; Zhao, F.F.; Wang, S.G.; Liu, F.F.; Liu, G.Z. Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environ. Pollut. 2019, 246, 509–517. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Thiel, M.; Prindiville, M.; Kiessling, T. Microplastic: What are the solutions. Freshw. Microplast. 2018, 273. [Google Scholar]
- Sudesh, K.; Iwata, T. Sustainability of biobased and biodegradable plastics. CLEAN Soil Air Water 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [Green Version]
- Vieyra, H.; Molina-Romero, J.M.; Calderón-Nájera, J.D.D.; Santana-Díaz, A. Engineering, Recyclable, and Biodegradable Plastics in the Automotive Industry: A Review. Polymers 2022, 14, 3412. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneira-Velasco, K.E.; Utreras-Silva, C.A.; Díaz-Barrios, A.; Sommer-Márquez, A.E.; Tafur, J.P.; Michell, R.M. Green nanocomposites based on thermoplastic starch: A review. Polymers 2021, 13, 3227. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Fauza, A.N.; Mardiyati, Y.; Santosa, S.P.; Shoimah, S.M.A. Facile preparation of cellulose bioplastic from Cladophora sp. algae via hydrogel method. Polymers 2022, 14, 4699. [Google Scholar] [CrossRef]
- Ahlgren, G.; Gustafsson, I.B.; Boberg, M. Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 1992, 28, 37–50. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Capone, C.; Di Landro, L.; Inzoli, F.; Penco, M.; Sartore, L. Thermal and mechanical degradation during polymer extrusion processing. Polym. Eng. Sci. 2007, 47, 1813–1819. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a dietary supplement to promote human health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresour. Technol. 2013, 135, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and nutritional evaluation of Chlorella and Auxenochlorella biomasses relevant for food application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Biology of microalgae. In Mycroalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 23–72. [Google Scholar]
- Hlima, H.B.; Bohli, T.; Kraiem, M.; Ouederni, A.; Mellouli, L.; Michaud, P.; Abdelkafi, S.; Smaoui, S. Combined effect of Spirulina platensis and Punica granatum peel extracts: Phytochemical content and antiphytophatogenic activity. Appl. Sci. 2019, 9, 5475. [Google Scholar] [CrossRef] [Green Version]
- Fernández, F.A.; Sevilla, J.M.F.; Grima, E.M. Costs analysis of microalgae production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–566. [Google Scholar]
- Pelizer, L.H.; de Carvalho, J.C.M.; de Oliveira Moraes, I. Protein production by Arthrospira (Spirulina) platensis in solid state cultivation using sugarcane bagasse as support. Biotechnol. Rep. 2015, 5, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, S.; Bhavan, P.S.; Seenivasan, C.; Muralisankar, T. Nutritional profile of Spirulina platensis, Chlorella vulgaris and Azolla pinnata to novel protein source for aquaculture feed formulation. Austin J. Aquac. Mar. Biol. 2017, 2, 1–8. [Google Scholar]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina platensis protein as sustainable ingredient for nutritional food products development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Liang, M.; Liang, P.; Fan, W.; Qian, C.; Xin, X.; Shi, J.; Nan, G. Thermo-rheological behavior and compatibility of modified asphalt with various styrene–butadiene structures in SBS copolymers. Mater. Des. 2015, 88, 177–185. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, S.; Gao, X.; Altarawneh, M.; Hayashi, J.I.; Zhang, M.; Dlugogorski, B.Z. Analytical procedure for proximate analysis of algal biomass: Case study for Spirulina platensis and Chlorella vulgaris. Energy Fuels 2019, 34, 474–482. [Google Scholar] [CrossRef]
- Chu, H.Z.; Liu, D.; Cui, Z.W.; Wang, K.; Qiu, G.X.; Liu, G.Y. Effect of crosslink density on solubility parameters of styrene butadiene rubber and the application in pre-screening of new potential additives. Polym. Test. 2020, 81, 106253. [Google Scholar] [CrossRef]
- Poole, J.W. Solubilities of Oils and Waxes in Organic Solvents—II. Ind. Eng. Chem. 1931, 23, 170–177. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid extraction methods from microalgae: A comprehensive review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef] [Green Version]
- Rubber Chemical Resistance Chart, Mykin Inc Manufactures O-rings, Custom Molded Rubber and Mechanical Seals, Rubber Chemical Resistance Chart, Rubber Compatibility Chart-Mykin Inc. Available online: https://mykin.com/rubber-chemical-resistance-chart (accessed on 28 January 2023).
