Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy
Abstract
:1. Introduction
2. CD-Based Polyrotaxanes
2.1. Anticancer Drug–Polyrotaxane Conjugate
2.2. Polyrotaxane-Based Nanoparticles as Anticancer Drug Carriers
2.3. Polyrotaxane-Based Delivery System for Cancer Gene Therapy
3. CD–Drug Polymeric Conjugate
3.1. NLG 207
3.2. CD-CPT Conjugate
3.3. CD–Dox Conjugate
4. CD-based Copolymer
4.1. CD-Grafted Copolymer with Linear Chains
4.2. CD-Based Star-Shaped Copolymers
4.2.1. Stimuli-Responsive Star-Shaped Copolymers
Oxidation- and Reduction-Responsive Copolymers
pH-Responsive Copolymers
Thermoresponsive Polymers
Dual-Responsive Copolymers
4.2.2. Tumor-Targeted Star-Shaped Copolymers
4.3. CDs Grafted on Polymer Chains or Complexed on Polymer Side Chains with Brush-like Structures
4.3.1. Brush-like Copolymers as Hosts (Polymers with Pendant CDs)
4.3.2. Brush-like Copolymers as Guests (Polymers with Pendant Guest Components)
5. CD-Based Crosslinked Structures
5.1. Covalently Crosslinked Structures
5.1.1. CD Crosslinked with Polymers
5.1.2. CD as Monomer
5.2. Crosslinked by Complex Interaction
5.3. Crosslinking by Electrostatic Interaction
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 96, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrin superstructures for drug delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103650. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef]
- Wei, H.; Yu, C.-Y. Cyclodextrin-functionalized polymers as drug carriers for cancer therapy. Biomater. Sci. 2015, 3, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Arisaka, Y.; Yui, N. Polyrotaxane-based biointerfaces with dynamic biomaterial functions. J. Mater. Chem. B 2019, 7, 2123–2129. [Google Scholar] [CrossRef]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef]
- Li, J.J.; Zhao, F.; Li, J. Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 2011, 90, 427–443. [Google Scholar] [CrossRef]
- Ooya, T.; Akutsu, M.; Kumashiro, Y.; Yui, N. Temperature-controlled erosion of poly(N-isopropylacrylamide)-based hydrogels crosslinked by methacrylate-introduced hydrolyzable polyrotaxane. Sci. Technol. Adv. Mater. 2005, 6, 447–451. [Google Scholar] [CrossRef]
- Murayama, H.; Bin Imran, A.; Nagano, S.; Seki, T.; Kidowaki, M.; Ito, K.; Takeoka, Y. Chromic slide-ring gel based on reflection from photonic bandgap. Macromolecules 2008, 41, 1808–1814. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Travelet, C.; Lapp, A.; Lindner, P.; Hadziioannou, G. Topological polymer networks with sliding cross-link points: The “sliding gels”. Relationship between their molecular structure and the viscoelastic as well as the swelling properties. Macromolecules 2007, 40, 535–543. [Google Scholar] [CrossRef]
- Moon, C.; Kwon, Y.M.; Lee, W.K.; Park, Y.J.; Chang, L.-C.; Yang, V.C. A novel polyrotaxane-based intracellular delivery system for camptothecin:In vitro feasibility evaluation. J. Biomed. Mater. Res. Part A 2008, 84, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, A.; Hanif, S.; Ouyang, J.; Tang, Z.; Kong, N.; Kim, N.Y.; Qi, B.; Patel, D.; Shi, B.; Tao, W. Stimuli-responsive prodrug-based cancer nanomedicine. Ebiomedicine 2020, 56, 102821. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Hou, M.; Shi, X.; Chen, J.; Ma, X.; Gao, Y.-E.; Wang, Y.; Xue, P.; Kang, Y.; Xu, Z. Reduction-active polymeric prodrug micelles based on α-cyclodextrin polyrotaxanes for triggered drug release and enhanced cancer therapy. Carbohydr. Polym. 2018, 193, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [Green Version]
- Ezrahi, S.; Aserin, A.; Garti, N. Basic principles of drug delivery and microemulsions-the case of paclitaxel. Adv. Colloid. Interfac. 2019, 263, 95–130. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Wang, X.; Zhen, X.; Zhang, Z.; Wu, W.; Jiang, X. Synthesis of Paclitaxel-Conjugated β-Cyclodextrin Polyrotaxane and Its Antitumor Activity. Angew. Chem. 2013, 125, 7413–7418. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Li, S.; Yin, C.; Li, C.; Wu, W.; Jiang, X. Modification of α-Cyclodextrin Polyrotaxanes by ATRP for Conjugating Drug and Prolonging Blood Circulation. ACS Biomater. Sci. Eng. 2017, 4, 1963–1968. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- He, B.; Gu, Z. Self-assembling polyrotaxanes: Drug carriers for anticancer drugs? Futur. Med. Chem. 2013, 5, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef]
- Tardy, B.L.; Dam, H.H.; Kamphuis, M.M.J.; Richardson, J.J.; Caruso, F. Self-Assembled Stimuli-Responsive Polyrotaxane Core–Shell Particles. Biomacromolecules 2014, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.L.; Tan, S.; Dam, H.H.; Ejima, H.; Blencowe, A.; Qiao, G.G.; Caruso, F. Nanoparticles assembled via pH-responsive reversible segregation of cyclodextrins in polyrotaxanes. Nanoscale 2016, 8, 15589–15596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Liu, R.; Lai, Y.; Li, Y.; Wang, G.; Chang, S.; Gu, Z. Supramolecular nanoparticles generated by the self-assembly of polyrotaxanes for antitumor drug delivery. Int. J. Nanomed. 2012, 7, 5249–5258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.W.; Yu, Y.L.; Chen, Y.H.; Hung, Y.T.; Yiang, G.T. Anticancer effects of methotrexate in combination with α-tocopherol and α-tocopherol succinate on triple-negative breast cancer. Oncol. Rep. 2019, 41, 2060–2066. [Google Scholar] [CrossRef]
- Zhang, L.G.; Su, T.; He, B.; Gu, Z.W. Self-assembly Polyrotaxanes Nanoparticles as Carriers for Anticancer Drug Methotrexate Delivery. Nano-Micro. Lett. 2014, 6, 108–115. [Google Scholar] [CrossRef]
- Tonegawa, A.; Tamura, A.; Yui, N. Emerging Nanoassembly of Polyrotaxanes Comprising Acetylated α-Cyclodextrins and High-Molecular-Weight Axle Polymer. ACS. Macro. Lett. 2019, 8, 826–834. [Google Scholar] [CrossRef]
- Tonegawa, A.; Tamura, A.; Zhang, S.; Yui, N. Hydrophobicity of acyl groups in α-cyclodextrin-threaded polyrotaxanes dominates the formation and stability of self-assembled nanoparticles. Polymer 2020, 200, 122537. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, D.; Pahle, J.; Walther, W. A Brief Introduction to Current Cancer Gene Therapy. Methods. Mol. Biol. 2022, 2521, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.; Mozhi, A.; Zhang, L.; Liu, Y.; Xu, X.; Xing, J.; Liang, X.; Ma, G.; Yang, J.; et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials 2014, 35, 6519–6533. [Google Scholar] [CrossRef]
- Arabi, F.; Mansouri, V.; Ahmadbeigi, N. Gene therapy clinical trials, where do we go? An overview. Biomed. Pharmacother. 2022, 153, 113324. [Google Scholar] [CrossRef]
- Van Hauwermeiren, F.; Vandenbroucke, R.E.; Grine, L.; Puimège, L.; Van Wonterghem, E.; Zhang, H.; Libert, C. Antisense oligonucleotides against TNFR1 prevent toxicity of TNF/IFNγ treatment in mouse tumor models. Int. J. Cancer 2014, 135, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Liang, X.-H.; Baker, B.F.; Crooke, R.M. Antisense technology: A review. J. Biol. Chem. 2021, 296, 100416. [Google Scholar] [CrossRef]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
- Sayed, N.; Allawadhi, P.; Khurana, A.; Singh, V.; Navik, U.; Pasumarthi, S.K.; Khurana, I.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022, 294, 120375. [Google Scholar] [CrossRef]
- Babu, A.; Muralidharan, R.; Amreddy, N.; Mehta, M.; Munshi, A.; Ramesh, R. Nanoparticles for siRNA-Based Gene Silencing in Tumor Therapy. IEEE Trans. Nanobiosci. 2016, 15, 849–863. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yang, Q.Q.; Zhou, Y.L. Non-Viral Delivery of Gene Therapy to the Tendon. Polymers 2022, 14, 3338. [Google Scholar] [CrossRef] [PubMed]
- Kanvinde, S.; Kulkarni, T.; Deodhar, S.; Bhattacharya, D.; Dasgupta, A. Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer. Biotech 2022, 11, 6. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Li, H.; Goh, S.H.; Li, J. Synthesis and characterization of polyrotaxanes consisting of cationic α-cyclodextrins threaded on poly[(ethylene oxide)-ran-(propylene oxide)] as gene carriers. Biomacromolecules 2007, 8, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, V.D.; Aicart, E.; Mondjinou, Y.A.; Johnson, M.A.; Bowman, V.D.; Thompson, D.H. Structure-property relationship for in vitro siRNA delivery performance of cationic 2-hydroxypropyl-β-cyclodextrin: PEG-PPG-PEG polyrotaxane vectors. Biomaterials 2016, 84, 86–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.-Q.; Qi, Y.; Li, R.-Q.; Cheng, G.; Zhao, N.; Xu, F.-J. High-performance cationic polyrotaxanes terminated with polypeptides as promising nucleic acid delivery systems. Polym. Chem. 2018, 9, 2281–2289. [Google Scholar] [CrossRef]
- Kulkarni, A.; DeFrees, K.; Schuldt, R.A.; Vlahu, A.; VerHeul, R.; Hyun, S.-H.; Deng, W.; Thompson, D.H. Multi-armed cationic cyclodextrin:poly(ethylene glycol) polyrotaxanes as efficient gene silencing vectors. Integr. Biol. 2012, 5, 115–121. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Taleghani, A.S.; Asare-Addo, K.; Nokhodchi, A. An updated review of folate-functionalized nanocarriers: A promising ligand in cancer. Drug Discov. Today 2021, 27, 471–489. [Google Scholar] [CrossRef]
- Martín-Sabroso, C.; Torres-Suárez, A.I.; Alonso-González, M.; Fernández-Carballido, A.; Fraguas-Sánchez, A.I. Active Targeted Nanoformulations via Folate Receptors: State of the Art and Future Perspectives. Pharmaceutics 2021, 14, 14. [Google Scholar] [CrossRef]
- Poggetto, G.D.; Troise, S.S.; Conte, C.; Marchetti, R.; Moret, F.; Iadonisi, A.; Silipo, A.; Lanzetta, R.; Malinconico, M.; Quaglia, F.; et al. Nanoparticles decorated with folate based on a site-selective αCD-rotaxanated PEG-b-PCL copolymer for targeted cancer therapy. Polym. Chem. 2020, 11, 3892–3903. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Wang, C.; Li, Y.; Lu, W.; Chen, S.; Luo, J.; Jiang, Y.; Chen, J. Receptor-mediated, tumor-targeted gene delivery using folate-terminated polyrotaxanes. Mol. Pharm. 2012, 9, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Hu, Y.; Xu, C.; Xu, F.-J. Reducible polyrotaxane-based pseudo-comb polycations via consecutive ATRP processes for gene delivery. Acta Biomater. 2016, 32, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Wolfgang, M.; Hwang, J.; Ryan, J.; Eliasof, S. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J. Control. Release 2011, 153, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, C.; Schluep, T.; Hwang, J.; Eliasof, S. CRLX101 (formerly IT-101)—A Novel Nanopharmaceutical of Camptothecin in Clinical Development. Curr. Bioact. Compd. 2011, 7, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. Design and development of IT-101, a cyclodextrin-containing polymer conjugate of camptothecin. Adv. Drug Deliv. Rev. 2009, 61, 1189–1192. [Google Scholar] [CrossRef]
- Dong, H.; Yang, D.; Hu, Y.; Song, X. Recent advances in smart nanoplatforms for tumor non-interventional embolization therapy. J. Nanobiotechnol. 2022, 20, 337. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Schluep, T.; Hwang, J.; Cheng, J.; Heidel, J.D.; Bartlett, D.W.; Hollister, B.; Davis, M.E. Preclinical efficacy of the camptothecin-polymer conjugate it-101 in multiple cancer models. Clin. Cancer Res. 2006, 12, 1606–1614. [Google Scholar] [CrossRef] [Green Version]