- Bumbac, M.; Nicolescu, C.M.; Olteanu, R.L.; Tiron, O.; Manea, E.E.; Bumbac, C.; Gorghiu, L.M.; Radulescu, C.; Stanescu, G.; Serban, B.C.; et al. UV-VIS analysis of granular activated algae chlorophyll content. J. Sci. Arts 2021, 57, 1111–1120. [Google Scholar] [CrossRef]
- Manrich, A.; de Oliveira, J.E.; Martins, M.A.; Mattoso, L.H.C. Physicochemical and Thermal Characterization of the Spirulina platensis. J. Agric. Sci. Technol. B 2020, 10, 298–307. [Google Scholar] [CrossRef] [PubMed]
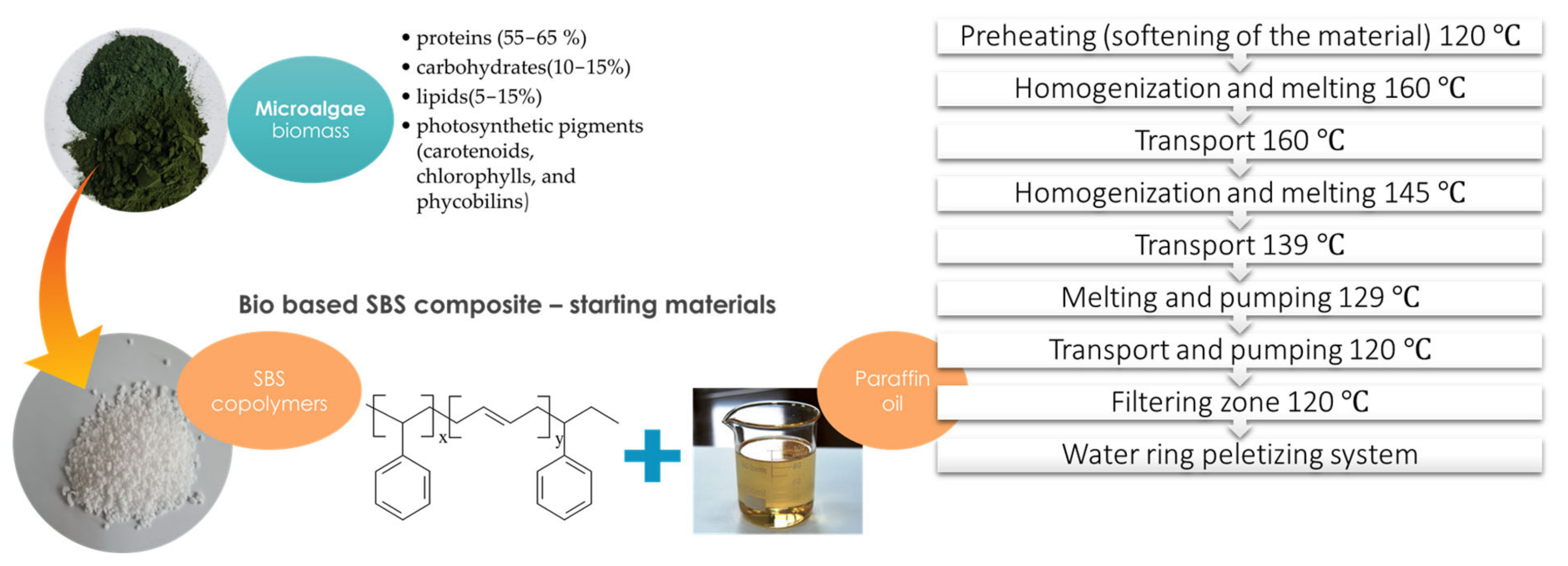
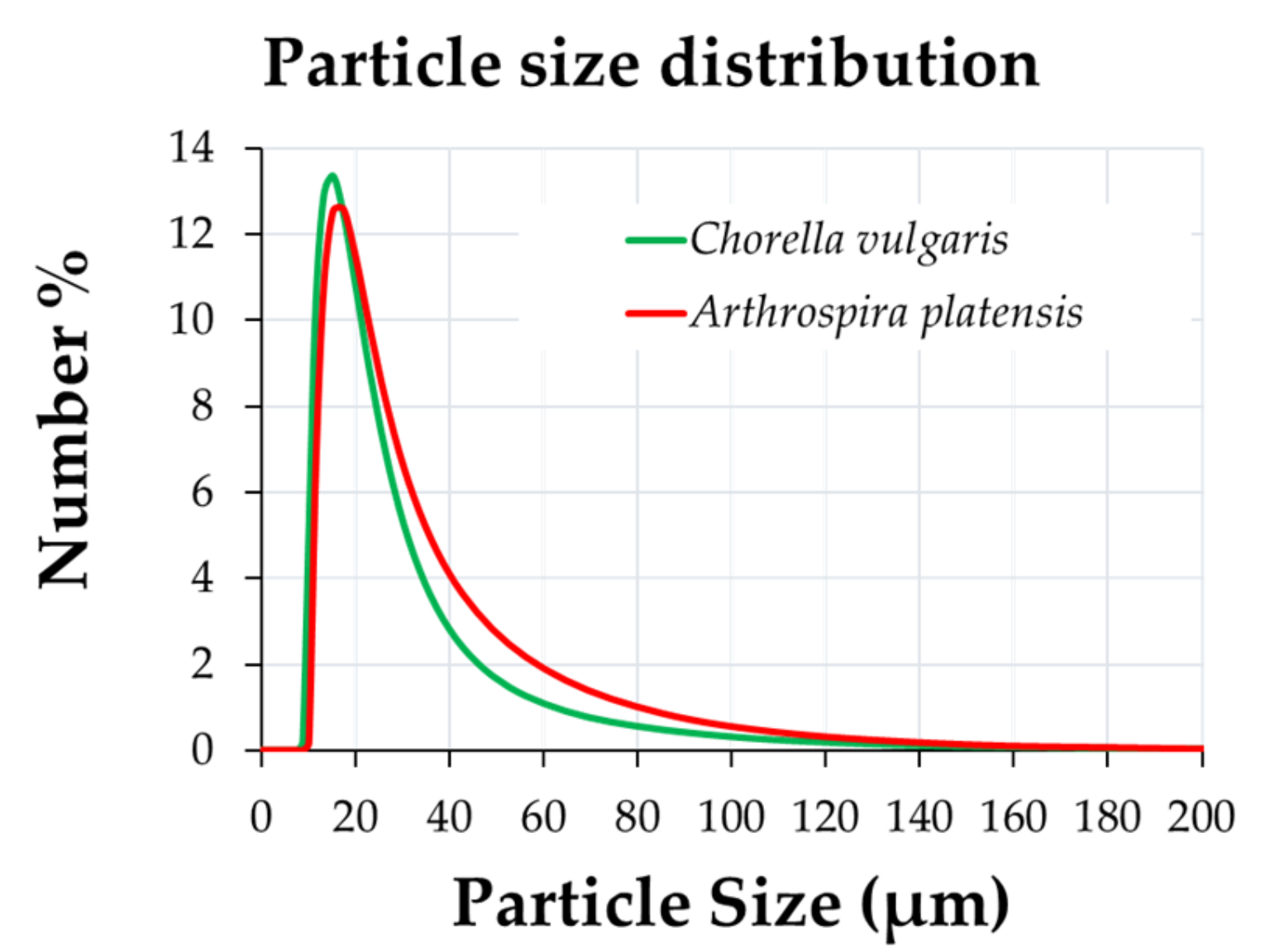
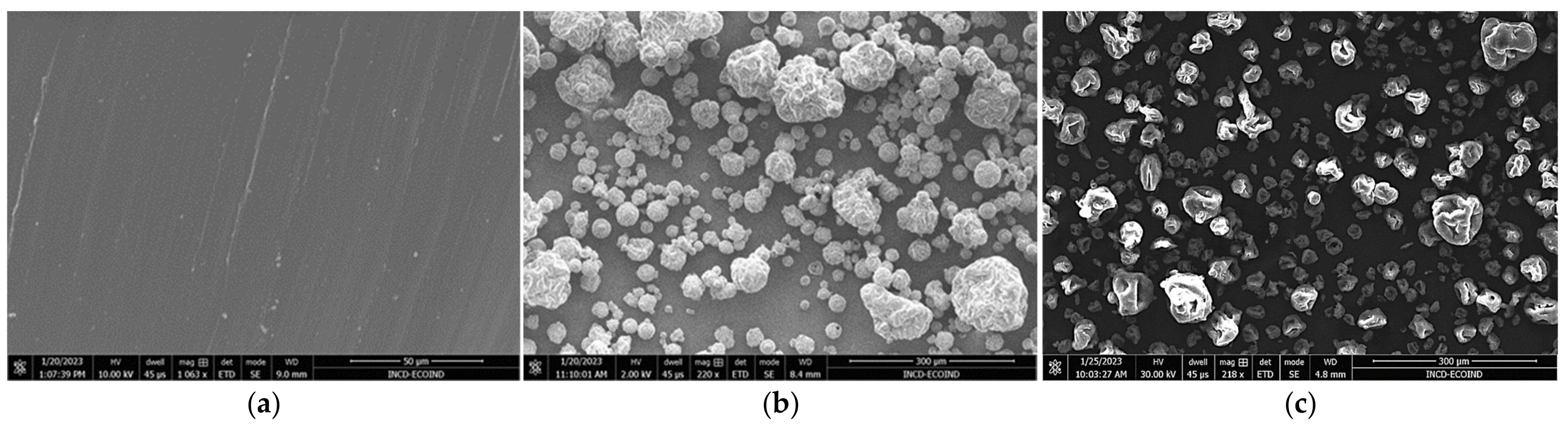
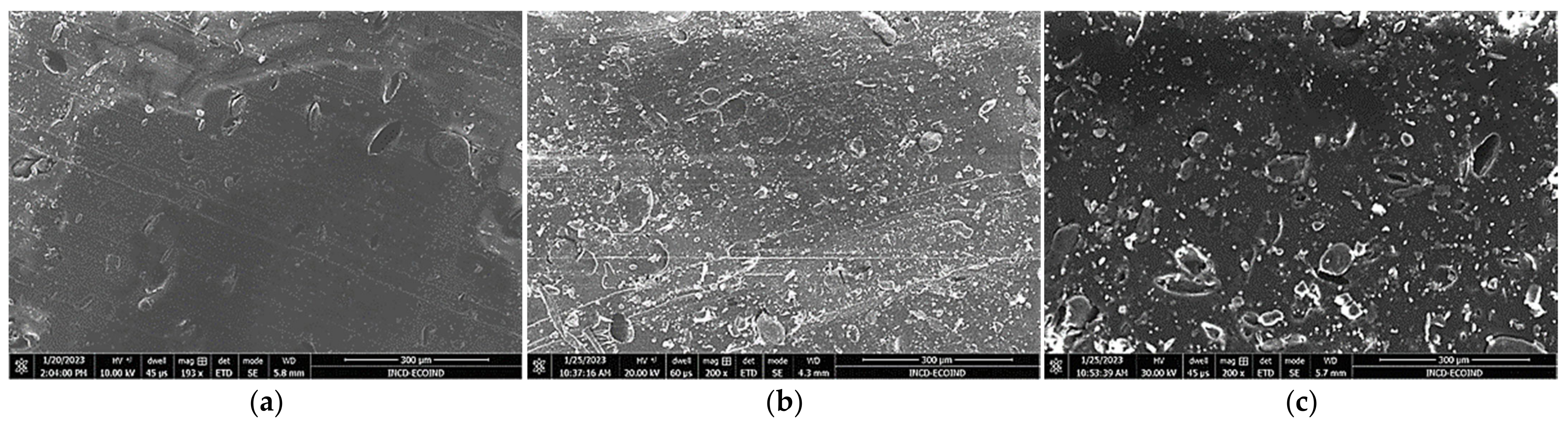




| Macroconstituent Content (g/100 g Dry Biomass) | |||
|---|---|---|---|
| Fats | Carbohydrates | Proteins | |
| Chlorella vulgaris | 10–15 | 15–25 | 55–65 |
| Arthrospira platensis | 5–10 | 10–20 | 60–70 |
| Copolymers 1 | SBSC 2 SBS1/SBS2/Paraffin Oil (25/50/25, w/w/w) | ||
|---|---|---|---|