- Eliasof, S.; Lazarus, D.; Peters, C.G.; Case, R.I.; Cole, R.O.; Hwang, J.; Schluep, T.; Chao, J.; Lin, J.; Yen, Y.; et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 15127–15132. [Google Scholar] [CrossRef] [Green Version]
- Gaur, S.; Chen, L.; Yen, T.; Wang, Y.; Zhou, B.; Davis, M.; Yen, Y. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 721–730. [Google Scholar] [CrossRef]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J.; et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.J.; Wiley, D.T.; Zuckerman, J.E.; Webster, P.; Chao, J.; Lin, J.; Yen, Y.; Davis, M.E. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc. Natl. Acad. Sci. USA 2016, 113, 3850–3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, J.; Lin, J.; Frankel, P.; Clark, A.J.; Wiley, D.T.; Garmey, E.; Fakih, M.; Lim, D.; Chung, V.; Luevanos, E.; et al. Pilot trial of CRLX101 in patients with advanced, chemotherapy-refractory gastroesophageal cancer. J. Gastrointest. Oncol. 2017, 8, 962–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, E.; Birrer, M.J.; Eliasof, S.; Garmey, E.G.; Lazarus, D.; Lee, C.R.; Man, S.; Matulonis, U.A.; Peters, C.G.; Xu, P.; et al. Translational impact of nanoparticle–drug conjugate crlx101 with or without bevacizumab in advanced ovarian cancer. Clin. Cancer Res. 2015, 21, 808–818. [Google Scholar] [CrossRef] [Green Version]
- Keefe, S.; Hoffman-Censits, J.; Cohen, R.; Mamtani, R.; Heitjan, D.; Eliasof, S.; Nixon, A.; Turnbull, B.; Garmey, E.; Gunnarsson, O.; et al. Efficacy of the nanoparticle–drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: Results of an investigator-initiated phase I–IIa clinical trial. Ann. Oncol. 2016, 27, 1579–1585. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Harris, B.; Saleem, S.; Cook, N.; Searle, E. Targeting hypoxia in solid and haematological malignancies. J. Exp. Clin. Cancer Res. 2022, 41, 318. [Google Scholar] [CrossRef]
- Tian, X.; Nguyen, M.; Foote, H.P.; Caster, J.M.; Roche, K.C.; Peters, C.G.; Wu, P.; Jayaraman, L.; Garmey, E.G.; Tepper, J.E.; et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res. 2017, 77, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Krasner, C.N.; Campos, S.M.; Young, C.L.; Chadda, K.R.; Lee, H.; Birrer, M.J.; Horowitz, N.S.; Konstantinopoulos, P.A.; D'Ascanio, A.M.; Matulonis, U.A.; et al. Sequential Phase II clinical trials evaluating CRLX101 as monotherapy and in combination with bevacizumab in recurrent ovarian cancer. Gynecol. Oncol. 2021, 162, 661–666. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Moon, D.H.; Moore, D.T.; Boles, J.; Bui, C.; Blackstock, W.; O'Neil, B.H.; Subramaniam, S.; McRee, A.J.; Carlson, C.; et al. Phase I/II trial of nano-camptothecin CRLX101 with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 189–195. [Google Scholar] [CrossRef]
- Shi, X.; Hou, M.; Ma, X.; Bai, S.; Zhang, T.; Xue, P.; Zhang, X.; Liu, G.; Kang, Y.; Xu, Z. Starburst Diblock Polyprodrugs: Reduction-Responsive Unimolecular Micelles with High Drug Loading and Robust Micellar Stability for Programmed Delivery of Anticancer Drugs. Biomacromolecules 2019, 20, 1190–1202. [Google Scholar] [CrossRef]
- Gao, Y.-E.; Bai, S.; Shi, X.; Hou, M.; Ma, X.; Zhang, T.; Xiao, B.; Xue, P.; Kang, Y.; Xu, Z. Irinotecan delivery by unimolecular micelles composed of reduction-responsive star-like polymeric prodrug with high drug loading for enhanced cancer therapy. Colloids Surf. B Biointerfaces 2018, 170, 488–496. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.-M.; Chen, Y.; Chen, J.-T.; Liu, Y. Polysaccharide-based Noncovalent Assembly for Targeted Delivery of Taxol. Sci. Rep. 2016, 6, 19212. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.-M.; Li, D.; Sun, H.-L.; Fan, H.-X.; Liu, Y. Camptothecin–Polysaccharide Co-assembly and Its Controlled Release. Bioconjugate Chem. 2016, 27, 2834–2838. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, Y.; Pan, Y.; Xue, X.; Zhang, X.; Kumar, A.; Liang, X.-J. Synergistically Enhanced Therapeutic Effect of a Carrier-Free HCPT/DOX Nanodrug on Breast Cancer Cells through Improved Cellular Drug Accumulation. Mol. Pharm. 2015, 12, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-E.; Bai, S.; Ma, X.; Zhang, X.; Hou, M.; Shi, X.; Huang, X.; Chen, J.; Wen, F.; Xue, P.; et al. Codelivery of doxorubicin and camptothecin by dual-responsive unimolecular micelle-based β-cyclodextrin for enhanced chemotherapy. Colloids Surf. B 2019, 183, 110428. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Qian, Y.; Hu, X.; Liu, S. Multifunctional pH-disintegrable micellar nanoparticles of asymmetrically functionalized β-cyclodextrin-based star copolymer covalently conjugated with doxorubicin and DOTA-Gd moieties. Biomaterials 2012, 33, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Huang, S.; Yang, C.; Wang, M. Unimolecular Micelles of Amphiphilic Cyclodextrin-Core Star-Like Copolymers with Covalent pH-Responsive Linkage of Anticancer Prodrugs. Mol. Pharm. 2017, 14, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hou, M.; Bai, S.; Ma, X.; Gao, Y.-E.; Xiao, B.; Xue, P.; Kang, Y.; Xu, Z.; Li, C.M. Acid-Activatable Theranostic Unimolecular Micelles Composed of Amphiphilic Star-like Polymeric Prodrug with High Drug Loading for Enhanced Cancer Therapy. Mol. Pharm. 2017, 14, 4032–4041. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.P.; Song, X.; Zhuo, L.; Bu, H.; Tian, W. Photo- and pH- Dual-Responsive β-Cyclodextrin-Based Supramolecular Prodrug Complex Self-Assemblies for Programmed Drug Delivery. Chem. Asian J. 2018, 13, 3903–3911. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Polymeric core-shell assemblies mediated by host-guest interactions: Versatile nanocarriers for drug delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 964–968. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, B.; Yao, H.; Lu, X.; Tan, Y.; Wang, B.; Wang, X.; Yang, Z. Schiff-Linked PEGylated Doxorubicin Prodrug Forming pH-Responsive Nanoparticles with High Drug Loading and Effective Anticancer Therapy. Front. Oncol. 2021, 1, 656717. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zheng, N.; Wang, Z. Tetraphenylsilane-Cored Star-Shaped Polymer Micelles with pH/Redox Dual Response and Active Targeting Function for Drug-Controlled Release. Biomacromolecules 2019, 20, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jiang, W.; Xie, X.; Jaiswal, Y.; Williams, L.; Wei, M.; Mo, Y.; Guan, Y.; Yang, H. Preparation and Release of pH-Sensitive β-Cyclodextrin Derivative Micelles Loaded with Paclitaxel. Polymers 2022, 14, 2482. [Google Scholar] [CrossRef]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef]
- Liang, J.; Yang, B.; Zhou, X.; Han, Q.; Zou, J.; Cheng, L. Stimuli-responsive drug delivery systems for head and neck cancer therapy. Drug Deliv. 2021, 28, 272–284. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Li, Z.; Li, Q. Folic acid-modified β-cyclodextrin nanoparticles as drug delivery to load DOX for liver cancer therapeutics. Soft Mater. 2019, 17, 437–447. [Google Scholar] [CrossRef]
- Hyun, H.; Lee, S.; Lim, W.; Jo, D.; Jung, J.S.; Jo, G.; Kim, S.Y.; Lee, D.-W.; Um, S.; Yang, D.H.; et al. Engineered beta-cyclodextrin-based carrier for targeted doxorubicin delivery in breast cancer therapy in vivo. J. Ind. Eng. Chem. 2018, 70, 145–151. [Google Scholar] [CrossRef]
- Bansal, D.; Yadav, K.; Pandey, V.; Ganeshpurkar, A.; Agnihotri, A.; Dubey, N. Lactobionic acid coupled liposomes: An innovative strategy for targeting hepatocellular carcinoma. Drug Deliv. 2014, 23, 140–146. [Google Scholar] [CrossRef]
- Yang, T.; Du, G.; Cui, Y.; Yu, R.; Hua, C.; Tian, W.; Zhang, Y. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int. J. Nanomed. 2019, 14, 1997–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Vrijsen, J.H.; Van de Reydt, E.; Junker, T. Tunable thermoresponsive β-cyclodextrin-based star Polymers. J. Polym. Sci. 2020, 58, 3402–3410. [Google Scholar] [CrossRef]
- Poorghorban, M.; Karoyo, A.H.; Grochulski, P.; Verrall, R.E.; Wilson, L.D.; Badea, I. A 1H NMR Study of Host/Guest Supramolecular Complexes of a Curcumin Analogue with β-Cyclodextrin and a β-Cyclodextrin-Conjugated Gemini Surfactant. Mol. Pharm. 2015, 12, 2993–3006. [Google Scholar] [CrossRef]
- Webber, M.J.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M. Redox-responsive nano-carriers as tumor-targeted drug delivery systems. Eur. J. Med. Chem. 2018, 157, 705–715. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; et al. Disulfide Bond-Driven Oxidation- and Reduction-Responsive Prodrug Nanoassemblies for Cancer Therapy. Nano Lett. 2018, 18, 3643–3650. [Google Scholar] [CrossRef]
- Tu, X.; Meng, C.; Liu, Z.; Sun, L.; Zhang, X.; Zhang, M.; Sun, M.; Ma, L.; Liu, M.; Wei, H. Synthesis and Phase Transition of Poly(N-isopropylacrylamide)-Based Thermo-Sensitive Cyclic Brush Polymer. Polymers 2017, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, J.; Chen, G.; Jiang, M. Dual responsive supramolecular hydrogel with electrochemical activity. Langmuir 2011, 27, 9602–9608. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Peng, J.; Cong, Y.; Dai, X.; Zhang, X.; Zhao, S.; Zhang, X.; Ma, L.; Wang, B.; Wei, H. Fabrication of supramolecular star-shaped amphiphilic copolymers for ROS-triggered drug release. J. Colloid Interface Sci. 2018, 514, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Chen, J.; Xu, R. Recent Progress in Bio-Responsive Drug Delivery Systems for Tumor Therapy. Front. Bioeng. Biotechnol. 2022, 10, 916952. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Gu, Z. Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery. Mater. Today 2016, 19, 274–283. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhang, Z.X.; Ping, Y.; Li, J.; Kang, E.T.; Neoh, K.G. Star-shaped cationic polymers by atom transfer radical polymerization from b-cyclodextrin cores for nonviral gene delivery. Biomacromolecules 2009, 10, 285–293. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, W.; Xu, F.J. New star-shaped carriers composed of β-cyclodextrin cores and disulfide-linked poly(glycidyl methacrylate) derivative arms with plentiful flanking secondary amine and hydroxyl groups for highly efficient gene delivery. ACS Appl. Mater. Interfaces 2012, 5, 703–712. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, W.; Zhao, N.-N.; Ma, J.; Yang, W.-T.; Xu, F.-J. Supramolecular pseudo-block gene carriers based on bioreducible star polycations. Biomaterials 2013, 34, 5411–5422. [Google Scholar] [CrossRef]
- Lv, J.; Liang, R.; Xia, Z.; Li, Y.; Lv, Z.; Hou, D.; Yu, L.; Chen, G.; Liu, Y.; Yang, F. Synthesis and characterization of amphiphilic star-shaped copolymers based on β-cyclodextrin for micelles drug delivery. Drug Dev. Ind. Pharm. 2019, 45, 1017–1028. [Google Scholar] [CrossRef]
- Serrano, A.G.; Pérez-Gil, J. Protein–lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids 2006, 141, 105–118. [Google Scholar] [CrossRef]
- Guagliardo, R.; Pérez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem. Commun. 2015, 51, 15768–15771. [Google Scholar] [CrossRef]