| SBS1 | SBS2 | ||
| Styrene-Butadiene Ratio | 30/70 | 40/60 | |
| Tensile strength (kg/cm2) | 152 | 203 | 75 |
| Elongation at break (%) | 750 | 700 | 1004 |
| Shore hardness (A) | 75 ± 7 | 90 ± 5 | 55 ± 2 |
| Melt flow rate (g.min−1) | 0.1–5 | 0.5–5 | 2.6–3.6 |
| Mass Loss on Drying (%, w/w) | |||||
|---|---|---|---|---|---|
| 0% | 5% | 10% | 20% | 30% | |
| Chlorella vulgaris MSBS | 3.68 ± 0.52 | 3.91 ± 0.58 | 3.73 ± 0.31 | 3.46 ± 0.42 | - |
| Arthrospira platensis MSBS | 2.88 ± 0.34 | 2.50 ± 0.16 | 3.31 ± 0.28 | 2.96 ± 0.14 | |
| Weight Loss (%, w/w) | ||||||
|---|---|---|---|---|---|---|
| Temp. (°C) | 0% | 5% | 10% | 20% | 30% | |
| Chlorella vulgaris MSBS | 180 °C | 0.61 ± 0.08 | 1.10 ± 0.09 | 1.98 ± 0.13 | 1.78 ± 0.21 | - |
| 200 °C | 2.13 ± 0.17 | 1.25 ± 0.11 | 3.54 ± 0.21 | 4.51 ± 0.34 | ||
| 250 °C | 2.46 ± 0.19 | 5.79 ± 0.37 | 4.79 ± 0.29 | 7.64 ± 0.65 | ||
| Arthrospira platensis MSBS | 180 °C | 0.61 ± 0.07 | 0.92 ± 0.11 | 1.54 ± 0.11 | 2.39 ± 0.17 | 2.86 ± 0.10 |