- He, X.; Li, J.; An, S.; Jiang, C. pH-sensitive drug-delivery systems for tumor targeting. Ther. Deliv. 2013, 4, 1499–1510. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Ge, Z.; Liu, S. Stimuli-responsive tertiary amine methacrylate-based block copolymers: Synthesis, supramolecular self-assembly and functional applications. Prog. Polym. Sci. 2014, 39, 1096–1143. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, Q.; Chen, J.; Wang, Y.; Zhang, Q. A novel cyclodextrin-containing pH-responsive star polymer for nanostructure fabrication and drug delivery. Polym. Chem. 2013, 4, 5086–5095. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, M.; Zhang, Z.; Shen, W.; Liu, L.; Zhang, Q. Anti-tumor drug delivery system based on cyclodextrin-containing pH-responsive star polymer: In vitro and in vivo evaluation. Int. J. Pharm. 2014, 474, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Li, H.; Wang, H.; Zhang, T.; Hutchinson, M.R.; Yin, H.; Wang, X. Small-Molecule Modulators of Toll-like Receptors. Acc. Chem. Res. 2020, 53, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yao, N.; Li, H.; Hanson, S.; Han, W.; Wang, C.; Zhang, L. Co-Delivery of Imiquimod and Plasmid DNA via an Amphiphilic pH-Responsive Star Polymer that Forms Unimolecular Micelles in Water. Polymers 2016, 8, 397. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Ma, X.; Hou, M.; Gao, Y.E.; Bai, S.; Xiao, B.; Xue, P.; Kang, Y.; Xu, Z.; Li, C.M. pH-Responsive unimolecular micelles based on amphiphilic star-like copolymers with high drug loading for effective drug delivery and cellular imaging. J. Mater. Chem. B 2017, 5, 6847–6859. [Google Scholar] [CrossRef]
- Jia, T.; Huang, S.; Yang, C.; Wang, M. Unimolecular micelles of pH-responsive star-like copolymers for co-delivery of anticancer drugs and small-molecular photothermal agents: A new drug-carrier for combinational chemo/photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 8514–8524. [Google Scholar] [CrossRef]
- Liu, D.; Sun, J. Thermoresponsive Polypeptoids. Polymers 2020, 12, 2973. [Google Scholar] [CrossRef]
- Geng, C.; Wang, S.; Wang, H. Recent Advances in Thermoresponsive OEGylated Poly(amino acid)s. Polymers 2021, 13, 1813. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, S.; Çakmak, A.S.; Gümüşderelioğlu, M. PNIPAAm-grafted thermoresponsive microcarriers: Surface-initiated ATRP synthesis and characterization. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.; Zheng, J.; Gan, J.; Wu, K.; Lu, M. Thermoresponsive and self-assembly behaviors of poly(oligo(ethylene glycol) methacrylate) based cyclodextrin cored star polymer and pseudo-graft polymer. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 471, 178–189. [Google Scholar] [CrossRef]
- Song, X.; Wen, Y.; Zhu, J.-L.; Zhao, F.; Zhang, Z.-X.; Li, J. Thermoresponsive Delivery of Paclitaxel by β-Cyclodextrin-Based Poly(N-isopropylacrylamide) Star Polymer via Inclusion Complexation. Biomacromolecules 2016, 17, 3957–3963. [Google Scholar] [CrossRef]
- Park, I.-K.; von Recum, H.A.; Jiang, S.; Pun, S.H. Supramolecular assembly of cyclodextrin-based nanoparticles on solid surfaces for gene delivery. Langmuir 2006, 22, 8478–8484. [Google Scholar] [CrossRef]
- Song, X.; Zhu, J.-L.; Wen, Y.; Zhao, F.; Zhang, Z.-X.; Li, J. Thermoresponsive supramolecular micellar drug delivery system based on star-linear pseudo-block polymer consisting of β-cyclodextrin-poly(N-isopropylacrylamide) and adamantyl-poly(ethylene glycol). J. Colloid Interface Sci. 2017, 490, 372–379. [Google Scholar] [CrossRef]
- Fan, X.; Cheng, H.; Wang, X.; Ye, E.; Loh, X.J.; Wu, Y.-L.; Li, Z. Thermoresponsive Supramolecular Chemotherapy by “V”-Shaped Armed β-Cyclodextrin Star Polymer to Overcome Drug Resistance. Adv. Health Mater. 2017, 7, e1701143. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Peng, S.; Tang, Z.; Tan, C.; Ling, J.; Lin, W.; Lin, X.; Zu, X.; Yi, G. pH/Reduction Dual-Stimuli-Responsive Cross-Linked Micelles Based on Multi-Functional Amphiphilic Star Copolymer: Synthesis and Controlled Anti-Cancer Drug Release. Polymers 2020, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Adeli, F.; Abbasi, F.; Babazadeh, M.; Davaran, S. Thermo/pH dual-responsive micelles based on the host–guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery. J. Nanobiotechnol. 2022, 20, 91. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, G.; Wang, N.; Guo, F.; Guo, L.; Liu, X. Synthesis of temperature/pH dual-sensitive supramolecular micelles from β-cyclodextrin-poly(N-isopropylacrylamide) star polymer for drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 136–142. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, F.; Wang, N.; Meng, M.; Li, G. Dual pH-sensitive supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int. J. Biol. Macromol. 2018, 116, 911–919. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Alsaab, H.O.; Sau, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surf. B Biointerfaces 2017, 157, 490–502. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Delgado, A.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, Y.; Furugen, A.; Nishimura, A.; Narumi, K.; Kobayashi, M.; Iseki, K. Evaluation of the effects of antiepileptic drugs on folic acid uptake by human placental choriocarcinoma cells. Toxicol. Vitr. 2018, 48, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, W.; Guo, F.; Yu, N.; Ying, S.; Lou, B.; Wu, J.; Gao, Y.; Ji, X.; Wang, H.; Li, A.; et al. A Novel Folic Acid Receptor-Targeted Drug Delivery System Based on Curcumin-Loaded β-Cyclodextrin Nanoparticles for Cancer Treatment. Drug Des. Dev. Ther. 2021, 15, 2843–2855. [Google Scholar] [CrossRef]
- O'Mahony, A.M.; Ogier, J.; Desgranges, S.; Cryan, J.F.; Darcy, R.; O'Driscoll, C.M. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: Co-formulation opportunities for siRNA delivery. Org. Biomol. Chem. 2012, 10, 4954–4960. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O'Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B 2021, 12, 378–393. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Li, S.; Chen, X.; Fan, X.; Hu, Z.; Wu, Y.-L.; Li, Z. Cyclodextrin based unimolecular micelles with targeting and biocleavable abilities as chemotherapeutic carrier to overcome drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110047. [Google Scholar] [CrossRef]
- Paulos, C.M.; Reddy, J.A.; Leamon, C.P.; Turk, M.J.; Low, P. Ligand binding and kinetics of folate receptor recycling in vivo: Impact on receptor-mediated drug delivery. Mol. Pharmacol. 2004, 66, 1406–1414. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Yin, H.; Zhang, Z.; Li, J. Folic acid modified cationic γ-cyclodextrin-oligoethylenimine star polymer with bioreducible disulfide linker for efficient targeted gene delivery. Biomacromolecules 2013, 14, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, H.; Li, J. Supramolecular self-assembly forming a multifunctional synergistic system for targeted co-delivery of gene and drug. Biomaterials 2014, 35, 1050–1062. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, F.; Zhang, D.; Li, J. Hyaluronic acid conjugated β-cyclodextrin-oligoethylenimine star polymer for CD44-targeted gene delivery. Int. J. Pharm. 2015, 483, 169–179. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, G.; Zhou, Z.; Gao, L.; Tao, Q. Sensitive complex micelles based on host-guest recognition from chitosan-graft-β-cyclodextrin for drug release. Int. J. Biol. Macromol. 2017, 105, 74–80. [Google Scholar] [CrossRef]
- Jain, V.; Bharatam, P.V. Pharmacoinformatic approaches to understand complexation of dendrimeric nanoparticles with drugs. Nanoscale 2013, 6, 2476–2501. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, C.; Zhang, Z.; Liu, K.L.; Chen, J.; Li, J. Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials 2011, 32, 8328–8341. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid–Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, e1803549. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.-M.; Chen, Y.; Chen, J.-T.; Liu, Y. Targeted polysaccharide nanoparticle for adamplatin prodrug delivery. J. Med. Chem. 2013, 56, 9725–9736. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, C.-P.; Chen, D.; Liu, C.-F.; Zhuo, L.-H.; Li, H.; Wang, C.; Bu, H.-T.; Tian, W. β-Cyclodextrin-modified hyaluronic acid-based supramolecular self-assemblies for pH- and esterase- dual-responsive drug delivery. Carbohydr. Polym. 2020, 246, 116654. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, J.; Wang, Y.; Zhong, Y.; Zhang, X.; Li, L.; Wang, J.; Lincoln, S.F.; Guo, X. Redox-Controlled Voltage Responsive Micelles Assembled by Noncovalently Grafted Polymers for Controlled Drug Release. Macromolecules 2019, 52, 1400–1407. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liu, X.; Chen, Y.; Duan, S.; Zhu, R.; Liu, Y.; Yin, L. Recent Advances on Reactive Oxygen Species-Responsive Delivery and Diagnosis System. Biomacromolecules 2019, 20, 2441–2463. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xu, H. Recent Progress in the Biological Applications of Reactive Oxygen Species-Responsive Polymers. Polym. Rev. 2019, 60, 114–143. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Tian, H.; Zhang, M.; He, J.; Ni, P. Facile construction of noncovalent graft copolymers with triple stimuli-responsiveness for triggered drug delivery. Polym. Chem. 2021, 12, 2152–2164. [Google Scholar] [CrossRef]
- Xiong, Q.; Cui, M.; Bai, Y.; Liu, Y.; Liu, D.; Song, T. A supramolecular nanoparticle system based on β-cyclodextrin-conjugated poly-l-lysine and hyaluronic acid for co-delivery of gene and chemotherapy agent targeting hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 155, 93–103. [Google Scholar] [CrossRef]
- Du, F.; Meng, H.; Xu, K.; Xu, Y.; Luo, P.; Luo, Y.; Lu, W.; Huang, J.; Liu, S.; Yu, J. CPT loaded nanoparticles based on beta-cyclodextrin-grafted poly(ethylene glycol)/poly (L-glutamic acid) diblock copolymer and their inclusion complexes with CPT. Colloids. Surf. B. Biointerfaces 2014, 113, 230–236. [Google Scholar] [CrossRef]
- Ren, S.; Chen, D.; Jiang, M. Noncovalently connected micelles based on a β-cyclodextrin-containing polymer and adamantane end-capped poly(ε-caprolactone) via host-guest interactions. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4267–4278. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.; Zhou, Z.; Guo, L.; Liu, X. Supramolecular assembly of poly(β-cyclodextrin) block copolymer and benzimidazole-poly(ε-caprolactone) based on host-guest recognition for drug delivery. Colloids Surf. B Biointerfaces 2017, 160, 364–371. [Google Scholar] [CrossRef]
- Fan, H.; Hu, Q.-D.; Xu, F.-J.; Liang, W.-Q.; Tang, G.-P.; Yang, W.-T. In vivo treatment of tumors using host-guest conjugated nanoparticles functionalized with doxorubicin and therapeutic gene pTRAIL. Biomaterials 2012, 33, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Rahimi, F.; Iranshahi, M.; Kahroba, H.; Zarebkohan, A.; Talebi, M.; Salehi, R.; Mousavi, H.Z. Co-delivery of doxorubicin and conferone by novel pH-responsive β-cyclodextrin grafted micelles triggers apoptosis of metastatic human breast cancer cells. Sci. Rep. 2021, 11, 21425. [Google Scholar] [CrossRef]