| 200 °C | 2.13 ± 0.13 | 1.59 ± 0.09 | 2.52 ± 0.16 | 3.12 ± 0.21 | 3.72 ± 0.27 | |
| 250 °C | 2.46 ± 0.13 | 5.39 ± 0.42 | 7.90 ± 0.38 | 9.89 ± 0.63 | 9.54 ± 0.67 | |
| Polymer Composite | Specific Gravity | Melt Flow Rate (g·min−1) | Tensile Strength (kg/cm2) | Elongation at Break (%) | Abrasion Resistance | Solvent Resistance | |||
|---|---|---|---|---|---|---|---|---|---|
| Water | Acetone 90% | Ethanol 90% | Methanol | ||||||
| SBSC | |||||||||
| Chlorella 5% | |||||||||
| Chlorella 10% | |||||||||
| Chlorella 20% | |||||||||
| Spirulina 5% | |||||||||
| Spirulina 10% | |||||||||
| Spirulina 20% | |||||||||
| Spirulina 30% | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| poor | excellent | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumbac, M.; Nicolescu, C.M.; Olteanu, R.L.; Gherghinoiu, S.C.; Bumbac, C.; Tiron, O.; Manea, E.E.; Radulescu, C.; Gorghiu, L.M.; Stanescu, S.G.; et al. Preparation and Characterization of Microalgae Styrene-Butadiene Composites Using Chlorella vulgaris and Arthrospira platensis Biomass. Polymers 2023, 15, 1357. https://doi.org/10.3390/polym15061357
Bumbac M, Nicolescu CM, Olteanu RL, Gherghinoiu SC, Bumbac C, Tiron O, Manea EE, Radulescu C, Gorghiu LM, Stanescu SG, et al. Preparation and Characterization of Microalgae Styrene-Butadiene Composites Using Chlorella vulgaris and Arthrospira platensis Biomass. Polymers. 2023; 15(6):1357. https://doi.org/10.3390/polym15061357
Chicago/Turabian StyleBumbac, Marius, Cristina Mihaela Nicolescu, Radu Lucian Olteanu, Stefan Cosmin Gherghinoiu, Costel Bumbac, Olga Tiron, Elena Elisabeta Manea, Cristiana Radulescu, Laura Monica Gorghiu, Sorina Geanina Stanescu, and et al. 2023. "Preparation and Characterization of Microalgae Styrene-Butadiene Composites Using Chlorella vulgaris and Arthrospira platensis Biomass" Polymers 15, no. 6: 1357. https://doi.org/10.3390/polym15061357