- Badwaik, V.; Liu, L.; Gunasekera, D.; Kulkarni, A.; Thompson, D.H. Mechanistic Insight into Receptor-Mediated Delivery of Cationic-β-Cyclodextrin:Hyaluronic Acid-Adamantamethamidyl Host:Guest pDNA Nanoparticles to CD44+ Cells. Mol. Pharm. 2016, 13, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.; VerHeul, R.; DeFrees, K.; Collins, C.J.; Schuldt, R.A.; Vlahu, A.; Thompson, D.H. Microfluidic Assembly of Cationic-β-Cyclodextrin:Hyaluronic Acid-Adamantane Host:Guest pDNA Nanoparticles. Biomater. Sci. 2013, 1, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Onodera, R.; Motoyama, K.; Okamatsu, A.; Higashi, T.; Kariya, R.; Okada, S.; Arima, H. Involvement of cholesterol depletion from lipid rafts in apoptosis induced by methyl-β-cyclodextrin. Int. J. Pharm. 2013, 452, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, W.-H.; Xu, X.-D.; Li, C.; Zhang, J.; Zhuo, R.-X.; Zhang, X.-Z. Design of a cellular-uptake-shielding “plug and play” template for photo controllable drug release. Adv. Mater. 2011, 23, 3526–3530. [Google Scholar] [CrossRef]
- Elamin, K.M.; Motoyama, K.; Higashi, T.; Yamashita, Y.; Tokuda, A.; Arima, H. Dual targeting system by supramolecular complex of folate-conjugated methyl-β-cyclodextrin with adamantane-grafted hyaluronic acid for the treatment of colorectal cancer. Int. J. Biol. Macromol. 2018, 113, 386–394. [Google Scholar] [CrossRef]
- Pace, G.; Ferri, V.; Grave, C.; Elbing, M.; von Hänisch, C.; Zharnikov, M.; Mayor, M.; Rampi, M.A.; Samorì, P. Cooperative light-induced molecular movements of highly ordered azobenzene self-assembled monolayers. Proc. Natl. Acad. Sci. USA 2007, 104, 9937–9942. [Google Scholar] [CrossRef] [Green Version]
- Elamin, K.M.; Yamashita, Y.; Higashi, T.; Motoyama, K.; Arima, H. Supramolecular Complex of Methyl-β-cyclodextrin with Adamantane-Grafted Hyaluronic Acid as a Novel Antitumor Agent. Chem. Pharm. Bull. 2018, 66, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.; Deng, W.; Hyun, S.H.; Thompson, D.H. Development of a low toxicity, effective pDNA vector based on noncovalent assembly of bioresponsive amino-β-cyclodextrin:adamantane-poly(vinyl alcohol)-poly(ethylene glycol) transfection complexes. Bioconjug. Chem. 2012, 23, 933–940. [Google Scholar] [CrossRef]
- Kulkarni, A.; DeFrees, K.; Hyun, S.H.; Thompson, D.H. Pendant polymer:amino-β-cyclodextrin:siRNA guest:host nanoparticles as efficient vectors for gene silencing. J. Am. Chem. Soc. 2012, 134, 7596–7599. [Google Scholar] [CrossRef]
- Kulkarni, A.; Badwaik, V.; DeFrees, K.; Schuldt, R.A.; Gunasekera, D.S.; Powers, C.; Vlahu, A.; Verheul, R.; Thompson, D.H. Effect of pendant group on pDNA delivery by cationic-β-cyclodextrin: Alkyl-PVA-PEG pendant polymer complexes. Biomacromolecules 2013, 15, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Gami, P.; Kundu, D.; Seera, S.D.K.; Banerjee, T. Chemically crosslinked xylan–β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 158, 18–31. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Xiong, J.; Wu, D.; Li, S.; Tao, Y.; Qin, Y.; Kong, Y. Facile synthesis of chitosan-grafted beta-cyclodextrin for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2019, 125, 941–947. [Google Scholar] [CrossRef] [PubMed]
- El-Zeiny, H.M.; Abukhadra, M.R.; Sayed, O.M.; Osman, A.H.; Ahmed, S.A. Insight into novel β-cyclodextrin-grafted-poly (N-vinylcaprolactam) nanogel structures as advanced carriers for 5-fluorouracil: Equilibrium behavior and pharmacokinetic modeling. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 586, 124197. [Google Scholar] [CrossRef]
- Ping, Y.; Hu, Q.; Tang, G.; Li, J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials 2013, 34, 6482–6494. [Google Scholar] [CrossRef] [PubMed]
- Giglio, V.; Viale, M.; Bertone, V.; Maric, I.; Vaccarone, R.; Vecchio, G. Cyclodextrin polymers as nanocarriers for sorafenib. Investig. New Drugs 2017, 36, 370–379. [Google Scholar] [CrossRef]
- Giglio, V.; Viale, M.; Monticone, M.; Aura, A.M.; Spoto, G.; Natile, G.; Intini, F.P.; Vecchio, G. Cyclodextrin polymers as carriers for the platinum-based anticancer agent LA-12. RSC Adv. 2016, 6, 12461–12466. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2022, 12, 803304. [Google Scholar] [CrossRef]
- Bognanni, N.; Viale, M.; Distefano, A.; Tosto, R.; Bertola, N.; Loiacono, F.; Ponassi, M.; Spinelli, D.; Pappalardo, G.; Vecchio, G. Cyclodextrin Polymers as Delivery Systems for Targeted Anti-Cancer Chemotherapy. Molecules 2021, 26, 6046. [Google Scholar] [CrossRef] [PubMed]
- Viale, M.; Tosto, R.; Giglio, V.; Pappalardo, G.; Oliveri, V.; Maric, I.; Mariggiò, M.A.; Vecchio, G. Cyclodextrin polymers decorated with RGD peptide as delivery systems for targeted anti-cancer chemotherapy. Investig. New Drugs 2019, 37, 771–778. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Cazaux, F.; Blanchemain, N.; Flament, M.-P.; Martel, B. New multifunctional pharmaceutical excipient in tablet formulation based on citric acid-cyclodextrin polymer. Int. J. Pharm. 2016, 511, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Malanga, M.; Manet, I.; Manoli, F.; Tuza, K.; Aykaç, A.; Ladavière, C.; Fenyvesi, E.; Vargas-Berenguel, A.; Gref, R.; et al. Citric acid–γ-cyclodextrin crosslinked oligomers as carriers for doxorubicin delivery. Photochem. Photobiol. Sci. 2013, 12, 1841–1854. [Google Scholar] [CrossRef]
- Daga, M.; de Graaf, I.A.; Argenziano, M.; Barranco, A.S.M.; Loeck, M.; Al-Adwi, Y.; Cucci, M.A.; Caldera, F.; Trotta, F.; Barrera, G.; et al. Glutathione-responsive cyclodextrin-nanosponges as drug delivery systems for doxorubicin: Evaluation of toxicity and transport mechanisms in the liver. Toxicol. Vitr. 2020, 65, 104800. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; Ullio, C.; Argenziano, M.; Dianzani, C.; Cavalli, R.; Trotta, F.; Ferretti, C.; Zara., G.P.; Gigliotti, C.L.; Ciamporcero, E.S.; et al. GSH-targeted nanosponges increase doxorubicin-induced toxicity "in vitro" and "in vivo" in cancer cells with high antioxidant defenses. Free Radic. Biol. Med. 2016, 97, 24–37. [Google Scholar] [CrossRef]
- Trotta, F.; Caldera, F.; Dianzani, C.; Argenziano, M.; Barrera, G.; Cavalli, R. Glutathione Bioresponsive Cyclodextrin Nanosponges. ChemPlusChem 2016, 81, 434. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Lombardi, C.; Ferrara, B.; Trotta, F.; Caldera, F.; Blangetti, M.; Koltai, H.; Kapulnik, Y.; Yarden, R.; Gigliotti, L.; et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget 2018, 9, 35813–35829. [Google Scholar] [CrossRef] [Green Version]
- Palminteri, M.; Dhakar, N.K.; Ferraresi, A.; Caldera, F.; Vidoni, C.; Trotta, F.; Isidoro, C. Cyclodextrin nanosponge for the GSH-mediated delivery of Resveratrol in human cancer cells. Nanotheranostics 2021, 5, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, J.; Prucková, Z.; Pospíšil, T.; Kašpárková, V.; Rouchal, M.; Vícha, R. A New Hyaluronan Modified with β-Cyclodextrin on Hydroxymethyl Groups Forms a Dynamic Supramolecular Network. Molecules 2019, 24, 3849. [Google Scholar] [CrossRef] [Green Version]
- Qian, Q.; Shi, L.; Gao, X.; Ma, Y.; Yang, J.; Zhang, Z.; Qian, J.; Zhu, X. A Paclitaxel-Based Mucoadhesive Nanogel with Multivalent Interactions for Cervical Cancer Therapy. Small 2019, 15, 1903208. [Google Scholar] [CrossRef]
- Namgung, R.; Lee, Y.M.; Kim, J.; Jang, Y.; Lee, B.-H.; Kim, I.-S.; Sokkar, P.; Rhee, Y.M.; Hoffman, A.S.; Kim, W.J. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat. Commun. 2014, 5, 3702. [Google Scholar] [CrossRef] [Green Version]
- Karpkird, T.; Manaprasertsak, A.; Penkitti, A.; Sinthuvanich, C.; Singchuwong, T.; Leepasert, T. A novel chitosan-citric acid crosslinked beta-cyclodextrin nanocarriers for insoluble drug delivery. Carbohydr. Res. 2020, 498, 108184. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, K.M.; Lee, S.C.; Cho, Y.W.; Lee, J.; Jeong, J.H.; Park, K. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Control. Release 2005, 101, 59–68. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Wang, J.; Li, H.; Yang, H. Glycyrrhetinic acid-cyclodextrin grafted pullulan nanoparticles loaded doxorubicin as a liver targeted delivery carrier. Int. J. Biol. Macromol. 2022, 216, 789–798. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, Y.I.; Kim, S.H.; Lee, Y.M.; Cho, C.S. Clonazepam release from coreshell type nanoparticles in vitro arch. Pharm. Res. 1997, 20, 324–329. [Google Scholar]
- Gao, H.; Wang, Y.-N.; Fan, Y.-G.; Ma, J.-B. Conjugates of poly(DL-lactide-co-glycolide) on amino cyclodextrins and their nanoparticles as protein delivery system. J. Biomed. Mater. Res. Part A 2006, 80A, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.H.; Peng, H.S.; Liu, C.; Lia, D.; Yin, Y.; Lu, B.; Zheng, H.; Wang, Q. Dual pHresponsive-charge-reversal micelle platform for enhanced anticancer therapy. Mater. Sci. Eng. C 2021, 118, 111527–111540. [Google Scholar] [CrossRef]
- Pop, E.; Loftsson, T.; Bodor, N. Solubilization and Stabilization of a Benzylpenicillin Chemical Delivery System by 2-Hydroxypropyl-β-cyclodextrin. Pharm. Res. 1991, 08, 1044–1049. [Google Scholar] [CrossRef]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Ansari, M.J.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Jamil, S.; Al-Shdefat, R.; Ali, B.E. Solubility and stability enhance-ment of curcumin through cyclodextrin complexation. IJBPAS 2014, 3, 2668–2675. [Google Scholar]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Carboxylate cross-linked cyclodextrin: A nanoporous scaffold for enhance-ment of rosuvastatin oral bioavailability. Eur. J. Pharm. Sci. 2018, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Agnes, M.; Yannakopoulou, K.; Fenyvesi, É.; Loftsson, T. Self-assembled cyclodextrin-based nanoparticles for meropenem stabilization. J. Drug Deliv. Sci. Technol. 2018, 45, 20–27. [Google Scholar] [CrossRef]

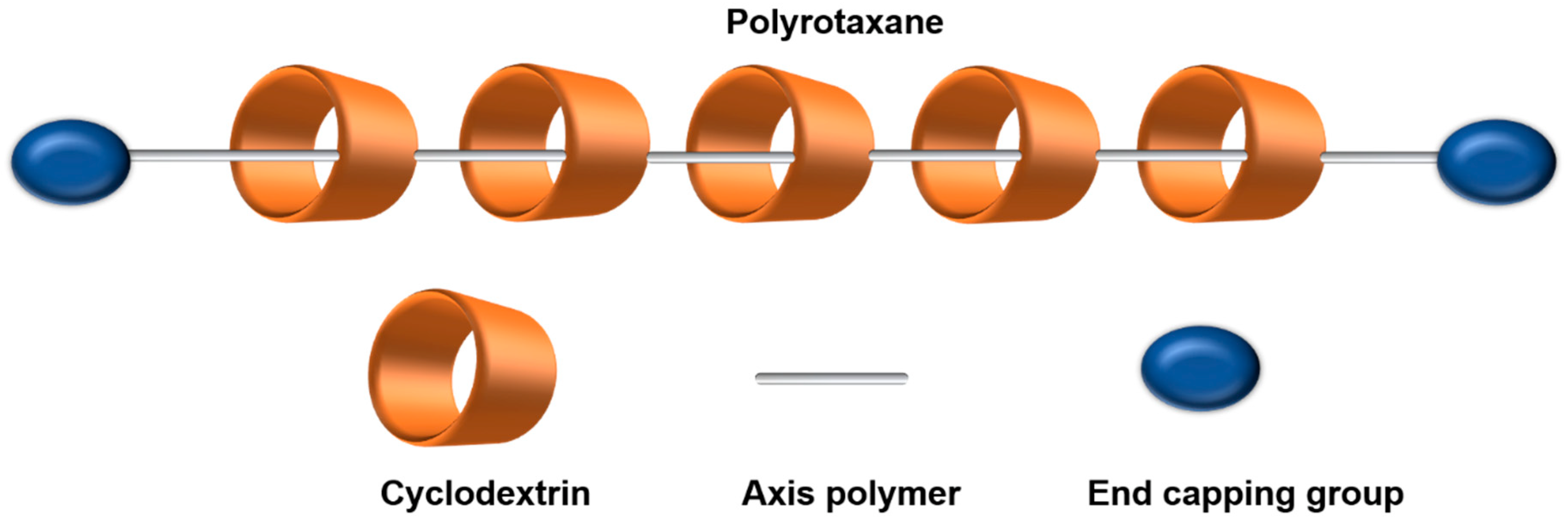


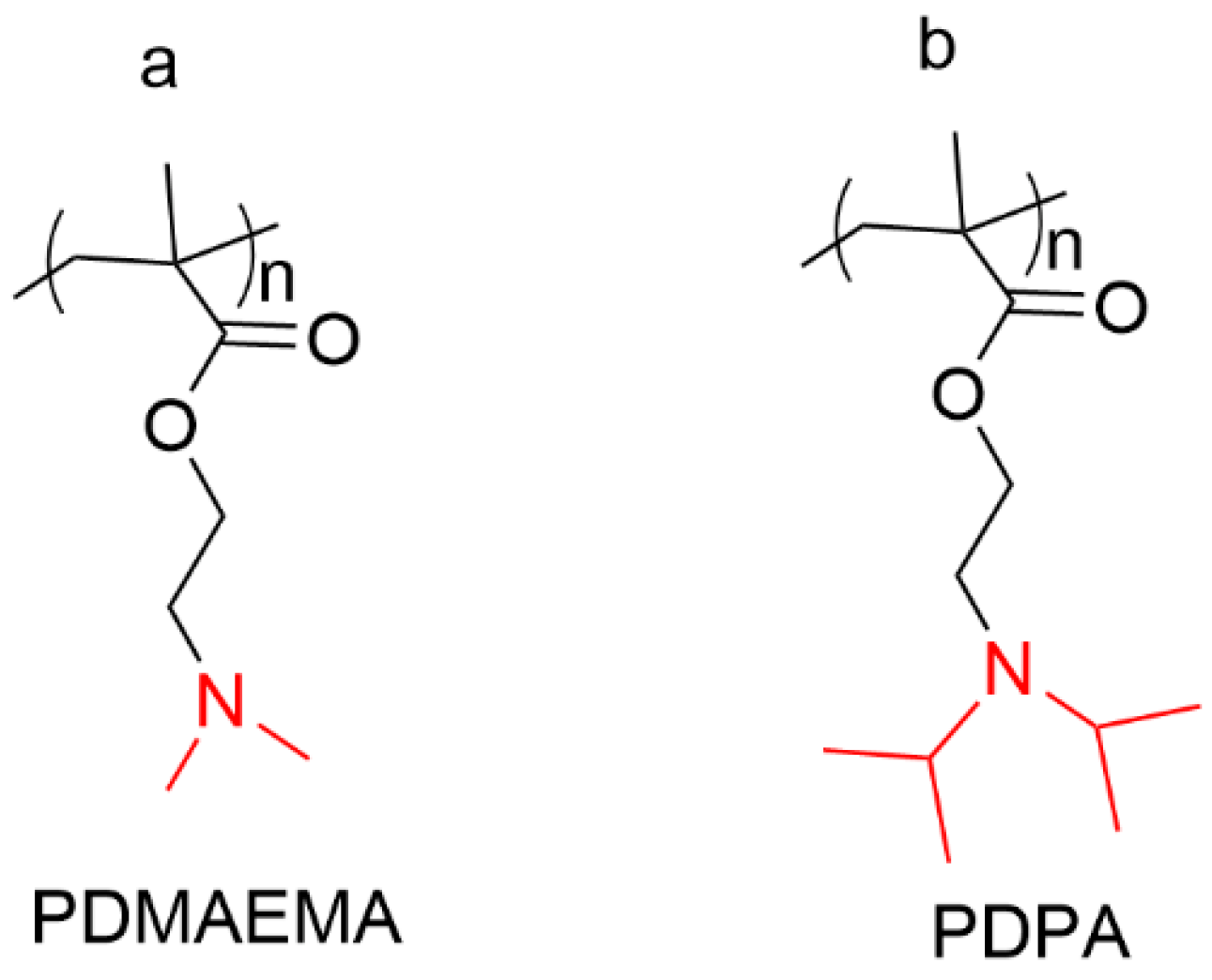
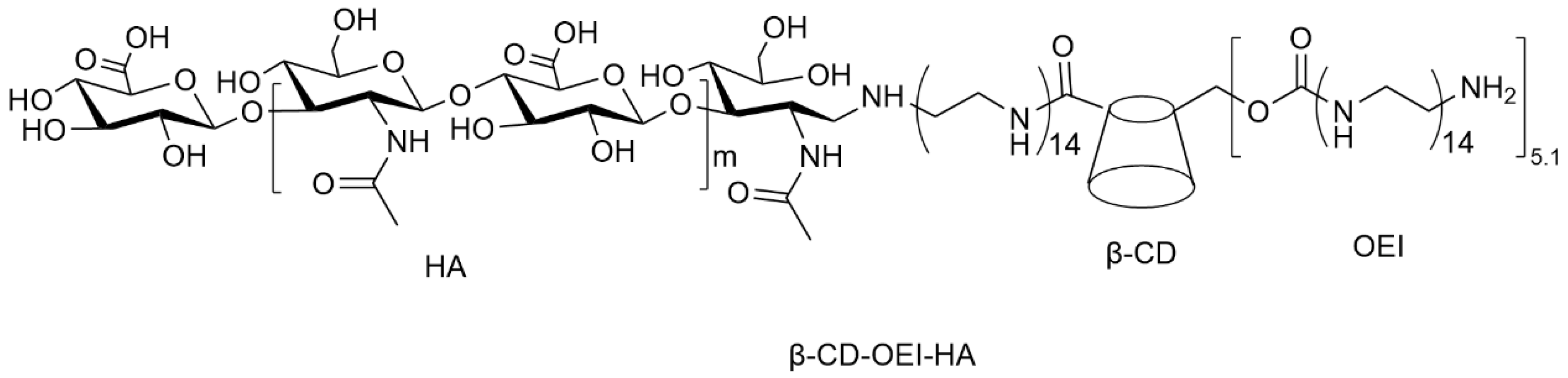
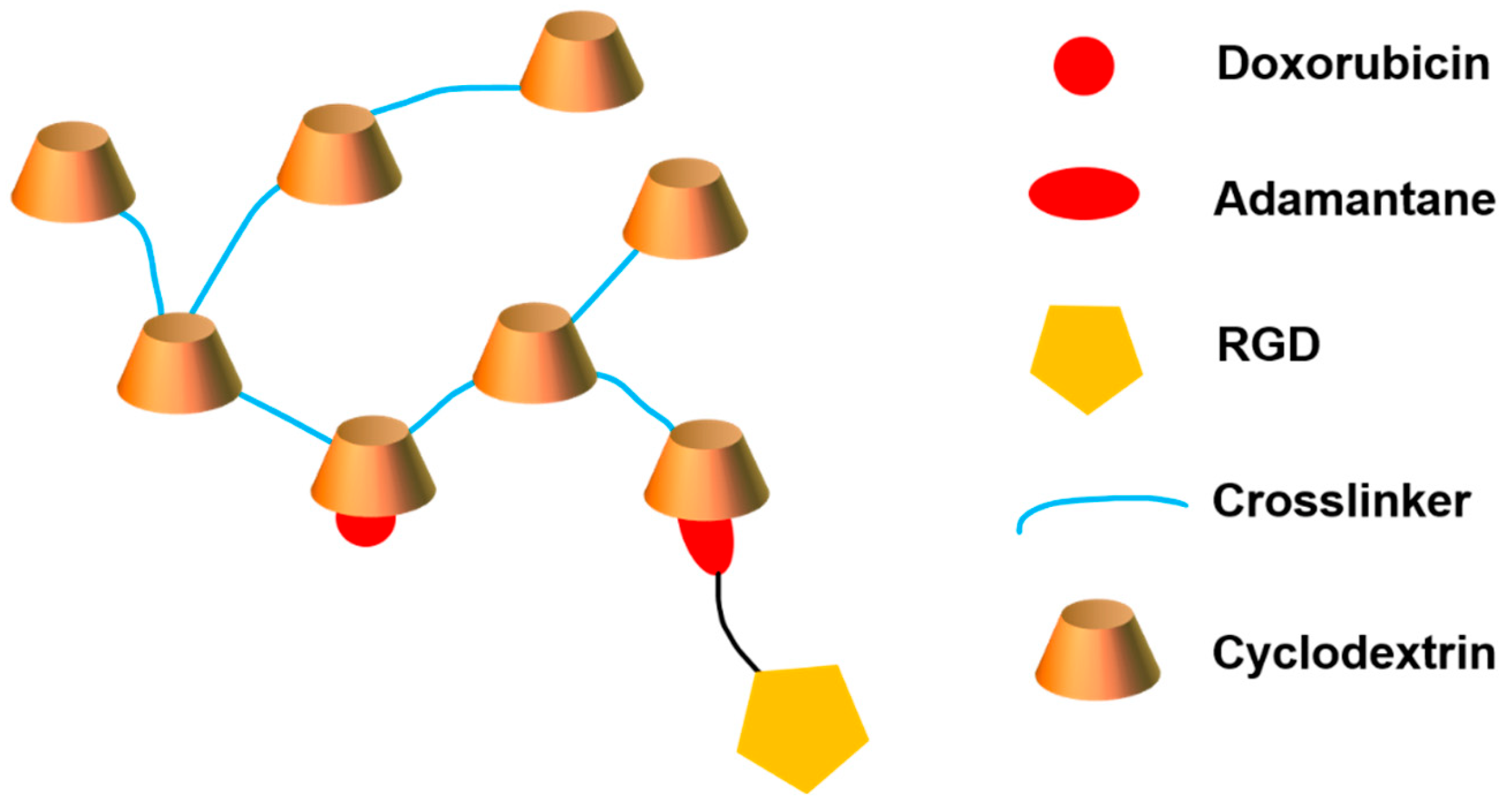
| Structure Characteristics | Drug Loading | Stimuli-Responsive | Targeting | ||
|---|---|---|---|---|---|
| Polyrotaxane | CD cavity occupied by polymers | Conjugated on polymer chains or CDs/encapsulated in the nanoparticles | Y b | Y c | |
| CDP conjugate a | Drug conjugated on CD polymer | Conjugated on CDs or polymers | Y b | Y c | |
| Copolymer | Linear-shaped | Linear chains | Encapsulated in the micelles | Y b | Y c |
| Star-shaped | CD as the core with multipolymer arms grafted on it | Encapsulated in the micelles or included in the CD cavity | |||
| Brush-shaped | CDs grafted on polymer chains or complex with guests grafted on polymer chains | Encapsulated in the nanoparticles or included in the CD cavity | |||
| Crosslinked polymer | Covalent crosslinking | CD as monomer or crosslinked with polymers | Encapsulated in the network or included in the CD cavity | Y b | Y c |
| Complex crosslinking | Crosslinked by complex effect | Conjugated on the polymer | |||
| Electrostatic crosslinking | Crosslinked by electrostatic effect | Included in the CD cavity | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, J.; Qiu, N. Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers 2023, 15, 1400. https://doi.org/10.3390/polym15061400
Li X, Liu J, Qiu N. Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers. 2023; 15(6):1400. https://doi.org/10.3390/polym15061400
Chicago/Turabian StyleLi, Xuebing, Junda Liu, and Neng Qiu. 2023. "Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy" Polymers 15, no. 6: 1400. https://doi.org/10.3390/polym15061400
APA StyleLi, X., Liu, J., & Qiu, N. (2023). Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers, 15(6), 1400. https://doi.org/10.3390/polym15061400





